Abstract
Objective
The scavenger receptors SR-A and CD36 have been implicated in macrophage foam cell formation during atherogenesis and in the regulation of inflammatory signaling pathways, including those leading to lesional macrophage apoptosis and plaque necrosis. To test the impact of deleting these receptors, we generated Apoe−/− mice lacking both SR-A and CD36 and fed them a Western diet for 12 weeks.
Methods and Results
We analyzed atheroma in mice, assessing lesion size, foam cell formation, inflammatory gene expression, apoptosis, and necrotic core formation. Aortic root atherosclerosis in Apoe−/− Cd36−/− Msr1−/− mice, as assessed by morphometry, electron microscopy, and immunohistochemistry, showed no decrease in lesion area or in vivo foam cell formation when compared to Apoe−/− mice. However, Apoe−/− Cd36−/− Msr1−/− lesions showed reduced expression of inflammatory genes and morphological analysis revealed a ≈30% decrease in macrophage apoptosis and a striking ≈50% decrease in plaque necrosis in aortic root lesions of these mice.
Conclusions
Although targeted deletion of SR-A and CD36 does not abrogate macrophage foam cell formation or substantially reduce atherosclerotic lesion area in Apoe−/− mice, loss of these pathways does reduce progression to more advanced necrotic lesions. These data suggest that targeted inhibition of these pathways in vivo may reduce lesional inflammation and promote plaque stability.
Keywords: scavenger receptor, atherosclerosis, apoptosis, necrosis, inflammation
Atherosclerosis is initiated by the retention of apolipoprotein B–containing lipoproteins in focal areas of the arterial subendothelium. Lipoprotein retention elicits a series of biological responses, notably a maladaptive, macrophage-dominant inflammatory response that leads to a gradual expansion of the subendothelial space with cells, lipids, and extracellular matrix.1–3 A prominent feature of these lesions is the macrophage foam cell, which results when subendothelial macrophages internalize retained lipoproteins. Although foam cell formation is one of the earliest events detected in the nascent plaque, it is the progression of these early lesions to more advanced stages that heralds the onset of clinical coronary events. However, only a minority of atherosclerotic lesions ultimately erode or rupture, exposing subendothelial prothrombogenic material to the bloodstream, triggering acute lumenal thrombosis and downstream tissue infarction. These “vulnerable” plaques are characterized by specific morphological features including thin fibrous caps, an abundance of inflammatory cytokines and matrix proteases, and large necrotic cores.4–6 Necrotic cores result from macrophage apoptosis coupled to inefficient clearance of the apoptotic cells, leading to large acellular regions within the arterial intima by which the necrotic cores are identified.7,8 Necrotic cores are thought to be particularly important for plaque vulnerability, because they are a source of inflammation, proteases, and procoagulants.9 In addition, the necrotic core structure is believed to induce heightened physical stress on the overlying fibrous cap, making it more prone to rupture.10
Macrophage scavenger receptors (SRs), particularly the type A scavenger receptor (SR-A) and CD36, have been implicated in processes that contribute both to early foam cell formation and to the progression toward more complex vulnerable plaques.1,11 Originally identified by their role in mediating the uptake and internalization of modified lipoproteins like oxidized LDL, the scavenger receptors have generally been viewed as essential for foam cell formation.12,13 This perspective is based on cell culture studies showing that SR-A and CD36 account for greater than 90% of the lipid accumulation in macrophages exposed to oxidized LDL.14 However, alternative models of foam cell formation, not involving SRs, have been investigated in vitro, and recent work from our laboratory indicated that deficiency of SR-A or CD36 did not prevent macrophage foam cell formation in vivo.15–20
Though less intensely investigated, the role of these same receptors in activating the apoptotic events that underlie necrotic core formation has also recently been explored. We have shown that SR-A plays a critical role in triggering apoptosis in macrophages undergoing endoplasmic reticulum (ER) stress, a process known to occur in advanced murine and human atheromata and to be associated with apoptosis and plaque necrosis in these lesions.21,22 On exposure to SR-A ligands, ER-stressed macrophages undergo SR-A–dependent apoptosis through a mechanism involving enhancement of apoptotic pathways and suppression of compensatory prosurvival pathways.21,23 Moreover, we found recently that CD36 ligands can also trigger apoptosis in ER-stressed macrophages, although by a different mechanism (Seimon and Tabas, unpublished data). Based on these data, we have postulated a role for SRs in advanced lesional macrophage apoptosis and its consequence, plaque necrosis.
The goal of the current study was to address the impact of combined SR-A and CD36 deficiency on aortic atherosclerosis, advanced lesional macrophage apoptosis, and plaque necrosis in C57BL/6 Apoe−/− mice. Our data establish that SR-A/CD36 deficiency does not decrease overall lesion area in the aortic root, nor does it diminish the intense foam cell formation in this region. However, combined receptor deficiency had a marked suppressive effect on inflammatory gene expression and advanced lesional macrophage apoptosis and plaque necrosis. These data establish an important alternative role for SRs in promoting atherogenesis.
Methods
Mice
Apoe−/− Msr1−/− Cd36−/− triple knock-out mice were generated by crossing previously established double knock-out lines (Cd36−/− Apoe−/− and Msr1−/− Apoe−/−) back-bred into the C57BL/6 background seven times.17 Apoe−/− Msr1−/− Cd36−/− triple knock-out mice and the control Apoe−/− Msr1+/+ Cd36+/+ mice were generated at the appropriate Mendelian ratios. The genetic backgrounds of the mice were assessed by genome scanning using 150 polymorphic markers that distinguish the C57BL6 and 129Sv genomes. This screening confirmed that all markers were derived from the C57BL/6 strain with the exception of those in closest proximity to the 129Sv gene that had been targeted for inactivation and selected for retention. All mice were housed in a pathogen-free environment in autoclaved filter-top cages with autoclaved water and kept on a 12-hour light/dark cycle. Animal care and use for all procedures was done in accordance with the Massachusetts General Hospital’s Subcommittee on Research Animal Care and the USDA Animal Welfare Act and the Public Health Survey Policy for the Humane Care and Use of Laboratory Animals.
At 6 weeks of age, mice were fed a high fat Western diet (Teklad Adjusted Calories 88137; 21% [w/w] fat; 0.15% [w/w] cholesterol; 19.5% [w/w] casein, no sodium cholate) for 12 weeks. Mice were fasted, weighed and blood was collected for analysis of total cholesterol (Thermo Electron Corp) and triglyceride (Sigma) by enzymatic assay.
Atherosclerotic Lesion Analysis
Atherosclerotic lesion area in the aortic root was quantified on cross sections of the aorta as we described.24–26 Every second section (5 µm thick) throughout the aortic sinus (400 µm) was taken for analysis. Sections stained with hematoxylin and oil red O were quantified using IP Laboratory Spectrum software version 3.9 (Scanalytics).17 Lesion area from 12 sections per mouse was determined independently at Massachusetts General Hospital or Columbia University by a single observer blinded to the genotype of the mice. Lesional necrotic areas were quantified from the average of 6 sections per mouse, spaced 30 µm apart, beginning at the base of the aortic root by tracing around all acellular and anuclear white areas. EM imaging of foam cells in aortic root lesions was performed as we described.17
Lesion area of the aorta en face was quantified using IPLab Spectrum software and expressed as a percent of the total aortic area or defined regions (aortic arch, thoracic, and abdominal aorta). Aortic cholesterol was then measured after methanol:chloroform (1:2) extraction. The chloroform layer was dried using nitrogen gas, resuspended in chloroform:Triton X-100, dried again, and finally resuspended in deionized water. Total and free cholesterol was determined using an enzymatic colorimetric assay (Wako) and normalized to total aortic area.
Gene Expression Profiling
Total RNA was prepared from the descending aortas of female mice (n=3 per genotype) using TRIzol reagent (Invitrogen) as we described.26 0.5 µg of aortic RNA from each mouse was reverse transcribed and quantitative RT-PCR (QRT-PCR) analysis of 84 atherosclerosis related genes was performed using Atherosclerosis RT2 Profiler PCR Arrays (SuperArray Inc) per the manufacturer’s protocol. The complete list of the genes analyzed is available online at http://www.superarray.com/rt_pcr_product/HTML/PAMM-038A.html. Data analysis was performed using the manufacturer’s integrated web-based software package for the PCR Array System using ΔΔCt based fold-change calculations.
Immunohistochemical Staining
Immunostaining for macrophage MOMA-2 (Rat IgG2b, Serotec Inc) and α-smooth muscle actin (DakoCytomation California Inc) was performed as we described.24–26 Immunostaining was quantified using IP Laboratory Spectrum software as a percentage of lesion area.
In Situ TUNEL Staining
Apoptotic cells in the intimal area of atherosclerotic lesions were labeled after proteinase K treatment by TUNEL (TdT-mediated dUTP nick-end labeling) using the in situ cell death detection kit TMR-red (Roche Diagnostics). TUNEL and DAPI staining was viewed using an Olympus IX-70 inverted fluorescent microscope and only TUNEL-positive cells that colocalized with DAPI-stained nuclei were counted as being positive.
Statistics
Statistical analysis was performed using a 2-way analysis of variance (ANOVA) or Student t test, as indicated.
Results
To determine the effect of targeted deletion of CD36 and SR-A on atherosclerotic lesion development, Apoe−/− Cd36−/− Msr1−/− and Apoe−/− control mice were fed a Western diet for 12 weeks. Over the course of the study, mean total serum cholesterol levels were modestly elevated in Apoe−/− Cd36−/− Msr1−/− mice as compared to Apoe−/− controls, but this difference was not statistically significant (supplemental Table I, available online at http://atvb.ahajournals.org). Further analysis of serum lipoproteins by fast protein liquid (FPLC) showed no difference in lipoprotein distribution between the 2 groups of mice (data not shown). Male and female Apoe−/− Cd36−/− Msr1−/− mice had significantly lower body weights than did their gender matched Apoe−/− controls (supplemental Table I). The triglyceride levels of the male TKO mice averaged 107 mg/dL and were significantly higher than those of the apo E null males. The higher serum triglyceride values were accounted for by increased VLDL triglyceride levels (data not shown). The female mice serum triglyceride levels did not differ between the 2 mouse groups. In data not presented in this work, in which we examined lipid levels of chow fed mice without SR-A or CD36, as well as data presented in our previous 8 week study of mice deficient in either SR-A or CD36, serum cholesterol levels have often differed from those of mice lacking only Apoe. These differences have not been consistent in their magnitude or direction. Given substantial variability in individual lipid levels of inbred mice fed high fat diets, it is difficult for us to come to any conclusion about mechanistic effects of the loss of CD36 and SR-A on overall serum lipid levels in mice (see lipid data in mice from Jackson labs at http://phenome.jax.org/pubcgi/phenome/mpdcgi?rtn=meas%2Fseveralcat&CATblood+lipidsqqqcholesterol=on.) The data presented in this study do indicate that although CD36 and SR-A may contribute modestly to the maintenance of serum lipid levels, the magnitude of the changes are likely too small to constitute a major influence on the atherosclerosis outcomes measured in this study.
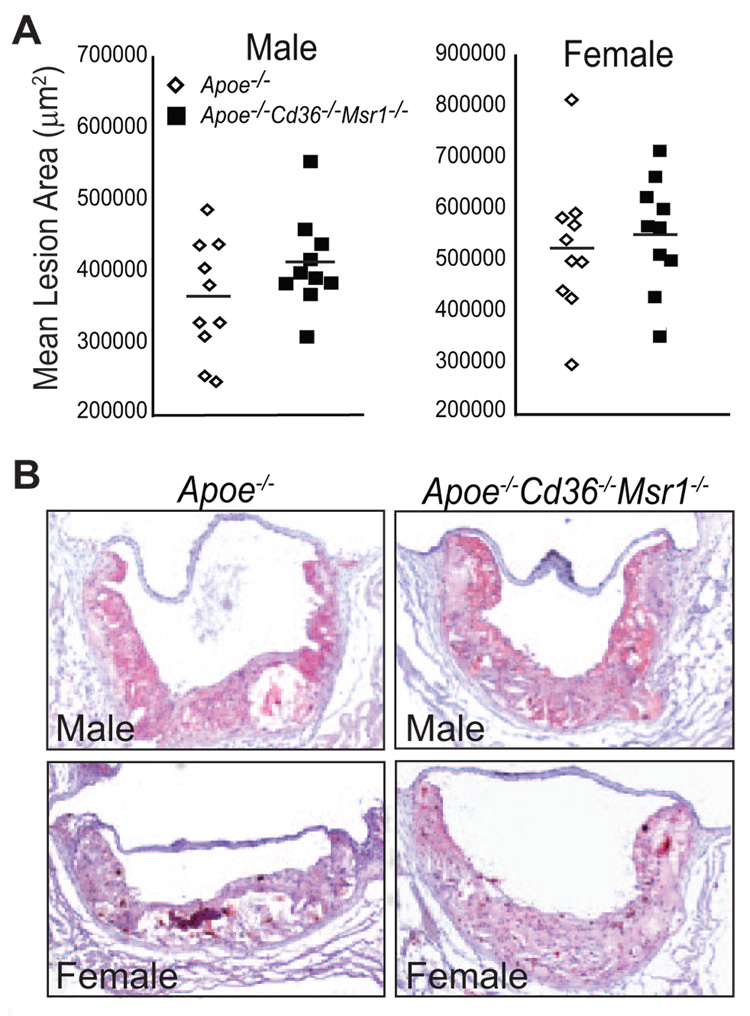
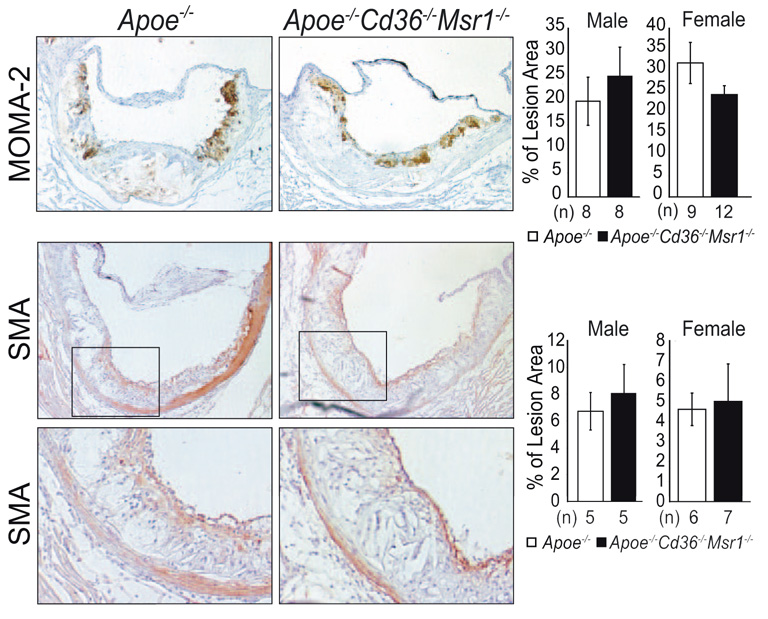
To determine whether the absence of CD36 and SR-A affected atherosclerotic lesion size, cross-sections of aortic root lesions were stained with the neutral lipid stain oil red O, and lesion area was measured (Figure 1A and 1B). The analysis revealed no significant difference in mean aortic root lesion area in male and female Apoe−/− Cd36−/− Msr1−/− mice (411 572±64 708 and 552 114±111 387 µm2, respectively), as compared to their Apoe−/− counterparts (362 268±80 957 and 524 216±138 423, respectively) (Figure 1A), although a trend toward more atherosclerosis was observed in Apoe−/− Cd36−/− Msr1−/− mice (male P=0.15; female P=0.63). These atherosclerosis measurements, conducted at Massachusetts General Hospital with the analyst blinded to genotype, were independently quantified by investigators at Columbia University with nearly identical findings (supplemental Figure I). Histological analysis of aortic root lesions revealed no significant differences in macrophage or smooth muscle cell content in atherosclerotic plaques of Apoe−/− and Apoe−/− Cd36−/− Msr1−/− mice (Figure 2).
Figure 1.
Disruption of Cd36 and Msr1 does not alter the extent of aortic root atherosclerosis in hyperlipidemic Apoe−/− mice. Aortic root atherosclerotic lesion area was quantified from 12 serial sections per mouse. Data are presented as (A) the mean lesion area of individual mice. N.S.; ANOVA. B, Representative photographs of oil red O–stained aortic root lesions. Magnification, × 100.
Figure 2.
Immunohistochemical characterization of aortic root atherosclerosis. Aortic root lesions were stained for macrophage (MOMA-2) or smooth muscle cell content (α-smooth muscle actin) and quantified as a percentage of lesion area (±SEM) using 3 sections per mouse. N.S.; Student t test. Magnification, ×100 (upper panel, middle panel), × 250 (B, lower panel).
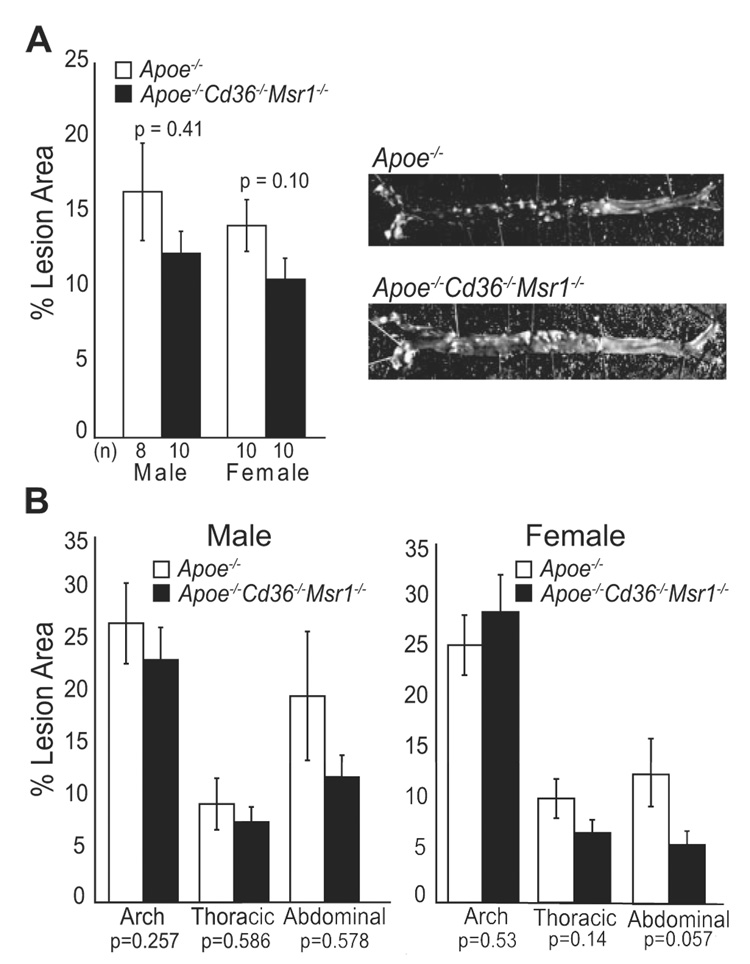
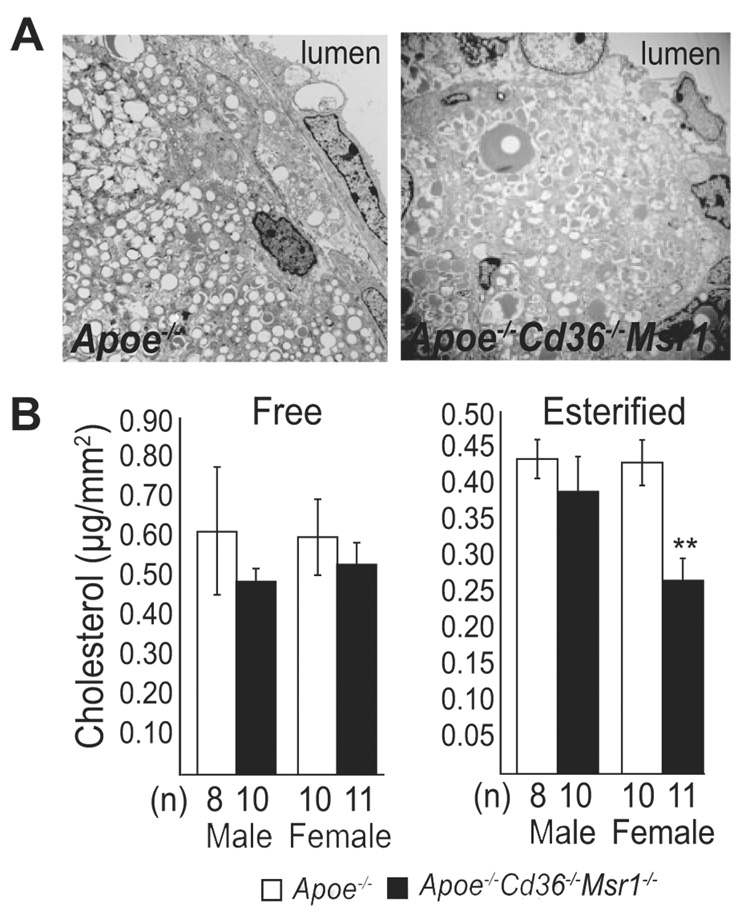
En face analysis of the aortic arch and descending aorta also showed no statistically significant differences in lesion area between the 2 groups of mice, although there was a trend toward decreased en face lesion area in Apoe−/− Cd36−/− Msr1−/− mice, particularly in the abdominal aorta of female mice (Figure 3). Further analysis of lipid accumulation in both the aortic root and aorta was performed using EM and cholesterol extraction, respectively. EM images of cross-sections of the aortic root showed the abundant presence of foam cells in the absence of both CD36 and SR-A, demonstrating that these scavenger receptors are not the only means through which lipid can accumulate in lesional macrophages (Figure 4A). As large aggregations of noninternalized lipoproteins were not detected, alternative lipoprotein clearance pathways must remain intact in the absence of CD36 and SR-A. Interestingly, measurement of cholesterol content in the entire aorta revealed a 38% decrease in esterified cholesterol in Apoe−/− Cd36−/− Msr1−/− versus Apoe−/− female mice (Figure 4B). These data, together with the trend toward decrease abdominal en face lesion area in female Apoe−/− Cd36−/− Msr1−/− mice, may reflect a partial region-specific role for the SR-A or CD36 in foam cell formation.
Figure 3.
Morphometric analysis of lesion area measured in the aorta en face. A, Total aortic lesion area in male and female Apoe−/− and Apoe−/− Cd36−/− Msr1−/− mice and representative photographs of en face aortae. B, Regional analysis of lesion distribution in the aorta. Data are expressed as mean % lesion area ± SEM.
Figure 4.
Analysis of lesional foam cell and cholesterol accumulation in the absence of CD36 and SR-A. A, Electron photomicrographs demonstrating intracellular lipid accumulation in the intima of aortic root lesions of Apoe−/− and Apoe−/− Cd36−/− Msr1−/− mice. Magnification, × 5000. B, Aortic free and esterified cholesterol content. **P<0.005; ANOVA.
In addition to their postulated role in foam cell formation, scavenger receptors have signaling roles that might influence gene expression in lesions. We analyzed expression of atherosclerosis-related genes in the aortas of Apoe−/− Cd36−/− Msr1−/− and Apoe−/− mice using Superarray Atherosclerosis PCR arrays. The array included 84 genes belonging to several subsets of gene families that have been implicated in athero-genesis, including inflammatory responses, apoptosis, adhesion molecules, lipid metabolism, cell growth, and transcription regulators. Two-thirds of the inflammatory genes analyzed were downregulated in the descending aorta of Apoe−/− Cd36−/− Msr1−/− mice, including the chemokines MCP-1 (Ccl2) and Gro-1 (KC; Cxcl1), and the cytokines interleukin (IL) 1α (IL1-α), tumor necrosis factor α (TNF-α) and transforming growth factor β (TGFβ; Table). In addition, the adhesion molecules intercellular adhesion molecule (ICAM1), e-selectin (Sele), p-selectin (Selpl), and integrin alpha X (Itgax) were also downregulated.
Table.
Expression Profiling of Aortas Reveals Reduced Inflammatory Gene Expression in the Absence of CD36 & SR-A
| Gene Name | Fold Up- or Downregulation (Apoe−/−Cd36−/−Msr1−/− vs Apoe−/−) |
P Value | Gene Description |
|---|---|---|---|
| Inflammatory response | |||
| Ccl2 | −3.38 | 0.15 | MCP-1; Chemokine (C-C motif) ligand 2 |
| Ccl5 | −14.81 | 0.16 | RANTES; Chemokine (C-C motif) ligand 5 |
| Ccr2 | −2.74 | 0.06 | Chemokine (C-C motif) receptor 2 |
| Cxcl1 | −3.15 | 0.10 | Gro-1; Chemokine (C-X-C motif) ligand 1 |
| Ifng | 1.30 | 0.51 | Interferon gamma |
| Il1a | −4.25 | 0.04 | Interleukin 1 alpha |
| II1b | −3.15 | 0.18 | Interleukin 1 beta |
| II2 | 1.27 | 0.74 | Interleukin 2 |
| Pparg | 1.43 | 0.40 | Peroxisome proliferator activated receptor gamma |
| Spp1 | −5.36 | 0.02 | Secreted phosphoprotein 1; early T-cell activation; osteopontin |
| Tgfb1 | −1.85 | 0.03 | Transforming growth factor, beta 1 |
| Tnf | −2.94 | 0.11 | Tumor necrosis factor alpha |
| Lipid metabolism | |||
| Apoa1 | 8.25 | 0.27 | Apolipoprotein A-I |
| Apob | 1.24 | 0.83 | Apolipoprotein B |
| Ldlr | −1.11 | 0.66 | Low density lipoprotein receptor |
| Ppara | 1.68 | 0.31 | Peroxisome proliferator activated receptor alpha |
| Ptgs1 | −1.11 | 0.54 | COX-1; Prostaglandin-endoperoxide synthase 1 |
| Lpl | −1.06 | 0.82 | Lipoprotein lipase |
| Adhesion molecules | |||
| Cdh5 | −1.44 | 0.10 | Cadherin 5 |
| Icam1 | −1.61 | 0.06 | Intercellular adhesion molecule |
| Vcam1 | −3.01 | 0.55 | Vascular cell adhesion molecule 1 |
| Itga2 | −1.90 | 0.11 | Integrin alpha 2 |
| Itgax | −4.46 | 0.01 | Integrin alpha X |
| Itgb2 | −1.90 | 0.11 | Integrin beta 2 |
| Fn1 | −1.31 | 0.42 | Fibronectin 1 |
| Sele | −3.79 | 0.07 | Selectin, endothelial cell |
| Sell | −1.44 | 0.43 | Selectin, lymphocyte |
| SelpI | −2.50 | 0.03 | Selectin, platelet (p-selectin) ligand |
| Thbs4 | 2.02 | 0.20 | Thrombospondin 4 |
| Apoptosis | |||
| Bcl2 | 2.99 | 0.06 | B-cell leukemia/lymphoma 2; anti-apoptotic |
| Bcl2l1 | 2.06 | 0.19 | Bcl2-like 1; anti-apoptotic |
| Bax | 1.21 | 0.64 | Bcl2-associated × protein; pro-apoptotic |
| Fas | 1.11 | 0.74 | TNF receptor superfamily member; pro-apoptotic |
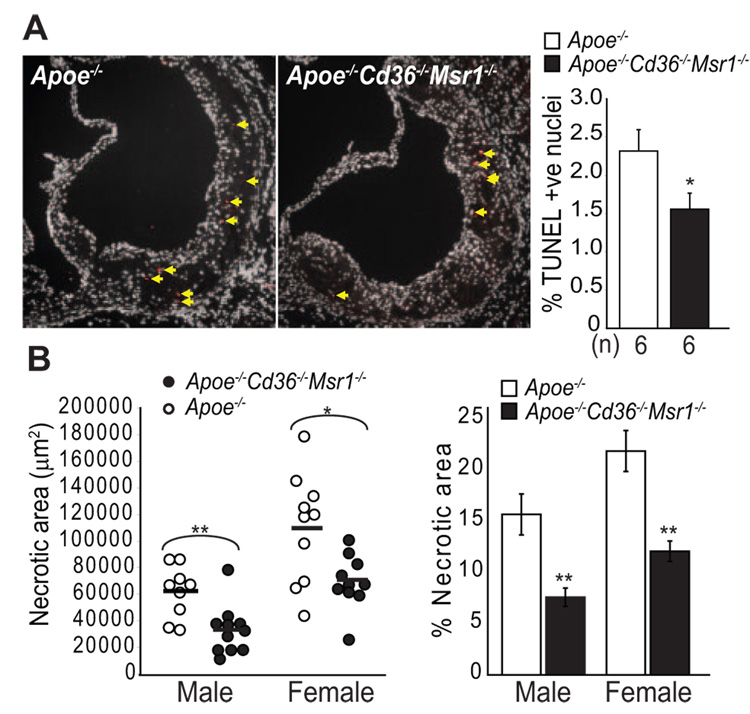
The array data also revealed that 2 antiapoptotic genes, B-cell leukemia/lymphoma (Bcl2) and Bcl2-like 1 (Bcl2l1), were upregulated when CD36 and SR-A were absent. These data suggested that scavenger receptor activity might increase apoptosis in atherosclerotic lesions, consistent with our in vitro observations that SR-A and CD36 ligands can trigger apoptosis in ER-stressed macrophages (Seimon et al, unpublished data).21,23 In this context, we analyzed atherosclerotic lesions for macrophage apoptosis and its consequence, plaque necrosis. We found that female Apoe−/− Cd36−/− Msr1−/− aortic root lesions had a 32% decrease in apoptotic cells as assessed by TUNEL staining (Figure 5A). Whereas no significant decrease in TUNEL staining was observed in male Apoe−/− Cd36−/− Msr1−/− mice, mean necrotic area and percent necrotic area were markedly decreased in aortic root lesions of both male and female Apoe−/− Cd36−/− Msr1−/− mice (Figure 5B). Taken together, these results are supportive of enhanced lesion stability in the Apoe−/− Cd36−/− Msr1−/− mice. Consistent with our previous mechanistic data in cultured macrophages, these results suggest an important role for SR-A or CD36 in advanced lesional macrophage apoptosis and subsequent plaque necrosis.
Figure 5.
Reduced apoptotic cell death and necrotic core formation in lesions of Apoe−/− Cd36−/− Msr1−/− mice. A, Lesional apoptotic cells in aortic root lesions of female mice were detected by TUNEL staining. Representative fluorescent images (×100 magnification) and quantitative data are shown. Red, TUNEL positive nuclei; white, DAPI-stained nuclei. *P<0.05; Student t test. B, Quantitative analysis of necrotic core size relative to total lesion area; n=9 to 11 per group; **P<0.005, *P<0.05; Student t test.
Discussion
The data in this study reveal 2 important points about the function of SR-A and CD36 in advanced aortic root atherosclerotic lesions of Apoe−/− mice. First, the receptors are not absolutely necessary for foam cell formation in a markedly hyperlipidemic animal, nor do they appear to contribute substantially to overall lesion size, despite the fact that they can account for the vast majority of oxidized LDL uptake in cultured macrophages. Second, the receptors contribute to inflammatory gene expression and appear to play an important role in the generation of advanced lesional macrophage apoptosis and plaque necrosis. These data are consistent with recent mechanistic studies from our laboratories linking scavenger receptors to downstream signaling pathways and their role in precipitating macrophage apoptosis.21,23,27–29
Since the first report of reduced atherosclerosis in Apoe−/− mice lacking SR-A by Suzuki and colleagues in 1997, the paradigm has been that scavenger receptors promote atherosclerosis primarily through their role in generating macrophage foam cells.30 However, additional studies of the role of SR-A in atherosclerosis have led to varied results, with some studies showing it to be proatherogenic and others suggesting an antiatherogenic effect.17,31–33 Studies of the impact of CD36 on atherosclerosis in Apoe−/− mice have been some-what more consistent, with deficiency of CD36 conferring protection against atherosclerotic lesion development in the descending aorta ranging from 30% to 80%.17,33,34 A more variable effect of CD36 on aortic root lesions has been reported, however these effects were modest compared with the benefit conferred on more distal aortic lesions.17,34 Thus, CD36 may evince region-specific influences on atherosclerosis. A key finding of the combined SR-A/CD36 knock-out study reported here is that the failure of prior studies of mice lacking either SR-A or CD36 to demonstrate elimination of aortic root foam cell formation cannot now be explained by compensation by the other receptor. While our study was being completed, Kuchibhotla et al reported that Apoe−/− mice lacking both SR-A and CD36, fed a western diet for 12-weeks, also retained foam cell formation in their aortic root lesions.33 Taken together, these data now clearly establish that foam cell formation can proceed in the absence of these scavenger receptor pathways.
How might foam cells form in vivo in the absence of SR-A and CD36? Other receptors such as scavenger receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX/ CXCL16) or lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) may internalize oxidized lipoproteins, and an increase in their expression could compensate for the loss of SR-A and CD36. We performed quantitative RT-PCR analysis of the expression of both SR-PSOX and LOX-1 in RNA taken from the aortas of female wild-type and TKO mice, but were unable to detect significantly increased expression for either receptor, using 4 to 5 animals per assay (data not shown). Foam cell formation can also be driven in cultured macrophage models using LDL that has not been oxidized (reviewed in35). Early studies by Khoo et al and Tabas and colleagues showed that aggregated LDL, known to be present in atherosclerotic lesions, is a potent scavenger receptor–independent inducer of foam cells in cultured macrophages.36,37 Other examples of scavenger receptor–independent foam cell–forming particles include sPLA2-modified LDL, chylomicron remnants, and platelet-derived vesicles.16,38 Furthermore, Kruth and colleagues have provided strong evidence that macrophages can internalize native lipoproteins via the process of macropinocytosis, a nonreceptor-mediated process that would likely take on increasing importance as the local concentration of lipoproteins increased.18 One important caveat of our work is that this study was conducted in Apoe−/− mice fed a Western diet. As these mice are markedly hyperlipidemic, the role of high affinity modified lipoprotein receptor uptake mechanisms in foam cell formation might well be less important in this setting than in situations of moderate hypercholesterolemia. Thus, this work does not preclude a more substantial role for SRA and CD36 in foam cell formation in other animal models or in human atherosclerosis.
Despite only a modest impact on overall atherosclerosis lesion area, we found that scavenger receptor deficiency causes substantial changes in atherosclerosis inflammatory gene expression. Decreased aortic expression of cytokines, chemokines and adhesion molecules indicated that the loss of the scavenger receptors was associated with a reduction in the inflammatory milieu. In agreement with this, Kuchibhotla et al reported decreased serum levels of inflammatory markers in their Apoe−/− Cd36−/− Msr1−/− mice, several of which overlap with our measurements of inflammatory gene expression in the aorta.33 In addition, we found that the combined absence of SR-A and CD36 markedly reduced both macrophage apoptosis and plaque necrosis in lesions of the aortic root. Plaque necrosis results when macrophages become apoptotic and then are not efficiently cleared by efferocytes. The observed decrease in lesional apoptosis is consistent with our previous in vitro studies showing that apoptosis in ER-stressed macrophages can be triggered by SRA and CD36 ligands (Seimon and Tabas, unpublished observations).21,23 Markers of ER stress correlate with advanced lesion stage and apoptosis in both animal and human coronary artery plaques, supporting a role for this process in triggering macrophage apoptosis and necrotic core formation.22 Moreover, gene targeting studies in mice have shown causal relationships among ER stress, advanced lesion macrophage apoptosis, and plaque necrosis.39 The finding of decreased necrotic cores in mice lacking the scavenger receptors might initially seem to be in conflict with our failure to demonstrate significant changes in lesion area or substantial differences in macrophage number in the atherosclerotic lesions. Necrotic core area, however, is a measurement that integrates events that are occurring over the entire time course of our study, and it reflects macrophage apoptosis, efferocytosis, and exit from the intima, as well as new macrophage recruitment to lesions. To fully understand the effects of the loss of scavenger receptors on lesion development and progression, it will be necessary to undertake a detailed analysis of these processes in future studies that assess these events in temporal sequence.
In summary, the data in this study demonstrate that scavenger receptors play a critical role in the evolution of atherosclerosis lesion complexity. This role appears to be mediated through their influence on apoptosis and inflammatory gene expression, rather than lipid uptake and foam cell formation. These findings also suggest that the analysis of more complex features of atherosclerosis, such as the quantification of necrotic core volumes, will increasingly be needed if we are to decipher the complex interplay between innate immune systems, apoptosis, and the evolution of the atherosclerotic plaque.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL45098 and RR20345 (M.W.F.), AG20255 (K.J.M.), HL75662 and HL57560 (I.T.), an American Heart Association Scientist Development Grant 0735594T (T.A.S.) and grant #200140/2006-0 from CNPq (J.L.).
Footnotes
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://atvb.ahajournals.org
Data Supplement (unedited) at: http://atvb.ahajournals.org/cgi/content/full/ATVBAHA.108.176644/DC1
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 2.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke M, Bennett M. The emerging role of vascular smooth muscle cell apoptosis in atherosclerosis and plaque stability. Am J Nephrol. 2006;26:531–535. doi: 10.1159/000097815. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 8.Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol. 2007;27:705–713. doi: 10.1161/01.ATV.0000261709.34878.20. [DOI] [PubMed] [Google Scholar]
- 10.Ohayon J, Dubreuil O, Tracqui P, Le Floc’h S, Rioufol G, Chalabreysse L, Thivolet F, Pettigrew RI, Finet G. Influence of residual stress/strain on the biomechanical stability of vulnerable coronary plaques: potential impact for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 2007;293:H1987–H1996. doi: 10.1152/ajpheart.00018.2007. [DOI] [PubMed] [Google Scholar]
- 11.Gerrity RG, Naito HK. Ultrastructural identification of monocyte-derived foam cells in fatty streak lesions. Artery. 1980;8:208–214. [PubMed] [Google Scholar]
- 12.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 14.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 15.Kruth HS. Sequestration of aggregated low-density lipoproteins by macrophages. Curr Opin Lipidol. 2002;13:483–488. doi: 10.1097/00041433-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Tabas I. Nonoxidative modifications of lipoproteins in atherogenesis. Annu Rev Nutr. 1999;19:123–139. doi: 10.1146/annurev.nutr.19.1.123. [DOI] [PubMed] [Google Scholar]
- 17.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruth HS, Jones NL, Huang W, Zhao B, Ishii I, Chang J, Combs CA, Malide D, Zhang WY. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2005;280:2352–2360. doi: 10.1074/jbc.M407167200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang WY, Gaynor PM, Kruth HS. Aggregated low density lipoprotein induces and enters surface-connected compartments of human monocytemacrophages. Uptake occurs independently of the low density lipoprotein receptor. J Biol Chem. 1997;272:31700–31706. doi: 10.1074/jbc.272.50.31700. [DOI] [PubMed] [Google Scholar]
- 20.Wooton-Kee CR, Boyanovsky BB, Nasser MS, de Villiers WJ, Webb NR. Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation. Arterioscler Thromb Vasc Biol. 2004;24:762–767. doi: 10.1161/01.ATV.0000122363.02961.c1. [DOI] [PubMed] [Google Scholar]
- 21.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 23.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunjathoor VV, Chiu DS, O’Brien KD, LeBoeuf RC. Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:462–468. doi: 10.1161/hq0302.105378. [DOI] [PubMed] [Google Scholar]
- 26.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 27.Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 28.Stuart LM, Bell SA, Stewart CR, Silver JM, Richard J, Goss JL, Tseng AA, Zhang A, El Khoury JB, Moore KJ. CD36 signals to the actin cytoskeleton and regulates microglial migration via a p130Cas complex. J Biol Chem. 2007;282:27392–27401. doi: 10.1074/jbc.M702887200. [DOI] [PubMed] [Google Scholar]
- 29.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 31.Babaev VR, Gleaves LA, Carter KJ, Suzuki H, Kodama T, Fazio S, Linton MF. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2000;20:2593–2599. doi: 10.1161/01.atv.20.12.2593. [DOI] [PubMed] [Google Scholar]
- 32.de Winther MP, Gijbels MJ, van Dijk KW, van Gorp PJ, Suzuki H, Kodama T, Frants RR, Havekes LM, Hofker MH. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis. 1999;144:315–321. doi: 10.1016/s0021-9150(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 33.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb NR, Moore KJ. Macrophage-derived foam cells in atherosclerosis: lessons from murine models and implications for therapy. Curr Drug Targets. 2007;8:1249–1263. doi: 10.2174/138945007783220597. [DOI] [PubMed] [Google Scholar]
- 36.Khoo JC, Miller E, McLoughlin P, Steinberg D. Enhanced macrophage uptake of low density lipoprotein after self-aggregation. Arteriosclerosis. 1988;8:348–358. doi: 10.1161/01.atv.8.4.348. [DOI] [PubMed] [Google Scholar]
- 37.Tabas I. Secretory sphingomyelinase. Chem Phys Lipids. 1999;102:123–130. doi: 10.1016/s0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 38.Boyanovsky BB, van der Westhuyzen DR, Webb NR. Group V secretory phospholipase A2-modified low density lipoprotein promotes foam cell formation by a SR-A- and CD36-independent process that involves cellular proteoglycans. J Biol Chem. 2005;280:32746–32752. doi: 10.1074/jbc.M502067200. [DOI] [PubMed] [Google Scholar]
- 39.Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.