Abstract
Rett Syndrome, an X-linked dominant neurodevelopmental disorder characterized by regression of language and hand use, is primarily caused by mutations in methyl-CpG-binding protein 2 (MECP2). Loss of function mutations in MECP2 are also found in other neurodevelopmental disorders such as autism, Angelman-like syndrome and non-specific mental retardation. Furthermore, duplication of the MECP2 genomic region results in mental retardation with speech and social problems. The common features of human neurodevelopmental disorders caused by the loss or increase of MeCP2 function suggest that even modest alterations of MeCP2 protein levels result in neurodevelopmental problems. To determine whether a small reduction in MeCP2 level has phenotypic consequences, we characterized a conditional mouse allele of Mecp2 that expresses 50% of the wild-type level of MeCP2. Upon careful behavioral analysis, mice that harbor this allele display a spectrum of abnormalities such as learning and motor deficits, decreased anxiety, altered social behavior and nest building, decreased pain recognition and disrupted breathing patterns. These results indicate that precise control of MeCP2 is critical for normal behavior and predict that human neurodevelopmental disorders will result from a subtle reduction in MeCP2 expression.
INTRODUCTION
Rett Syndrome (RTT, OMIM #312750) is an X-linked neurodevelopmental disorder characterized by regression of language and hand use after a period of normal initial cognitive development (1). During this regression, autistic features can manifest, sometimes leading to the misdiagnosis of autism. After regression, characteristic clinical features such as distinctive hand stereotypies, movement abnormalities, breathing irregularities, autonomic dysfunction, seizures and sleep disruption become prominent. The disorder primarily affects girls at a frequency of 1:10 000–20 000 live female births (2).
Mutations in Methyl-CpG-Binding Protein 2 (MECP2) are found in over 95% of typical RTT (3), with ∼70% of these cases caused by point mutations. MeCP2 primarily functions as a transcriptional repressor (4,5) by recruiting histone deacetylases to DNA that contains the epigenetic mark of methylated cytosines (6). Point mutations in MECP2 behave similarly to a complete deletion of the coding sequence of MECP2, indicating that they are complete or partial loss of function alleles (3). In females, these point mutations cause features characteristic of RTT; in contrast, the same point mutations may result in severe infantile encephalopathy and early death in males (7). Additionally, a number of point mutations in MECP2 not associated with RTT have been identified in males with moderate mental retardation, movement abnormalities and psychiatric features (8–16). These mutations are often found in X-linked mental retardation (XLMR) families with the female carriers displaying mild mental retardation or learning disabilities.
The discovery that mutations in MECP2 cause the neurodevelopmental disorder RTT led to the analysis of MECP2 in other neurodevelopmental disorders with similar clinical features. For example, MECP2 mutations have subsequently been discovered in girls with a number of disorders such as idiopathic autism (17), Angelman-like syndrome (15) and mental retardation (18). In addition, MeCP2 expression is decreased in the brains of individuals with neurodevelopmental disorders such as autism, Down syndrome, Angelman syndrome and Prader–Willi syndrome (19,20). Interestingly, duplications of the genomic region spanning MECP2 have been discovered in humans who have neurological abnormalities (21–26). Features of this disorder are typically found in males and are characterized by hypotonia, cognitive impairment, autistic features and language deficits. Of note, the human disorder was initially predicted by abnormalities observed in transgenic mice that over-express MeCP2 2-fold (27). These results point to the importance of MeCP2 expression levels and suggest that neurodevelopmental disorders may result from either increases or decreases in MeCP2 levels.
To determine whether a slight reduction in MeCP2 level resulted in phenotypic abnormalities, we made use of a conditional allele of Mecp2 (‘floxed’) that retains a neomycin selection cassette and polyadenylation sequence within the 3′-UTR (28) which disrupts the Mecp2 3′-UTR isoform that predominates in the brain (29). In many instances, retention of selection cassettes has resulted in the generation of hypomorphic alleles of the engineered locus (30). When tested, we found that the ‘floxed’ allele of Mecp2 caused reduced expression of both Mecp2 mRNA and MeCP2 protein by ∼50% and resulted in a broad spectrum of phenotypic abnormalities. This demonstrates that precise MeCP2 levels are critical for neuronal function and that slight reduction in MeCP2 levels in humans is likely to result in clinical abnormalities.
RESULTS
The Mecp2Flox allele decreases mRNA levels of both Mecp2 isoforms and reduces MeCP2 protein level in the brain
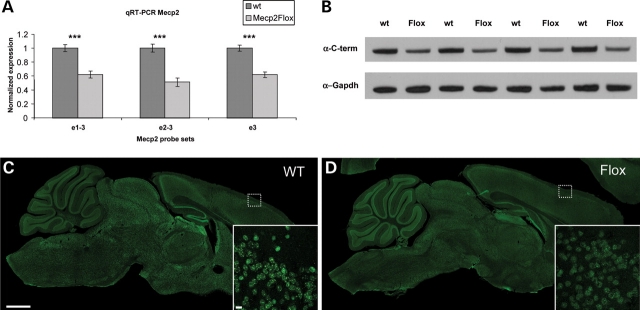
To investigate the molecular consequences of the ‘floxed’ Mecp2 allele, we used quantitative real-time PCR (qRT-PCR) to measure the expression level of Mecp2 mRNA in F1 129S6.B6 Mecp2Flox/y animals and wild-type (WT) littermate controls at 26–27 weeks of life. Mecp2 is expressed as two isoforms that differ in the choice of start sites (31,32). In the e1 isoform, exon 1 provides the start site and is spliced to the common exon 3. The e2 isoform is formed by utilizing the start site in exon 2, which is then spliced to exon 3. We designed qRT-PCR primers and probes that either span the exon 1–3 boundary (e1–3) or span the exon 2–3 boundary (e2–3) to quantify the expression of these Mecp2 isoforms. Additionally, we designed a qRT-PCR primer/probe set that is entirely contained within the common exon 3, allowing us to quantify the total Mecp2 transcript level. We find that the Mecp2Flox allele results in an ∼50% decrease in the brain in isoform e1, isoform e2 and exon 3 (Fig. 1A).
Figure 1.
The expression of Mecp2 is decreased in Mecp2Flox/y mice at both the mRNA and the protein level. The mRNA level as measured by qRT-PCR (A) is decreased in mutant animals. Notably, the e1 isoform (measured by a probe spanning the exons 1–3 boundary, e1–3), the e2 isoform (measured by a probe spanning exons 2–3 boundary, e2–3) and the common exon 3 (probe e3) all are decreased by ∼50% in Mecp2Flox/y mice compared with wild-type littermates. The overall protein levels of MeCP2 measured by western blot (B) are decreased in Mecp2Flox/y animals compared with wild-type littermates. MeCP2 was detected by an antibody specific to the common carboxy-terminus (α-C-term). The bottom blot in (B) is a loading control probed with an antibody to Gapdh. Each lane represents biological replicates of the respective genotypes. The decrease in MeCP2 protein level is also observed by immunofluorescence (C, D). Whereas the expression of MeCP2 is clearly discernable as relatively bright nuclear foci in wild-type animals (C), in Mecp2Flox/y animals the signal is weaker (D). The small dashed box shows the cortical region represented at higher magnification in the inset in (C) and (D). The higher magnification reveals decreased MeCP2 levels, but a similar cellular distribution of MeCP2. The scale bar in the large figure in (C) represents 1 mm and within the inset represents 10 µm.
To assess whether the reduction in Mecp2 mRNA also resulted in a comparable decrease in protein expression, we analyzed whole brain extracts by western immunoblotting (Fig. 1B) from F1 129S6.B6 Mecp2Flox/y animals and WT littermate controls at 26–27 weeks of life. We used an antibody that recognizes the common carboxy-terminus (C-term) of MeCP2. MeCP2 protein levels are decreased in Mecp2Flox/y animals compared with WT littermate controls. Quantification of band intensity normalized to Gapdh revealed that MeCP2 levels in Mecp2Flox/y animals are decreased by 42% compared with WT controls (P < 0.001).
MeCP2 is decreased by immunofluorescence but the cellular pattern is unchanged
As a complementary approach to examine MeCP2 protein levels and to determine whether the reduction in MeCP2 expression was uniform throughout the whole brain, we analyzed sagittal sections of brain tissue obtained from Mecp2Flox/y animals. Indeed, when identical settings were used to capture confocal images of a WT littermate control animal (Fig. 1C) and an F1 129S6.B6 Mecp2Flox/y animal (Fig. 1D), the overall MeCP2 expression is markedly attenuated in the mutant animals. Higher power magnification (inset in Fig. 1C and D) reveals that MeCP2 retains the same overall punctuate nuclear staining pattern in the mutant animals as the WT animals, but the expression intensity is reduced. We observed similar attenuated MeCP2 levels in animals in the F1 129S6.FVB strain (data not shown).
Mecp2Flox/y animals have normal survival and slight weight alterations in certain genetic strain backgrounds
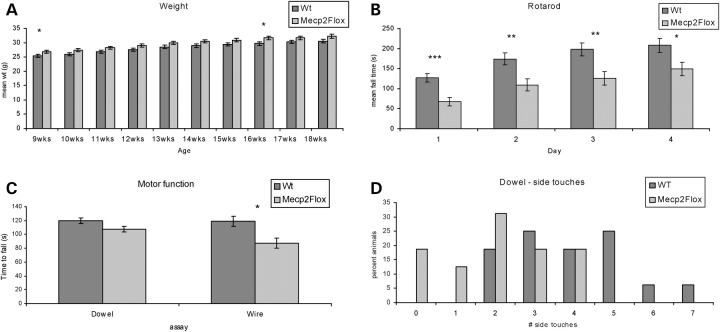
Because the complete absence of Mecp2 results in death between 8 and 12 weeks of life (28,33), we first assessed whether the ∼50% reduction of MeCP2 in the Mecp2Flox allele has any effect on lifespan. We did not observe early mortality in Mecp2Flox/y animals in either a pure 129S6/SvEvTac (129S6) background, an F1 129S6/SvEvTac.C57BL/6J (F1 129S6.B6), or an F1 129S6/SvEvTac.FVB/N (F1 129S6.FVB). Furthermore, we did not observe any of the overt abnormalities seen in the Mecp2–null animals, such as gross body tremor or hindlimb clasping, in Mecp2Flox/y animals. However, on the F1 129S6.B6 background, the Mecp2Flox/y animals are ∼1 g heavier than WT littermate control animals (Fig. 2A) at 9 and 16 weeks of life. This mild increase in body weight was not observed in the F1 129.FVB background (not shown).
Figure 2.
Mecp2Flox/y mice are heavier and perform poorly on motor tasks. Mecp2Flox/y mice (F1 129S6.B6, n = 16 for each genotype) are ∼1 g heavier than littermate wild-type (WT) controls, although this finding is only significant at 9 and 16 weeks of life (A). The mice perform poorly on a variety of motor tasks including accelerating rotarod (B), dowel walking (C and D) and wire hanging (C). On the accelerating rotarod, the mutant mice perform poorly on Day 1, which indicates an inherent coordination deficit. The Mecp2Flox/y mice fall off the wire sooner than controls (C, P = 0.002, Mann–Whitney) but not on the dowel (P = 0.12, Mann–Whitney). Additionally, the Mecp2Flox/y animals have fewer side touches in both the dowel task (D, P = 0.013 Mann–Whitney) and the wire hang task (not shown, P = 0.002 Mann–Whitney). *P < 0.05, **P < 0.01, ***P < 0.001.
Mecp2Flox/y animals have decreased motor performance
Despite the lack of lethality and absence of overt hindlimb clasping, we assessed the performance of the mutant mice on a variety of motor tasks. In the accelerating rotating rod task, a test for motor coordination and learning, the animal is subjected to four trials a day for 4 days. The mean latency to fall for each day is recorded. F1 129S6.B6 Mecp2Flox/y animals spend less time on the rod on Day 1, indicating a baseline deficit in motor coordination (Fig. 2B). Although the mutant animals continue to fall off the rod sooner than WT littermate controls on all 4 days, they exhibit the expected increase in the time spent on the rod over the successive days, demonstrating that they are capable of motor learning. This problem with motor coordination was not apparent in F1 129S6.FVB animals (not shown).
The coordination deficit is also observed when the mutant animals are subjected to two additional motor tasks, the dowel walking task and the wire hanging task. F1 129S6.B6 Mecp2Flox/y animals fell off the wire sooner than WT littermate controls (Fig. 2C). The mutant animals also have fewer number of side touches during the wire hanging task (not shown) and for the dowel walking task (Fig. 2D). These differences in motor coordination were not observed in F1 129S6.FVB animals (not shown).
Mecp2Flox/y animals have decreased pain recognition but intact pain sensitivity
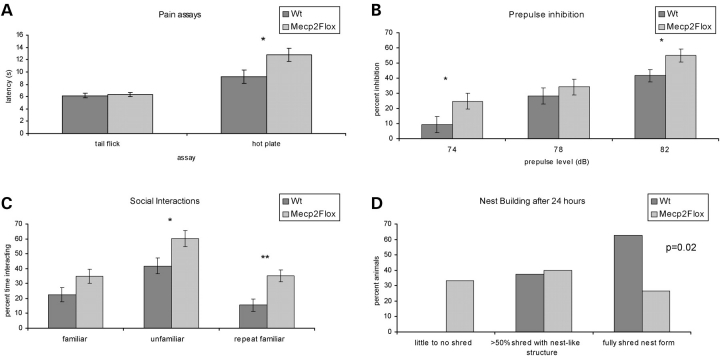
Individuals with RTT have altered pain thresholds (D. Glaze, personal communication); therefore, we tested F1 129S6.B6 Mecp2Flox/y animals to determine whether they have alterations in these neurological systems. In the tail flick assay, which assesses the pain sensitivity and is based on a nociceptive response (34), a reflex is generated within the spinal column that does not depend on the pain recognition systems in the brain. Mecp2Flox/y animals showed a normal response to the tail flick assay at 40, 50 and 60°C, indicating that the nociceptive reflex is intact (Fig. 3A). However, they show an increased latency to respond when exposed to the hot plate assay, a measurement of pain recognition (Fig. 3A). This requires the transmission of pain sensation between the spinal cord and the brain to generate a muscle response leading to paw withdrawal. Also, 5 out of 15 WT mice (33%) licked their hindlimbs in response to the heated plate. In contrast, mutant mice only displayed hindlimb paw withdrawal. The dissociation between the tail flick and the hot plate seen in these animals suggests a primary deficit in pain recognition rather than a problem with the peripheral sensation of pain. When these assays were performed on F1 129S6.FVB animals, similar results were observed with no difference between mutant WT animals in pain sensitivity, but decreased pain recognition in the mutant animals (not shown).
Figure 3.
Mecp2Flox/y mice have altered pain sensitivity, prepulse inhibition, social interactions and nest building. Mecp2Flox/y mice (F1 129S6.B6, n = 16 for each genotype) have increased latency of paw withdrawal on the hotplate (A, P = 0.002); however, they show normal response to tail flick at 40 (A), 50 and 60°C (not shown). The Mecp2Flox/y animals have a diminished startle response (not shown, P = 0.001) but increased inhibitory gating when presented with a 74 or 82 dB sound prepulse (B). Mecp2Flox/y mice (F1 129.B6, n = 16 for each genotype) spent a greater percentage of time at the partition than WT littermate controls when both an unfamiliar mouse was placed in the adjacent chamber or when the mice were re-exposed to a familiar mouse (C, *P < 0.05, **P < 0.01). When singly housed with a fresh nesting material, many Mecp2Flox/y mice (n = 15) did not show any attempt to form a nest and only a small percentage had a fully formed nest after 14 h, in contrast to the large percentage (D, χ2 P = 0.02) of WT littermate control animals (n = 16).
Mecp2Flox/y animals have decreased acoustic startle and prepulse inhibition
Sensorimotor gating involves the inhibition of a startle response to a stimulus when that stimulus is shortly preceded by a less intense stimulus (the prepulse). Alterations in this gating system have been characterized in neuropsychiatric conditions such as schizophrenia (35,36). To assess any abnormalities in sensorimotor gating in Mecp2Flox/y animals, we used the prepulse inhibition assay. In this assay the animals are exposed to a stimulus (120 dB white noise) and their startle response is recorded. The animals are also exposed to increasing levels of prepulse (74, 78 and 82 dB) shortly before the stimulus and their startle response is recorded. The amount of startle to the stimulus after hearing the prepulse is then expressed as a percentage of the startle without the prepulse. F1 129S6.B6 animals have a diminished startle response compared with WT littermate controls (P = 0.001, not shown). However, Mecp2Flox/y animals show increased inhibitory gating at 74 and 82 dB (Fig. 3B). Similar results were observed in the F1 129S6.FVB animals (not shown).
Mecp2Flox/y animals have altered social behavior
To assess social behavior, we used the partition test, a test of social interaction without physical contact (37). In this test, the test animal is singly housed for 4 days in a standard mouse cage divided into two equal halves by a partition. The partition is clear and has multiple holes that allow the mouse to see and smell the adjacent chamber. On the fifth day of single housing, an adult conspecific male mouse is placed in the adjacent chamber. The pair is co-housed for at least 18 h. The once novel mouse partner is now considered ‘familiar’ by the test subject. The following day, the time the test mouse spends at the partition engaged in social interest directed at the partner mouse is recorded in three sequential 5 min test encounters: test animal versus the ‘familiar’ mouse, test animal versus a novel ‘unfamiliar’ mouse and test animal versus the same ‘familiar’ mouse. The first two test encounters assess social interest. The last test encounter is a measurement of social recognition and tests the ability of the test animal to recognize their original co-house partner. Using this assay, F1 129S6.B6 Mecp2Flox/y animals spend a larger fraction of the time at the partition interacting with both the unfamiliar mouse and the second exposure to the familiar mouse (Fig. 3C). We have also observed this behavior in Mecp2Flox/y animals in the F1 129.FVB strain background (not shown).
Mecp2Flox/y animals have a deficit in nest-building behavior
Previous work demonstrated that another Mecp2 allele (Mecp2308) shows deficits in nest-building behavior (37); therefore, we tested this skill in F1 129S6.B6 Mecp2Flox/y animals. Fourteen hours after being presented with nest building material, 5 out of 15 mutant animals (33%) showed no nest building compared with 0 out of 16 of the WT littermate controls. Similarly, only 4 of the 15 mutant animals (27%) had fully formed nests, compared with 10 of the 16 WT animals (Fig. 3D).
Mecp2Flox/y animals have hippocampal and amgydala-dependent learning problems
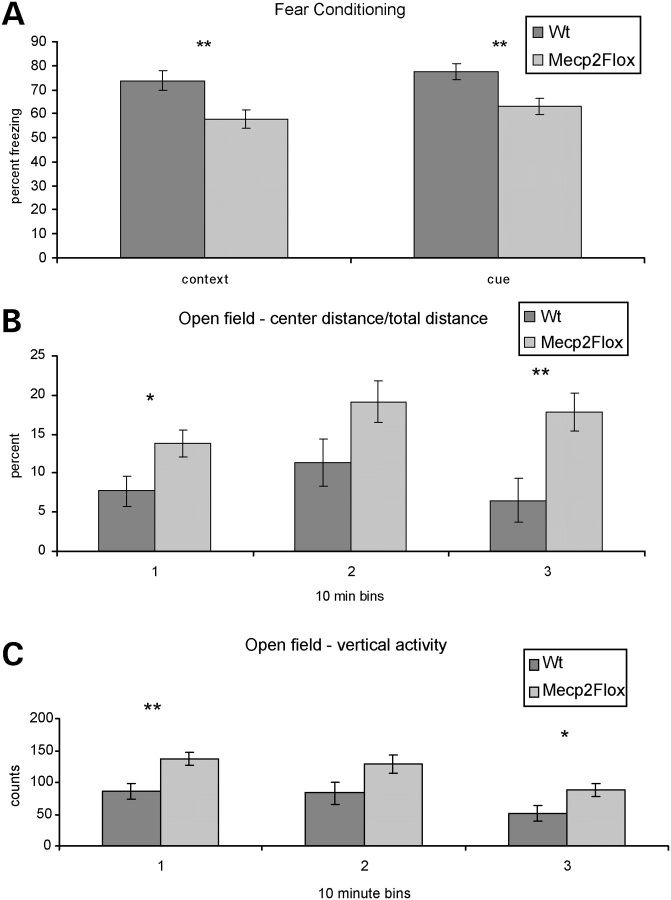
To characterize the effect of Mecp2Flox allele on learning, we tested F1 129S6.FVB Mecp2Flox/y animals and WT littermate controls on the fear conditioning task. In this task, the animal is placed in a chamber (the context) exposed to a 30 s sound pulse (the cue) before receiving a mild electrical shock. During the training day, the animal is exposed to two cue/shock pairings. The following day the animal is re-introduced into the training chamber (context) and the percent time spent freezing (a fear behavior in mice which indicates memory of the context) is recorded. The animal is then exposed to a chamber with altered visual and odor stimuli. The cue is presented and the percent time freezing is recorded. Mecp2Flox/y animals have decreased freezing (Fig. 4A) both when re-exposed to the context (hippocampal dependent) and to the cue (amgydala- and hippocampal-dependent). The learning deficits were not apparent in F1 129S6.B6 animals (not shown).
Figure 4.
Mecp2Flox/y mice have altered learning and decreased anxiety. The Mecp2Flox/y mice (F1 129S6.FVB, n = 16 per genotype) have decreased learning in a fear conditioning task both when exposed to the context (A, P = 0.006) or to the cue stimulus (A, P = 0.004). Additionally, they appear to be less anxious as measured by the distance traveled within the center of an open field chamber compared with the total distance (B) during the first and third 10 min intervals. This decreased anxiety is also apparent by the increased vertical exploratory movements that the mutant animals under took during the first and third 10 min intervals in the open field chamber (C). *P < 0.05, **P < 0.01.
Mecp2Flox/y animals have decreased anxiety
F1 129S6.FVB Mecp2Flox/y animals and WT littermate controls were tested for anxiety and locomotor activity using the open-field assay. Mutant animals did not show any difference in the distance traveled, movement time or speed (not shown) compared with WT animals. However, mutant animals did have a greater ratio of center/total distance traveled compared with the WT animals in the first and last 10 min intervals (Fig. 4B). The willingness of rodents to explore the open center of the chamber reflects decreased anxiety. An additional measure of anxiety is the amount of vertical exploratory movements that a mouse makes during the test. Anxious mice perform less vertical explorations than non-anxious mice. The mutant mice show significantly more vertical activity during the first and last 10 min intervals (Fig. 4C) compared with WT mice. Finally, during the task, mutant mice had more stereotypy counts than WT control animals (2047 versus 1708 beam break counts, P < 0.05, not shown).
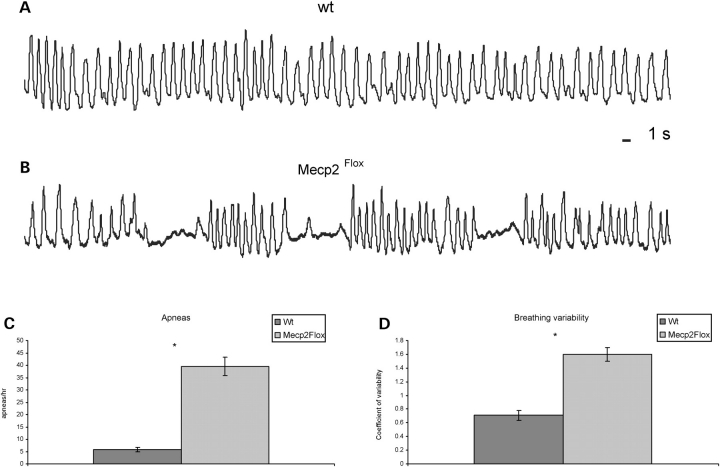
Mecp2Flox/y animals have altered respiratory patterns
Because girls with RTT (38) and RTT mouse models (39–41) have disrupted breathing patterns, we analyzed breathing in F1 129S6.FVB Mecp2Flox/y animals and WT littermate controls at 4 months of life. The respiratory pattern shows qualitative differences between WT and mutant animals (Fig. 5A and B). There was a marked increase in the incidence of apneas (defined as ∼1 s duration with at least two missed breaths) in Mecp2Flox/y animals (39.5 ± 3.7 per hour) relative to WT (5.8 ± 0.9 per hour) mice (Fig. 5C). Additionally, the coefficient of variability of the respiratory rhythm was significantly higher in Mecp2Flox/y animals (1.6 ± 0.1) relative to WT (0.71 ± 0.07) mice (Fig. 5D).
Figure 5.
Respiration is altered in Mecp2Flox/y mice. Representative plethysmographic recordings showing the irregular rhythm and periods of apnea in F1 129S6.FVB Mecp2Flox/y mice (B) relative to WT mice (A). Population data showing the incidence of apneas (C, n = 4 for each genotype). Population data showing the coefficients of variability of respiratory frequency (D, n = 4 for each genotype). *P < 0.05.
DISCUSSION
Detailed characterization of the Mecp2Flox/Y animals revealed a surprising array of phenotypes associated with subtle alterations of MeCP2 levels. The ‘floxed’ allele of Mecp2 results in an ∼50% reduction of MeCP2 levels, which is likely secondary to the presence of the neomycin cassette within the 3′-UTR (28,30). This reduction creates a hypomorphic allele of Mecp2, which results in a variety of behavioral and physiological changes such as learning problems, movement abnormalities and breathing dysfunction. These results further underscore the critical importance of the tight regulation of MeCP2 levels. The phenotypic abnormalities of transgenic animals with a mild over-expression of MeCP2 (27) and people with duplications of the MECP2 locus (21–26) indicate that 2-fold increases in MeCP2 levels are detrimental. The results presented here indicate that reducing MeCP2 levels by ∼50% are also detrimental. The critical need for precise MeCP2 expression levels suggests that this protein plays a dynamic role in neuronal function rather than a static role in repression of non-neuronal genes.
Furthermore, the finding that this conditional allele is hypomorphic underscores the need to design conditional knock-out experiments carefully to insure the correct interpretation of the results. Given that this conditional allele results in a large array of abnormalities, any analysis of Mecp2 conditional knock-out animals must also include a commensurate analysis of the ‘floxed’ allele, preferably using littermate controls. Without these controls, it will be uncertain if any observed effect is a direct effect of the conditional knock-out or a non-specific effect of the hypomorphic ‘floxed’ allele. Equally important, the non-specific effect of a Cre-transgene should also be considered and the phenotypic effect of that genetic engineering event should not be discounted. The most straightforward approach to manage such uncertainty is to conduct these conditional knock out experiments in a manner to include phenotypic characterization of all possible genotypes.
Of the 11 neurobehavioral assays performed, 8 were performed on two strain backgrounds. Although the hypomorphic allele caused a variety of behavioral phenotypes on these two different mouse strain backgrounds, it is noteworthy that some features were enhanced in one strain and subdued in the other. These behavioral differences between the strains does not impact the overall conclusion of the paper that a 50% reduction of MeCP2 results in behavioral abnormalities, rather this suggests that such a reduction in MeCP2 is sensitive to modifier effects. This observation is likely to extend to humans and suggests that the array of phenotypes associated with subtle reduction in MeCP2 levels is likely to be broad and that inter-individual clinical variability will be common.
This work also has important ramifications concerning potential therapeutic strategies for RTT. Recently, novel compounds that allow translational read-through of premature stop-codons have been used to treat animal models of muscular dystrophy (42). Because many common RTT causing MECP2 mutations create such premature stop codons (3), interest has developed in utilizing this approach for treatment of RTT. The difference is that whereas the restoration of 40–50% of the expression of a structural protein such as dystrophin might be sufficient to improve the function of muscles, functional restoration in RTT might require expression much closer to WT endogenous levels.
Importantly, this study predicts that human neurodevelopmental disorders will result from a decrease of MeCP2 levels by as little as 50%. This decrease in expression may be the result of sequence changes in the MECP2 locus that occur either in cis (enhancers, promoter or within the 3′-UTR), or possibly via the trans-factors that regulate MeCP2 expression. This has been suggested by studies that found reduced MeCP2 levels in the brain of a number of neurodevelopmental disorders (19,20), but the concern has been that the decreased MeCP2 levels in these post-mortem samples of neurodevelopmental disorders reflects a non-specific decrease in neuronal function rather than a specific finding of the disorders. Other work has identified sequence polymorphisms in the 3′-UTR or MECP2 in individuals with autism (43–45). The challenge with that work has been establishing the functional significance of the sequence polymorphisms. The work described here demonstrates that a 50% decrease in MeCP2 levels might indeed cause disease, and that misregulation of MeCP2 may be a common feature of many neurodevelopmental disorders.
MATERIALS AND METHODS
Animal husbandry
Mice were maintained on a 12 h light:12 h dark cycle with standard mouse chow and water ad libitum. All of the mice used in these experiments were generated by crossing heterozygous female Mecp2Flox/+ mice that had been backcrossed to 129S6/SvEvTac for at least five generations to male mice on either a pure FVB/N background or male mice on a pure C57BL/6J background to generate isogenic F1 animals. Only male animals were used for all the experiments listed with WT littermate males serving as the controls. Mecp2Flox/Y and WT littermate controls were co-housed immediately after weaning. Behavioral experiments were performed with 15–20 mice per genotype with the exception of the open-field assay, which was performed with 10–12 mice per genotype. All research and animal care procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Behavior assays
Accelerating rotating rod
Mice were placed on an accelerating rotating rod apparatus (Ugo Basile North America, Inc., Schwenksville, PA, USA) for 16 trials (four trials per day for four consecutive days). The rod accelerated linearly from 3.6 to 36 rpm for the first 4.5 min. Each trial lasted a maximum of 5 min with a 30–60 min inter-trial interval. The latency for each mouse to fall from the rod was recorded for each trial. The mean fall time for each day was calculated. Data were analyzed using a one-way ANOVA (genotype).
Dowel walking
Mice at 11–12 weeks of life were placed in the center of a 0.9 cm wooden dowel balanced between the two wooden poles. The latency to fall, the latency to reach either end of the wooden dowel defined as a ‘side touch’ and the number of side touches were recorded. In the event of a side touch, the timer was stopped, the side touch recorded and the mouse was returned to the center of the dowel. Trials lasted for a maximum of 2 min. Data were analyzed using the Mann–Whitney U-test.
Wire hanging
Mice at 11–12 weeks of life were suspended by their forepaws on a 2 mm wire and the time remaining on the wire, the time to first side touch and the number of side touches, as described above, was recorded. Maximum time for each trial was 2 min, and similarly to the dowel, whenever a mouse touched the side wall it was replaced to the center of the wire. Data were analyzed using the Mann–Whitney U-test for fall time and number of side touches.
Tail flick
Mice at 16–17 weeks of life were tested for pain sensitivity using the Tail-Flick Analgesia Meter (Columbus Instruments, Columbus, OH, USA). Animals were allowed to acclimate to a plexiglass restraint for 2 min and tails were placed over the sensing groove. Testing commenced upon activation of an intense light beam directed at the tails 4 cm from their base. The latency to observe a tail flick in response to the light beam was recorded. Each animal was tested using three different light beam temperature settings (40, 50 and 60°C) presented in random order.
Hot plate
Mice at 16–17 weeks of life were tested for pain recognition using the Hot-Plate Analgesia Meter (Columbus Instruments). Animals were placed on a 55°C heated surface. A response to the discomfort served as a functional readout of pain recognition. Responses included hindlimb licking, shaking or twitching. The latency to respond to the heated surface was recorded and the data were analyzed using ANOVA (genotype).
Prepulse inhibition
Mice at 15–16 weeks of life were subjected to acoustic prepulse inhibition. The acoustic prepulse inhibition task consists of presenting the animal with two closely paired sound pulses: a prepulse at +0 dB, +4 dB (74 dB), +8 dB (78 dB), +12 dB (82 dB) and over background followed 100 ms later by a pulse of 120 dB. The amount of startle the pulse induces in the animal is recorded using a startle chamber for mice (SR-Lab, San Diego Instruments, San Diego, CA, USA) which records activity for 65 ms after the pulse. The maximum amplitude recorded over the 65 ms is recorded and compared using an ANOVA (genotype) at each prepulse level.
Partition test
Test mice at 20 weeks of age were individually housed in standard housing cages for 4 days. Each cage was separated into two compartments by a perforated barrier which allows social interaction without direct physical contact. On Day 5 of individual housing, age- and gender-matched C57BL/6J partner mice were placed into the compartment opposite the test mice. Paired mice were co-housed in the separate halves of the partitioned cage for at least 18 h. Following this period of induced familiarity, the time that test mice displayed directed interest in their partner mice was recorded during three different paradigms: test subject versus familiar partner, test subject versus unfamiliar partner and repeated test subject versus familiar partner. Each behavioral paradigm was assessed during three 5-min intervals and was performed in sequential order. Data were analyzed using a one-way ANOVA (genotype).
Nest-building assay
Singly-housed mice at 19 weeks of life were tested for their ability to build nests. Nest material (Kimwipes, Kimberly Clark, Dallas, TX, USA) was placed in each cage 1 h prior to the onset of the dark cycle and were left undisturbed for 14 h. Nest-building was assessed based on a three-point scale (1 = 0–25% of material shredded, 2 = less than 50% shredded with material gathered in a nest, 3=fully shredded) with material gathered in a nest. Data were analyzed using χ2 analysis.
Fear conditioning
Mice were tested at 22 weeks of life in a chamber that contains a grid floor that can deliver an electric shock (Actimetrics chamber system, Med Associates, St. Albans, VT, USA). On Day 1 of the test, mice were placed within the chamber and left undisturbed for 2 min after which a 30 s white noise sound pulse (‘cue’) was delivered. At the end of the cue, the mouse was shocked (2 s, 0.4 mA). Two minutes later, a second pairing of sound cue followed by shock was delivered. Thirty seconds after the final shock, the animal was removed and replaced in the home cage. The following day, the animals were replaced to the same chamber (‘context test’) and freezing behavior was recorded for 6 min. Freezing behavior was recorded automatically by the instrument. One hour after the context test, the animals were placed into a chamber which had been cleaned with an unfamiliar agent (ethanol) and the wall color, the chamber shape and the odor (artificial vanilla) had been changed to remove the contextual cues of the chamber. The animals were then monitored for 3 min. After 3 min, the white noise cue was started and lasted 3 min. The amount of freezing was recorded separately for the first 3 min and for the last 3 min (cue test). The number of freezing intervals was converted to a percentage of freezing for both the context test and the cue test, and the data were analyzed using a one-way ANOVA (genotype).
Open-field analysis
Mice at 12 weeks of life were placed in the center of chamber (40 × 40 × 30 cm) and the activity was measured by photobeams connected to a computer-operated Digiscan optical animal activity system (AccuScan, Columbus, OH, USA). This system measures both XY position as well as z-activity (rearing). The test was performed with 60 dB white noise and 150 lux illumination. The activity was measured for 30 min and data were analyzed as three 10 min intervals. The analysis of data was performed using a one-way ANOVA (genotype).
Quantitative real-time polymerase chain reaction
Freshly dissected whole brains (n = 4 per genotype) from 26–27-week-old mice were placed in 2 ml Trizol (Invitrogen, Carlsbad, CA, USA) on ice and immediately homogenized using a Polytron homogenizer at half maximal. The resultant homogenates were processed per the manufacturer's instructions. RNA was DNAse digested and cleaned using the Qiagen RNeasy Mini kit per manufacturer's instructions (Qiagen Inc., Valencia, CA, USA). First-strand cDNA was synthesized from 5 µg of the purified RNA using SuperScript III (Invitrogen). Quantitative PCR was performed using Applied Biosystems 7300 Real Time PCR System (Applied Biosystems, Foster City, CA) according to manufactures instructions using Taqman gene expression assays. Primers and Taqman probes were designed to assess Mecp2 transcript levels using the Primer Express v2.0 software program (Applied Biosystems). The primers and probe sequences are as follows:
Mecp2e1–3 (forward primer 5′-AGGAGGAGAGACTGGAGGAAAAG-3′, reverse primer 5′-CTTTCTTCGCCTTCTTAAACTTCAG-3′; probe 5′-FAM-AAGACCAGGATCTCCAGGGCCTCAGA-TAMRA-3′)
Mecp2e2–3 (forward primer 5′-GATTCCATGGTAGCTGGGATGT-3′; reverse primer 5′- TCTGAGGCCCTGGAGATCCT-3′; probe 5′-FAM- AGGGCTCAGGGAGGAAAAGTCAGAAGA-BHQ-3′)
Mecp2e3 forward primer 5′-TACAACCTTCAGCCCACCATT-3′; reverse primer 5′-CTGAGCTTTCTGATGTTTCTGCTT-3′; probe 5′-FAM-TGCAGAGCCAGCAGAGGCAGGC-BHQ-3′)
Plethysmographic measurements
Whole-body plethysmographic measurements of the frequency and depth of breathing were made from unrestrained male mice at 12 weeks of life. Pressure changes associated with breathing were measured with a 260-ml chamber (with fresh room air flowing at the room temperature of ∼23°C), a pressure transducer (model DP103; Validyne Engineering, Northridge, CA, USA), and a signal conditioner (CD-15; Validyne). Animals were allowed to acclimate within the recording chamber for 20 min prior to recordings. The mean, standard error of the mean and coefficient of variability (standard deviation/mean) were calculated for the respiratory rate for each subject. Statistical significance was tested using paired difference Student's t-test; significance was accepted at P-values <0.05.
Western blotting
Freshly dissected whole brains from 26- to 27-week-old mice were Dounce homogenized in ice-cold RIPA buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1% Triton-X-100, 0.1% SDS) with Roche complete protease inhibitors and 1 mm PMSF. Samples were rotated for 10 min at 4°C and then spun at maximum speed in a microcentrifuge for 10 min at 4°C to pellet insoluble material. Forty micrograms of each sample were boiled in a sample buffer, loaded onto a NuPAGE Bis-Tris 4–12% gel (Invitrogen) and transferred to nitrocellulose for western blotting. Rabbit anti-C-terminal Mecp2 (Upstate, Charlottesville, VA, USA) was used at 1:1000 dilution, and mouse anti-GAPDH clone 6C5 (Advanced Immunochemical, Long Beach, CA, USA) was used at 1:10 000. HRP-conjugated Anti-rabbit secondary (BioRad, Hercules, CA, USA) was used at 1:5000 and HRP-conjugated anti-mouse secondary (GE Healthcare, UK) was used at 1:2000.
Immunofluorescence
Animals were anesthetized (Avertin) and transcardially perfused with 4% paraformaldehyde (PFA) for 8 min. Brains were dissected and post-fixed in 4% PFA overnight at 4°C. After rinsing in 1× phosphate-buffered saline (PBS), brains were cryoprotected in 30% sucrose and then embedded in O.C.T. and stored at −80°C. Fifty micrograms of mid-sagittal sections were cut and suspended in 24-well tissue culture plates containing 1× PBS. Sections were blocked in 2% normal goat sera with 0.3% triton X-100 for 1 h at 4°C followed by a 65 h 4°C incubation in a 1:100 dilution of anti-MeCP2 (Upstate cat# 07–013). Sections were washed four times for 20 min in 1× PBS and then incubated for 48 h at 4°C in 1:500 Alexa 488 labeled goat-anti rabbit (Molecular Probes). Sections were washed an additional four times for 20 min in 1× PBS and then mounted with ProLong Gold antifade mounting medium (Invitrogen cat# P36930). Images were collected from optical sections using a Zeiss 510 (Carl Zeiss, Thornwood, NY, USA) confocal microscope and processed using ImageJ software (http://rsb.info.nih.gov/ij/).
FUNDING
Autism Speaks (R.C.S.), Cure Autism Now (H.Y.Z.), National Institutes of Health/National Institute of Neurological Disorders and Stroke (NS052240 to J.L.N.), (NS057819 to H.Y.Z.), National Institute of Child Health and Human Development Mental Retardation and Developmental Disabilities Research Center (HD024064) and Howard Hughes Medical Institute (H.Y.Z.).
ACKNOWLEDGEMENTS
The authors thank Adrian Bird for the Mecp2Flox mice S. Maricich for comments on the manuscript and the Baylor Mouse Neurobehavior Core.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Hagberg B., Aicardi J., Dias K., Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann. Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 2.Neul J.L., Zoghbi H.Y. Rett syndrome: a prototypical neurodevelopmental disorder. Neuroscientist. 2004;10:118–128. doi: 10.1177/1073858403260995. [DOI] [PubMed] [Google Scholar]
- 3.Neul J.L., Fang P., Barrish J., Lane J., Caeg E., Smith E.O., Zoghbi H., Percy A., Glaze D. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett Syndrome. Neurology. 70 doi: 10.1212/01.wnl.0000291011.54508.aa. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nan X., Campoy F.J., Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- 5.Nan X., Cross S., Bird A. Gene silencing by methyl-CpG-binding proteins. Novartis Found. Symp. 1998;214:6–16. doi: 10.1002/9780470515501.ch2. Discussion 16–21, 46–50. [DOI] [PubMed] [Google Scholar]
- 6.Nan X., Meehan R.R., Bird A. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res. 1993;21:4886–4892. doi: 10.1093/nar/21.21.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kankirawatana P., Leonard H., Ellaway C., Scurlock J., Mansour A., Makris C.M., Dure L.S.t., Friez M., Lane J., Kiraly-Borri C., et al. Early progressive encephalopathy in boys and MECP2 mutations. Neurology. 2006;67:164–166. doi: 10.1212/01.wnl.0000223318.28938.45. [DOI] [PubMed] [Google Scholar]
- 8.Meloni I., Bruttini M., Longo I., Mari F., Rizzolio F., D'Adamo P., Denvriendt K., Fryns J.P., Toniolo D., Renieri A. A mutation in the rett syndrome gene, MECP2, causes X-linked mental retardation and progressive spasticity in males. Am. J. Hum. Genet. 2000;67:982–985. doi: 10.1086/303078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couvert P., Bienvenu T., Aquaviva C., Poirier K., Moraine C., Gendrot C., Verloes A., Andres C., Le Fevre A.C., Souville I., et al. MECP2 is highly mutated in X-linked mental retardation. Hum. Mol. Genet. 2001;10:941–946. doi: 10.1093/hmg/10.9.941. [DOI] [PubMed] [Google Scholar]
- 10.Dotti M.T., Orrico A., De Stefano N., Battisti C., Sicurelli F., Severi S., Lam C.W., Galli L., Sorrentino V., Federico A. A Rett syndrome MECP2 mutation that causes mental retardation in men. Neurology. 2002;58:226–230. doi: 10.1212/wnl.58.2.226. [DOI] [PubMed] [Google Scholar]
- 11.Klauck S.M., Lindsay S., Beyer K.S., Splitt M., Burn J., Poustka A. A mutation hot spot for nonspecific X-linked mental retardation in the MECP2 gene causes the PPM-X syndrome. Am. J. Hum. Genet. 2002;70:1034–1037. doi: 10.1086/339553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleefstra T., Yntema H.G., Oudakker A.R., Romein T., Sistermans E., Nillessen W., van Bokhoven H., de Vries B.B., Hamel B.C. De novo MECP2 frameshift mutation in a boy with moderate mental retardation, obesity and gynaecomastia. Clin. Genet. 2002;61:359–362. doi: 10.1034/j.1399-0004.2002.610507.x. [DOI] [PubMed] [Google Scholar]
- 13.Winnepenninckx B., Errijgers V., Hayez-Delatte F., Reyniers E., Frank Kooy R. Identification of a family with nonspecific mental retardation (MRX79) with the A140V mutation in the MECP2 gene: is there a need for routine screening? Hum. Mutat. 2002;20:249–252. doi: 10.1002/humu.10130. [DOI] [PubMed] [Google Scholar]
- 14.Yntema H.G., Oudakker A.R., Kleefstra T., Hamel B.C., van Bokhoven H., Chelly J., Kalscheuer V.M., Fryns J.P., Raynaud M., Moizard M.P., et al. In-frame deletion in MECP2 causes mild nonspecific mental retardation. Am. J. Med. Genet. 2002;107:81–83. doi: 10.1002/ajmg.10085. [DOI] [PubMed] [Google Scholar]
- 15.Imessaoudene B., Bonnefont J.P., Royer G., Cormier-Daire V., Lyonnet S., Lyon G., Munnich A., Amiel J. MECP2 mutation in non-fatal, non-progressive encephalopathy in a male. J. Med. Genet. 2001;38:171–174. doi: 10.1136/jmg.38.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen D., Lazar G., Couvert P., Desportes V., Lippe D., Mazet P., Heron D. MECP2 mutation in a boy with language disorder and schizophrenia. Am. J. Psychiatry. 2002;159:148–149. doi: 10.1176/appi.ajp.159.1.148-a. [DOI] [PubMed] [Google Scholar]
- 17.Carney R.M., Wolpert C.M., Ravan S.A., Shahbazian M., Ashley-Koch A., Cuccaro M.L., Vance J.M., Pericak-Vance M.A. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr. Neurol. 2003;28:205–211. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 18.Orrico A., Lam C., Galli L., Dotti M.T., Hayek G., Tong S.F., Poon P.M., Zappella M., Federico A., Sorrentino V. MECP2 mutation in male patients with non-specific X-linked mental retardation. FEBS Lett. 2000;481:285–288. doi: 10.1016/s0014-5793(00)01994-3. [DOI] [PubMed] [Google Scholar]
- 19.Samaco R.C., Nagarajan R.P., Braunschweig D., LaSalle J.M. Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum. Mol. Genet. 2004;13:629–639. doi: 10.1093/hmg/ddh063. [DOI] [PubMed] [Google Scholar]
- 20.Nagarajan R.P., Hogart A.R., Gwye Y., Martin M.R., LaSalle J.M. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:e1–e11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Gaudio D., Fang P., Scaglia F., Ward P.A., Craigen W.J., Glaze D.G., Neul J.L., Patel A., Lee J.A., Irons M., et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet. Med. 2006;8:784–792. doi: 10.1097/01.gim.0000250502.28516.3c. [DOI] [PubMed] [Google Scholar]
- 22.Friez M.J., Jones J.R., Clarkson K., Lubs H., Abuelo D., Bier J.A., Pai S., Simensen R., Williams C., Giampietro P.F., et al. Recurrent infections, hypotonia, and mental retardation caused by duplication of MECP2 and adjacent region in Xq28. Pediatrics. 2006;118:e1687–e1695. doi: 10.1542/peds.2006-0395. [DOI] [PubMed] [Google Scholar]
- 23.Lugtenberg D., de Brouwer A.P., Kleefstra T., Oudakker A.R., Frints S.G., Schrander-Stumpel C.T., Fryns J.P., Jensen L.R., Chelly J., Moraine C., et al. Chromosomal copy number changes in patients with non-syndromic X linked mental retardation detected by array CGH. J. Med. Genet. 2006;43:362–370. doi: 10.1136/jmg.2005.036178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meins M., Lehmann J., Gerresheim F., Herchenbach J., Hagedorn M., Hameister K., Epplen J.T. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J. Med. Genet. 2005;42:e12. doi: 10.1136/jmg.2004.023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Esch H., Bauters M., Ignatius J., Jansen M., Raynaud M., Hollanders K., Lugtenberg D., Bienvenu T., Jensen L.R., Gecz J., et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet. 2005;77:442–453. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox J.J., Holden S.T., Dee S., Burbridge J.I., Raymond F.L. Identification of a 650 kb duplication at the X chromosome breakpoint in a patient with 46,X,t(X;8)(q28;q12) and non-syndromic mental retardation. J. Med. Genet. 2003;40:169–174. doi: 10.1136/jmg.40.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins A.L., Levenson J.M., Vilaythong A.P., Richman R., Armstrong D.L., Noebels J.L., David Sweatt J., Zoghbi H.Y. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- 28.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 29.Coy J.F., Sedlacek Z., Bachner D., Delius H., Poustka A. A complex pattern of evolutionary conservation and alternative polyadenylation within the long 3′-untranslated region of the methyl-CpG-binding protein 2 gene (MeCP2) suggests a regulatory role in gene expression. Hum. Mol. Genet. 1999;8:1253–1262. doi: 10.1093/hmg/8.7.1253. [DOI] [PubMed] [Google Scholar]
- 30.Lewandoski M. Conditional control of gene expression in the mouse. Nat. Rev. Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- 31.Mnatzakanian G.N., Lohi H., Munteanu I., Alfred S.E., Yamada T., MacLeod P.J., Jones J.R., Scherer S.W., Schanen N.C., Friez M.J., et al. A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nat. Genet. 2004;36:339–341. doi: 10.1038/ng1327. [DOI] [PubMed] [Google Scholar]
- 32.Kriaucionis S., Bird A. The major form of MeCP2 has a novel N-terminus generated by alternative splicing. Nucleic Acids Res. 2004;32:1818–1823. doi: 10.1093/nar/gkh349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R.Z., Akbarian S., Tudor M., Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 34.Karl T., Pabst R., von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- 35.Takao K., Miyakawa T. Investigating gene-to-behavior pathways in psychiatric disorders: the use of a comprehensive behavioral test battery on genetically engineered mice. Ann. N. Y. Acad. Sci. 2006;1086:144–159. doi: 10.1196/annals.1377.008. [DOI] [PubMed] [Google Scholar]
- 36.Geyer M.A., McIlwain K.L., Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol. Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 37.Moretti P., Bouwknecht J.A., Teague R., Paylor R., Zoghbi H.Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mo.l Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 38.Glaze D.G. Neurophysiology of Rett syndrome. J. Child Neurol. 2005;20:740–746. doi: 10.1177/08830738050200090801. [DOI] [PubMed] [Google Scholar]
- 39.Viemari J.C., Roux J.C., Tryba A.K., Saywell V., Burnet H., Pena F., Zanella S., Bevengut M., Barthelemy-Requin M., Herzing L.B., et al. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J. Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stettner G.M., Huppke P., Brendel C., Richter D.W., Gartner J., Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J. Physiol. 2007;4:4. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogier M., Wang H., Hong E., Wang Q., Greenberg M.E., Katz D.M. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J. Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch E.M., Barton E.R., Zhuo J., Tomizawa Y., Friesen W.J., Trifillis P., Paushkin S., Patel M., Trotta C.R., Hwang S., et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 43.Shibayama A., Cook E.H., Jr, Feng J., Glanzmann C., Yan J., Craddock N., Jones I.R., Goldman D., Heston L.L., Sommer S.S. MECP2 structural and 3′-UTR variants in schizophrenia, autism and other psychiatric diseases: a possible association with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;128:50–53. doi: 10.1002/ajmg.b.30016. [DOI] [PubMed] [Google Scholar]
- 44.Xi C.Y., Ma H.W., Lu Y., Zhao Y.J., Hua T.Y., Zhao Y., Ji Y.H. MeCP2 gene mutation analysis in autistic boys with developmental regression. Psychiatr. Genet. 2007;17:113–116. doi: 10.1097/YPG.0b013e3280114a5c. [DOI] [PubMed] [Google Scholar]
- 45.Coutinho A.M., Oliveira G., Katz C., Feng J., Yan J., Yang C., Marques C., Ataide A., Miguel T.S., Borges L., et al. MECP2 coding sequence and 3′-UTR variation in 172 unrelated autistic patients. Am J Med Genet B Neuropsychiatr. Genet. 2007;144:475–483. doi: 10.1002/ajmg.b.30490. [DOI] [PubMed] [Google Scholar]