Abstract
Investigations from basic biology suggest that activation of the Rho/Rho kinase pathway reduces the bioavailability of nitric oxide (NO) and thereby promotes atherosclerosis and its clinical complications. Yet, little information is available about the relationship of the Rho/Rho kinase pathway to NO bioavailability in humans with atherosclerosis. Accordingly, we determined whether inhibition of Rho kinase augments NO bioavailability and improves endothelial function in human subjects with coronary artery disease (CAD). Thirteen CAD subjects and 16 age- and sex-matched healthy controls were randomly assigned to receive the Rho kinase inhibitor, fasudil, or placebo for 1 month each in a double-blind crossover trial. Flow-mediated, endothelium-dependent and nitroglycerin-induced, endothelium-independent vasodilation were assessed by brachial artery ultrasonography. Rho kinase activity was measured in peripheral leukocytes. Fasudil increased endothelium-dependent vasodilation in CAD subjects from 9.4±1.9% to 13.4±1.9% (P=0.001) but not in healthy controls (from 11.3±1.4% to 7.7±1.1%; P=0.07). Endothelium-independent vasodilation was not affected by fasudil in either CAD or healthy subjects. Fasudil reduced Rho kinase activity by 59±18% in CAD subjects (P=0.001) but not in healthy subjects (by 3±6%; P=0.60). The change in endothelium-dependent vasodilation achieved with fasudil relative to placebo was inversely proportional to Rho kinase inhibition (ie, greater Rho kinase inhibition was associated with larger improvement in endothelium-dependent vasodilation) (r=−0.48; P=0.01). These findings suggest that Rho/Rho kinase activation promotes endothelial dysfunction in humans with atherosclerosis. Inhibition of the Rho/Rho kinase pathway should provide a useful strategy to restore NO bioavailability in humans with atherosclerosis.
Keywords: atherosclerosis, endothelium, nitric oxide, rho kinase, vasodilation
The endothelium synthesizes a variety of autocrine and paracrine factors, including nitric oxide (NO), that regulate vascular tone, maintain blood cell/vessel wall interaction, prevent thrombosis, and limit smooth muscle cell proliferation.1 Reduced NO bioavailability, or endothelial dysfunction, is a hallmark of atherosclerosis and predicts adverse cardiovascular events.2,3 A more complete understanding of the molecular mechanisms that impair NO bioavailability in humans with atherosclerosis will facilitate better strategies to reduce the progression and the overt clinical manifestations of this disease.
The small GTP-binding protein Rho, and its downstream effector, Rho kinase, have been implicated in many of the pathologic processes that underlie atherosclerosis including endothelial dysfunction, vasoconstriction, inflammation, cellular migration and proliferation, and a procoagulant state.4,5 Basic studies have shown that inhibition of Rho/Rho kinase augments the expression and activity of endothelial nitric oxide synthase (eNOS),6–8 whereas overactivity of the Rho/Rho kinase pathway, as occurs in experimental atherosclerosis, reduces NO bioavailability.9,10 It is unknown, however, whether the Rho/Rho kinase pathway is overactive in human atherosclerosis and, hence, whether inhibition of the Rho/Rho kinase pathway can improve NO bioavailability in this setting. The development of a selective inhibitor of Rho kinase, fasudil, for safe clinical use,10,11 coupled with an assay of Rho kinase activity,6 have permitted us to examine the role of this pathway in endothelial dysfunction in humans with atherosclerosis. We specifically tested the hypothesis that fasudil reverses endothelial dysfunction in patients with coronary artery disease (CAD) and that this improvement in endothelial function correlates with Rho kinase inhibition.
Patients and Methods
Patients
The Human Research Committee at Brigham and Women’s Hospital approved this study. We investigated 13 subjects with CAD and 16 healthy age- and sex-matched controls. Subjects were recruited by advertisement and from the cardiology clinics at our institution. A complete history, physical examination, ECG, and laboratory evaluation were performed for each subject. The presence of CAD was determined by ≥50% stenosis in at least 1 coronary artery at cardiac catheterization, by history of prior myocardial infarction, prior angioplasty, or coronary artery bypass graft surgery. Exclusion criteria for CAD subjects included unstable angina, revascularization, or severe heart failure within 3 months of study enrollment, malignancy, chronic inflammatory disease, pregnancy, breast feeding, hypoxia with room air saturation <90%, low-density lipoprotein (LDL) cholesterol <100 mg/dL off statin therapy, fasting glucose >120 mg/dL, creatinine >2.0 mg/dL, liver transaminases >2 times the upper limit of normal, and reluctance to discontinue statin therapy. CAD subjects with elevated fasting blood glucose were excluded because Rho kinase may interfere with insulin signaling, potentially affecting endothelial function independently of atherosclerosis. 12 To minimize the risk associated with the study, subjects were encouraged to continue all cardiac medications, except statins. Subjects were, however, asked to hold all medications until after the research procedures on study days. Healthy subjects did not have any risk factors for CAD, symptoms, or ECGs suggestive of atherosclerosis or documented atherosclerosis. They were not consuming any vasoactive medications.
Study Design
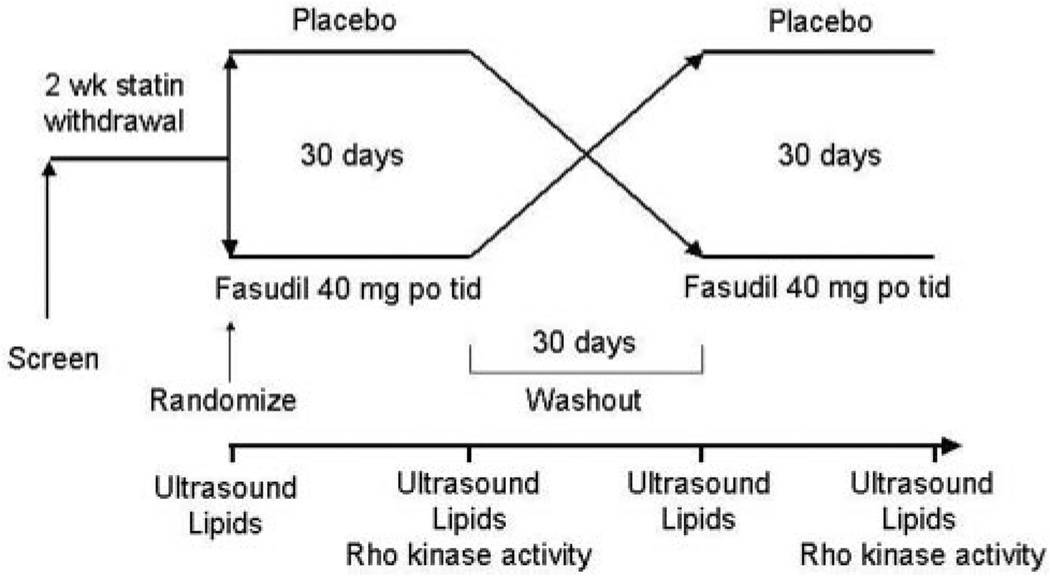
The study was designed as a randomized, double-blind, placebo-controlled crossover trial (Figure 1). After an initial screening visit, subjects who signed informed consent and met inclusion/exclusion criteria were asked to discontinue statin therapy for a minimum of 2 weeks. This period is sufficient to restore endothelial function and lipid profiles to prestatin treatment levels.13 Subjects were then randomized to receive 1 month of fasudil (40 mg orally 3 times daily) or placebo. This dose of fasudil is safe and well tolerated in humans with stable effort angina.4,11 After a subsequent 30-day washout period, subjects crossed over to the alternate treatment arm for an additional month. Brachial artery ultrasonography was performed to evaluate endothelial function before and after each treatment. Blood for fasting lipid profiles and safety assessment was collected before and after each treatment period. Blood for assay of Rho kinase activity was collected at the end of each treatment period.
Figure 1.
Study protocol.
Assessment of Endothelial Function
An ultrasound scanner (Toshiba model Power Vision 8000) equipped with a high-resolution broadband linear array transducer (7.5 to 12.5 MHz) was used to image the brachial artery. Endothelium-dependent and -independent dilation of the brachial artery was determined according to established guidelines.14 To assess endothelium-dependent vasodilation, brachial artery diameter was measured under basal conditions and during reactive hyperemia following 5 minutes of an ischemic stimulus. A blood pressure cuff placed on the upper arm was inflated to suprasystolic pressures for 5 minutes to induce forearm ischemia. We have found that following cuff deflation, the maximal increase in brachial artery diameter occurs at 1 minute of reactive hyperemia and that NO mediates this dilation.15,16 To assess endothelium-independent vasodilation, brachial artery diameter was measured under basal conditions and following the administration of sublingual nitroglycerin (0.4 mg). Maximal brachial artery dilation occurs 3 to 4 minutes after the administration of sublingual nitroglycerin.14
Acquisition and analysis of the digitized images were performed using software from Information Integrity Inc. The vessel wall/lumen interface was determined by derivative based edge detection algorithm following identification of the region of the anterior and posterior walls by the investigator. The maximum diameter of the vessel was then determined.17 We have found that this method is associated with an interobserver variability of 0.05±0.16% and intraobserver variability of 0±0.15%. The same arm and site were used for all measurements.
Measurement of Rho Kinase Activity
Rho kinase activity was assayed in peripheral blood leukocytes as the amount of phospho-Thr853 in the myosin-binding subunit (MBS) of myosin light chain (MLC) phosphatase.6 Blood was collected at room temperature in heparinized tubes (20 U/mL) with 10 mmol/L fasudil (Asahi Chemical Industry Co Ltd, Tokyo, Japan ). Fasudil was added to inhibit Rho kinase activity and hence further formation of phospho-Thr853 MBS ex vivo. We have found that at room temperature appreciable dephosphorylation of phospho-Thr853 MBS does not occur (data not shown). After adding an equal volume of 2% dextran, the sample was kept at room temperature for 30 minutes. The supernatant was spun at 1450 rpm for 10 minutes. Red blood cells in the resulting cell pellet were lysed with the addition of water and spun at 1450 rpm for 10 minutes after the addition of Hank’s balanced salt solution (Hyclone, Logan, Utah). The resulting leukocyte pellet was resuspended in medium 199(Sigma Chemical Co) and counted using a hematocytometer. Cells were fixed in 10% trichloroacetic acid and 10 mmol/L dichlorodiphenyltrichloroethane. After centrifugation, the cell pellets were stored at −80°C for Western blot analysis.
Cells pellets were dissolved in 10 µL of 1 mol/L Tris base and then mixed with 100 µL of extraction buffer (8 mol/L urea, 2% sodium dodecyl sulfate, 5% sucrose, and 5% 2-mercaptoethanol). Equal amounts of cell extracts were subjected to 7.5% SDS-PAGE and transferred onto PVDF membrane (Immobilon-P, Millipore, Billerica, Mass). NIH 3T3 cell lysates were used as a positive control and to standardize the results of Western blot analyses from several membranes. After serum starvation for 20 hours, confluent cells were stimulated with 10 µmol/L lysophosphatidic acid for 10 minutes, then subsequently fixed and harvested in 10% trichloroacetic acid and 10 mmol/L dichlorodiphenyltrichloroethane. Following centrifugation at 1450 rpm for 10 minutes at 4°C, precipitates were dissolved in 10 µL of 1 mol/L Tris base and mixed with 100 µL of extraction buffer. An equal volume of positive control cell lysate was used for each gel. Membranes were incubated with rabbit anti–phospho-specific Thr853–MBS polyclonal antibody (generous gift of Dr Ikebe, University of Massachusetts Medical School, Worcester, Mass)18 or rabbit anti-MBS polyclonal antibody (Covance Laboratories, Evansville, Ind). Bands were visualized with the use of the ECL detection kit (Amersham Pharmacia Biotech). Rho kinase activity was expressed as the ratio of phospho-Thr853–MBS in each sample per phosphoThr853–MBS in each positive control divided by MBS in each sample per MBS in each positive control.
Measurement of Lipids
Fasting serum lipids (total and high-density lipoprotein [HDL] cholesterol and triglycerides) were measured with an Olympus AU400 analyzer using enzymatic methods. LDL cholesterol was calculated according to the methods of Friedewald et al.19
Drugs
Fasudil hydrochloride hydrate and matching placebo tablets were obtained from Berlex Pharmaceuticals (Montville, NJ). Nitroglycerin was obtained from Pfizer (Morris Plains, NJ). Berlex Pharmaceuticals established the randomization code and drug was administered in a double-blinded fashion by the investigational drug pharmacy at Brigham and Women’s Hospital.
Statistical Analysis
Descriptive and experimental measures are expressed as mean±SE or median (25th percentile, 75th percentile) as appropriate. Baseline characteristics were compared between CAD and healthy subjects using unpaired Student’s t test or Wilcoxon rank sum test for continuous variables and Fisher’s exact test for discrete variables. Flow-mediated endothelium-dependent vasodilation and nitroglycerin-mediated endothelium-independent vasodilation for each subject group were compared using paired Student’s t test for normally distributed data and Sign rank test for not normally distributed data. Because both flow-mediated, endothelium-dependent and nitroglycerin-mediated, endothelium-independent vasodilation were similar within each group after each washout period, all reported comparisons were made between the postplacebo and postfasudil measurements. Comparisons between groups were made using an independent t test or Wilcoxon rank sum test. Similar analyses were used to assess the affect of fasudil on brachial artery diameter, mean arterial pressure, lipid profiles, and Rho kinase activity. The change in vasodilation with fasudil relative to placebo was correlated with the change in Rho kinase activity using Spearman’s correlation coefficient. A probability value of <0.05 was considered to be statistically significant.
Results
Baseline Characteristics
CAD and healthy subjects were age and sex matched (Table 1). Resting heart rate and mean arterial pressure were similar in both groups (Table 1). As expected, compared with healthy individuals, cardiac risk factors were more prevalent in the CAD subjects (Table 1). Even though diabetic subjects were excluded, mean fasting glucose was higher in CAD subjects compared with healthy individuals (P=0.03). Consistent with the inclusion and exclusion criteria, fasting lipids including total cholesterol (P=0.0006), LDL (P<0.0001), and triglycerides (P<0.05) were higher in CAD subjects. All CAD subjects were on aspirin and statin therapy at the time of enrollment. Eleven (85%) of the CAD patients were being treated with β blockers, 6 (46%) with angiotensin-converting enzyme inhibitors and 2 (15%) with calcium channel blockers. Two (13%) of the healthy individuals took aspirin at the time of enrollment.
TABLE 1.
Baseline Characteristics
| CAD (n=13) |
Healthy (n=16) |
p | |
|---|---|---|---|
| Age (y) | 65±2 | 60±2 | 0.07 |
| Male/female (n) | 9/4 | 9/7 | 0.70 |
| HR, bpm | 67±2 | 71±2 | 0.18 |
| MAP, mm Hg | 94±3 | 95±1 | 0.67 |
| Smokers (n) | 4 | 0 | 0.03 |
| Glucose, mmol/L | 5.9±0.3 | 5.2±0.2 | 0.03 |
| Total cholesterol, mmol/L | 6.1±0.3 | 4.6±0.2 | 0.0006 |
| LDL cholesterol, mmol/L | 3.8±0.2 | 2.6±0.2 | <0.0001 |
| HDL cholesterol, mmol/L | 1.3±0.1 | 1.4±0.1 | 0.45 |
| Triglycerides, mmol/L | 2.1±0.7 | 1.2±0.2 | 0.05 |
Data are presented as mean±SE. HR indicates heart rate; MAP, mean arterial pressure.
Effect of Fasudil on Vascular Function
Baseline arterial diameters were similar in the 2 groups and also within each group on fasudil compared with placebo (Table 2). The increase in blood-flow velocity with reactive hyperemia was similar during placebo administration in CAD subjects and healthy controls (P=0.33). These values were not altered significantly during fasudil treatment compared with placebo in either group (Table 2).
TABLE 2.
Brachial Artery Parameters
| CAD (n=13) | Healthy (n=16) | |||||
|---|---|---|---|---|---|---|
| Placebo | Fasudil | P | Placebo | Fasudil | P | |
| Baseline Diameter, mm | 3.5±0.2 | 3.5±0.2 | 0.11 | 3.6±0.2 | 3.6±0.2 | 0.69 |
| FMD, % | 9.4±1.9 | 13.4±1.9 | 0.001 | 11.3±1.4 | 7.7±1.1 | 0.07 |
| TNG mediated dilation, % | 15.6±2.4 | 15.3±2.3 | 0.89 | 14.4±1.7 | 13.9±1.5 | 0.94 |
| RH, % (increase) | 616±81 | 489±37 | 0.22 | 651±47 | 756±80 | 0.23 |
| TNG hyperemia, % (increase) | 96±5.8 | 92±8.9 | 0.74 | 103 6.5 | 91±5.8 | 0.27 |
Data are presented as mean±SE. FMD indicates flow-mediated dilation; TNG, nitroglycerin; RH, reactive hyperemia.
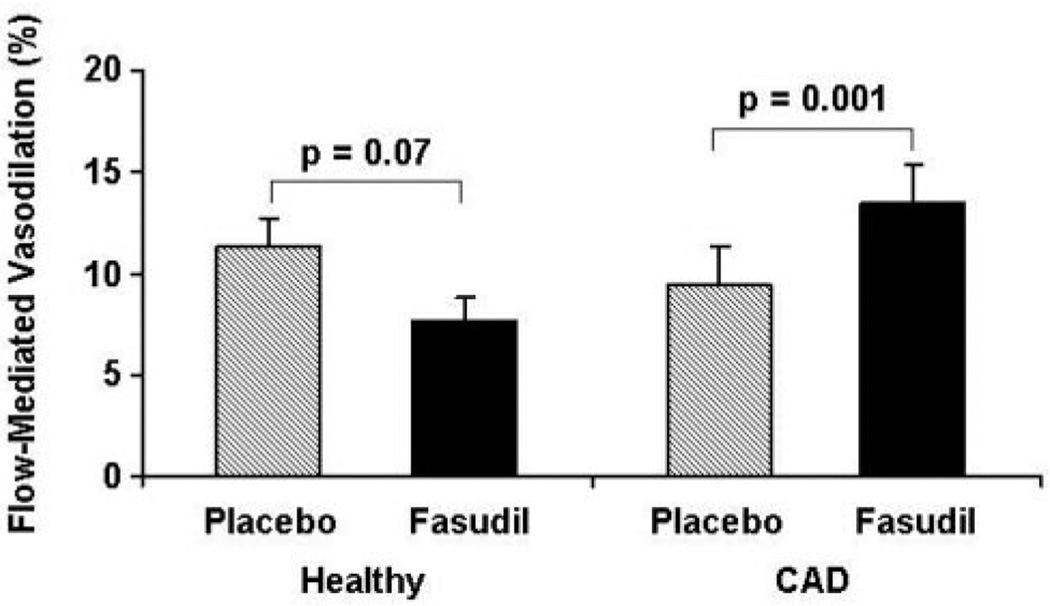
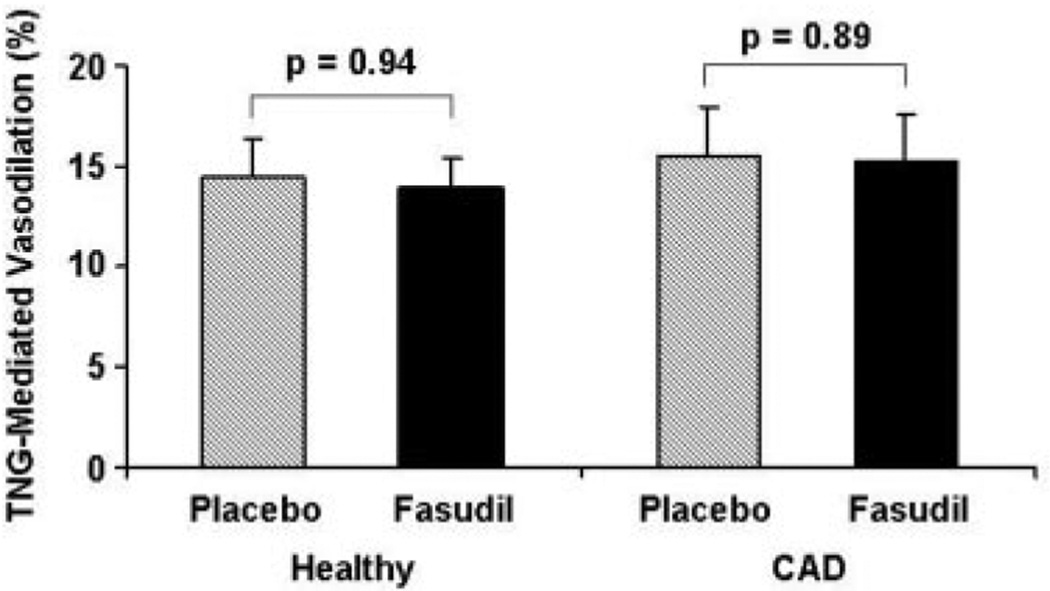
As expected, flow-mediated, endothelium-dependent vasodilation tended to be lower, although not significantly, between CAD and healthy subjects during placebo treatment (9.4±1.9% versus 11.3±1.4%, respectively; P=0.23). Fasudil augmented flow-mediated vasodilation in CAD subjects from 9.4±1.9% to 13.4±1.9% (P=0.001) but not in healthy controls (11.3±1.4% to 7.7±1.1%; P=0.07) (Figure 2). Nitroglycerin-induced, endothelium-independent vasodilation did not differ significantly between CAD subjects and healthy controls after placebo treatment (15.6±2.4% versus 14.4±1.9%; P±0.70). Fasudil did not alter nitroglycerin-mediated vasodilation in either CAD (P=0.89) or healthy subjects (P=0.94) (Figure 3).
Figure 2.
Effect of fasudil on flow-mediated vasodilation. Results are expressed as mean±SE. The percentage increase in brachial artery diameter 1 minute after cuff release compared with the baseline is illustrated. Fasudil increased flow-mediated, endothelium-dependent vasodilation significantly in the CAD subjects (P=0.001) but not in healthy controls (P=0.07).
Figure 3.
Effect of fasudil on nitroglycerin-mediated vasodilation. Results are expressed as mean±SE. The percentage increase in brachial artery diameter 3 minutes after sublingual nitroglycerin administration compared with baseline is illustrated. Fasudil did not significantly alter nitroglycerin-mediated, endothelium-independent vasodilation in either CAD or healthy subjects
Mean arterial blood pressure was not changed by fasudil treatment compared with placebo in either CAD (93±4 to 90±3 mm Hg; P=0.41) or healthy subjects (88±2 to 88±2 mm Hg; P=0.93).
Effect of Fasudil on Rho Kinase Activity
Rho kinase activity (measured as percentage relative staining of phospho-Thr853–MBS/MBS) after placebo treatment tended to be higher in CAD subjects compared with healthy individuals (P=0.16) (Table 3). Fasudil reduced Rho kinase activity by 59±18% in CAD subjects (P=0.001) but not in healthy controls (by 3%±6%; P=0.60) (Table 3). Notably, fasudil reduced Rho kinase activity in the CAD subjects to the same level observed in the healthy controls (Table 3).
TABLE 3.
Effect of Fasudil on Rho Kinase Activity
| Placebo | Fasudil | P | |
|---|---|---|---|
| CAD (n=12) | |||
| Rho kinase activity, % | 129±24.3 | 78.9±12.0 | 0.001 |
| (pThr853-MBS/MBS) | 109.8 [84.6, 144.0] | 73.9 [36.7, 110.7] | |
| Healthy (n=16) | |||
| Rho kinase activity, % | 87.3±8.7 | 84.4±8.2 | 0.60 |
| (pThr853-MBS/MBS) | 94.1 [57.5, 113.0] | 85.7 [56.9, 107.8] |
Data are presented as mean±SE and median [25th percentile, 75th percentile]. pThr853-MBS indicates phosphorylated myosin-binding subunit of myosin light chain phosphatase at residue Thr853; MBS, of MLC phosphatase.
Correlation of Rho Kinase Inhibition With Change in Endothelium-Dependent Dilation
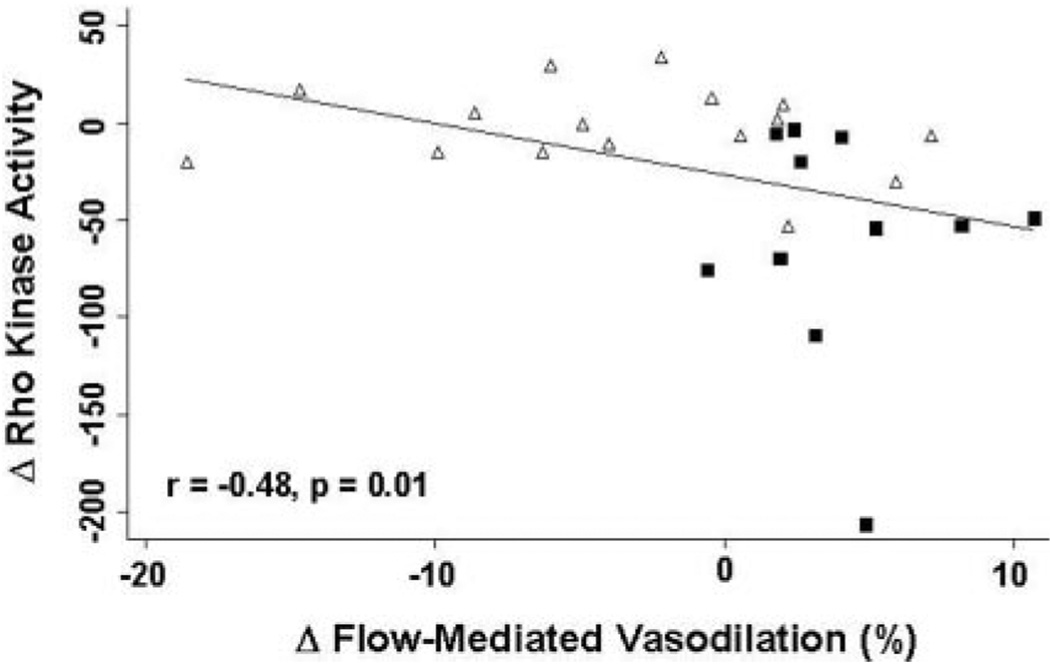
To confirm that fasudil augmented flow-mediated dilation via inhibition of Rho kinase, we correlated change in flow-mediated dilation with change in Rho kinase activity. For the 28 subjects in whom Rho kinase measurements were available, the change in endothelium-dependent vasodilation after fasudil relative to placebo was inversely proportional to the change in Rho kinase activity (r=−0.48; P=0.01) (Figure 4).
Figure 4.
Correlation of Rho kinase inhibition with fasudil and change in endothelium-dependent vasodilation. For the entire cohort of 28 patients, the change in flow-mediated, endothelium-dependent vasodilation after fasudil relative to placebo was inversely proportional to the extent of Rho kinase inhibition with fasudil (r=−0.48, P=0.01). Squares represent CAD and triangles represent healthy subjects, respectively.
Effect of Fasudil on Lipids
As expected, fasudil treatment did not change total cholesterol, HDL, LDL, or triglyceride levels compared with placebo in either subject group (Table 4).
TABLE 4.
Effect of Fasudil on Lipids
| Placebo | Fasudil | P | |
|---|---|---|---|
| CAD (n=13) | |||
| Total cholesterol, mmol/L | 6.2±0.4 | 4.7±0.4 | 0.35 |
| LDL cholesterol, mmol/L | 3.7±0.2 | 3.7±0.2 | 0.59 |
| HDL cholesterol, mmol/L | 1.2±0.1 | 1.2±0.1 | 0.81 |
| Triglycerides, mmol/L | 2.8±1.1 | 2.3±0.7 | 0.09 |
| Healthy (n=16) | |||
| Total cholesterol, mmol/L | 4.7±0.2 | 4.7±0.2 | 0.95 |
| LDL cholesterol, mmol/L | 2.8±0.2 | 2.8±0.2 | 0.90 |
| HDL cholesterol, mmol/L | 1.4±0.1 | 1.4±0.1 | 0.78 |
| Triglycerides, mmol/L | 1.0±0.1 | 1.1±0.2 | 0.62 |
Data are presented as mean±SE.
Discussion
The principal new findings reported in this study are that in patients with CAD: (1) fasudil inhibits Rho kinase activity; (2) fasudil augments flow-mediated endothelium-dependent vasodilation; and (3) the improvement in endothelial function induced by fasudil is related to the extent of Rho kinase inhibition. These results suggest that activation of Rho kinase contributes to the reduced NO bioavailability and endothelial dysfunction seen in humans with atherosclerosis.
Our laboratory and other investigators have previously shown that endothelial function is impaired in patients with atherosclerosis2,16,20 and its risk factors, including hypercholesterolemia. 21–23 However, the mechanisms that reduce NO bioavailability in atherosclerosis are only partially understood. Accumulating evidence has implicated the Rho/Rho kinase pathway as a powerful mediator of endothelial dysfunction in vitro. In human endothelial cells in vitro, activation of Rho kinase results in reduced eNOS expression and activity, which are reversed by the addition of the Rho kinase inhibitor hydroxyfasudil.6 Rho is also a target of 3-hydroxy-3-methyl glutaryl coenzyme A reductase inhibitors (statins).9 Inhibition of Rho by statins augments eNOS expression via stabilization of eNOS mRNA24 and stimulates eNOS activity (Ser1177 phosphorylation) via activation of the phosphatidylinositol 3-kinase/Akt pathway.8,25
Rho kinase activity is increased in animals with experimental atherosclerosis,26,27 hypertension,28 and diabetes.29 Furthermore, risk factors for atherosclerosis including oxidized LDL,30 hyperglycemia,18 and nicotine31 increase Rho/Rho kinase activity in vitro. However, it is not known whether the Rho/Rho kinase pathway is upregulated in humans with atherosclerosis and whether this leads to reduced NO bioavailability. Accordingly, we tested the hypothesis that overactivity of Rho kinase in humans with atherosclerosis leads to reduced NO production, manifest as improved endothelium-dependent vasodilation with Rho kinase inhibition by fasudil. Consistent with this hypothesis, we found that fasudil augments flow-mediated, endothelium-dependent dilation in patients with CAD but has no such effect in healthy individuals in whom Rho kinase is presumably not “overactive.” To our knowledge, this is also the first study to directly measure Rho kinase activity in humans, in peripheral blood leukocytes. The CAD subjects in this study tended to have higher Rho kinase activity along with impaired endothelial function at baseline compared with healthy controls. However, these values did not achieve statistical significance probably because of relatively small numbers of subjects and the older age of the control subjects32,33 and potentially because of the prevalence of background medications such as angiotensin-converting enzyme inhibitors that have previously been shown to improve endothelial function in patients with CAD.34 Despite this, fasudil significantly reduced Rho kinase activity in CAD subjects relative to healthy controls, and accordingly the CAD subjects experienced a significant improvement in endothelium-dependent vasodilation compared with healthy individuals. Moreover, the improvement in endothelium-dependent vasodilation was inversely correlated with the extent of Rho kinase inhibition. These results obtained in a Western cohort with CAD are consistent with findings of improved endothelial function with Rho kinase inhibition in Japanese cohorts with smoking, 35 and hypertension.36
In contrast to the findings in CAD subjects, endothelium-dependent vasodilation in healthy subjects tended to worsen with fasudil therapy compared with placebo. There are several potential explanations for these findings. In animal models, Rho expression is regulated by a negative feedback mechanism mediated by the actin cytoskeleton.37 Under physiological conditions, such as in healthy individuals in whom basal Rho kinase activity is not up regulated, inhibition of Rho kinase may lead to decreased negative feedback and thus to increased transcription of Rho. This in turn may lead to a subsequent compensatory increase in the downstream effects of Rho including suppression of endothelial NO production. Alternatively, Rho kinase inhibition in healthy individuals may lead to an excess of NO production, resulting in the formation of peroxynitrite, which in turn might lead to eNOS uncoupling and worsening endothelial function.38 Our results suggest that like NO, optimal levels of Rho/Rho kinase activity are probably required for the maintenance of vascular homeostasis. Based on our results the effects of Rho/Rho kinase inhibition in healthy subjects warrant further investigation.
Rho kinase enhances MLC phosphorylation and thereby cellular contraction by inhibiting MLC phosphatase through phosphorylation of its regulatory MBS.39 Hence, Rho kinase inhibition might be expected to result in vascular smooth muscle vasodilation. Despite this well-established effect, fasudil at the dose used in our study did not alter baseline brachial artery diameter, endothelium-independent vasodilation to nitroglycerin or systemic blood pressure. Studies in Japanese cohorts also found no effect of fasudil on endothelium-independent vasodilation among smokers or hypertensive subjects.35,36,40 Absence of an effect of fasudil on flow-mediated, endothelium-dependent dilation in healthy controls and lack of an effect on vascular smooth muscle reactivity in all of our subjects is consistent with the possibility that fasudil, in doses used, selectively and beneficially acted on the cells with an “overactive” Rho kinase—the dysfunctional endothelium in atherosclerosis.
In this study, the Rho kinase inhibitor fasudil, increased flow-mediated vasodilation without altering lipid levels in patients with elevated baseline total and LDL cholesterol. Thus, it appears that the beneficial effects of Rho kinase inhibition on vascular function in subjects with CAD occur independently of risk factor modification. These findings are in concert with basic observations suggesting that the non–lipid-lowering effects of statin therapy on endothelial function may be mediated through inhibition of the Rho/Rho kinase pathway.24 Whether statins inhibit Rho/Rho kinase activity in humans with atherosclerosis remains to be determined.
Potential Limitations
We used fasudil to inhibit Rho kinase.10,41 Within 20 minutes, fasudil is metabolized to hydroxyfasudil, a more potent inhibitor of Rho kinase.4,10,41 As the half-life of hydroxyfasudil is far longer than that fasudil, approximately 8 hours, fasudil functions largely as a prodrug for hydroxyfasudil. 4,10,41 Compared with inhibiting other kinases, hydroxyfasudil is relatively selective for Rho kinase with the Ki value of 0.17 µmol, 18 µmol for protein kinase C and 140 µmol for MLC kinase.10 Nonetheless, the possibility cannot be excluded that fasudil and hydroxyfasudil might inhibit other kinases42 and confound the results of our study. In that respect, it is important that the improvement in endothelium-dependent dilation observed in this study correlated significantly with Rho kinase inhibition. Other Rho kinase inhibitors are in development, and key findings with fasudil will need to be reinforced with structurally unrelated inhibitors as they become available.
This study is an important first step to demonstrate that Rho kinase may be overactive in human atherosclerosis and that this overactivity may contribute to the endothelial dysfunction seen in this disease state. Although this study did not directly evaluate the exact mechanism by which Rho kinase overactivity decreases NO bioavailability, it provides the rationale for future investigations.
Conclusions
Our study demonstrates that fasudil inhibits Rho kinase and improves flow-mediated, endothelium-dependent vasodilation in subjects with CAD compared with healthy individuals. Furthermore, the improvement in endothelial function is inversely proportional to the degree of Rho kinase inhibition. Fasudil, does not impact endothelium-independent vasodilation in either CAD or healthy subjects. Moreover, the effects of fasudil on endothelial function appear to be independent of lipid lowering. These results suggest that overactivity of the Rho/Rho kinase pathway may contribute to the endothelial dysfunction seen in atherosclerotic patients. In light of the prognostic implications of impaired endothelial NO bioavailability, Rho kinase inhibition may provide a novel strategy to improve endothelial function in patients with atherosclerosis.
Acknowledgments
We gratefully acknowledge Berlex Pharmaceuticals (Montville, NJ) for supplying the fasudil and placebo tablets.
Sources of Funding
A.N. is supported by an American Heart Association, Northeast Affiliate Scientist Development Award (no. 0335439T). J.K.L. is supported by a grant from the National Heart, Lung, and Blood Institute (HL52233). P.G. is supported by a grant from the National Heart, Lung, and Blood Institute (PO1 HL-48743). M.A.K. is the Simon C. Fireman Scholar of Medicine at Brigham and Women’s Hospital.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/static/html/reprints.html
Disclosures
A.N., P.G., and J.K.L. have research grant support from Pfizer Inc to examine whether Atorvastatin inhibits Rho/Rho kinase. A.N. also has a grant from the Doris Duke Cardiovascular Foundation to study the role of Rho/Rho kinase in atherosclerosis. J.K.L. is a consultant for and receives research support for a study on Rho kinase in ischemic stroke from Asahi-Kasei Inc.
References
- 1.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 2.Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 3.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 4.Shimokawa H, Hiramori K, Iinuma H, Hosoda S, Kishida H, Osada H, Katagiri T, Yamauchi K, Yui Y, Minamino T, Nakashima M, Kato K. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002;40:751–761. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 7.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laufs U, Liao JK. Targeting Rho in cardiovascular disease. Circ Res. 2000;87:526–528. doi: 10.1161/01.res.87.7.526. [DOI] [PubMed] [Google Scholar]
- 10.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 11.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, Bittar N, Weiss RJ, Morales-Ballejo H, Thadani U. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 12.Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. Active Rho kinase (ROK-alpha) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:6214–6222. doi: 10.1074/jbc.M110508200. [DOI] [PubMed] [Google Scholar]
- 13.Stroes ES, Koomans HA, de Bruin TW, Rabelink TJ. Vascular function in the forearm of hypercholesterolaemic patients off and on lipid-lowering medication. Lancet. 1995;346:467–471. doi: 10.1016/s0140-6736(95)91322-x. [DOI] [PubMed] [Google Scholar]
- 14.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 15.Uehata A, Lieberman EH, Gerhard MD, Anderson TJ, Ganz P, Polak JF, Creager MA, Yeung AC. Noninvasive assessment of endothelium-dependent flow-mediated dilation of the brachial artery. Vasc Med. 1997;2:87–92. doi: 10.1177/1358863X9700200203. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 17.Stadler RW, Karl WC, Lees RS. New methods for arterial diameter measurement from B-mode images. Ultrasound Med Biol. 1996;22:25–34. doi: 10.1016/0301-5629(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 18.Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261–3268. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 21.Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 22.Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95:2617–2622. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- 23.Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, Dzau VJ. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 25.Wolfrum S, Dendorfer A, Schutt M, Weidtmann B, Heep A, Tempel K, Klein HH, Dominiak P, Richardt G. Simvastatin acutely reduces myocardial reperfusion injury in vivo by activating the phosphatidylinositide 3-kinase/Akt pathway. J Cardiovasc Pharmacol. 2004;44:348–355. doi: 10.1097/01.fjc.0000137162.14735.30. [DOI] [PubMed] [Google Scholar]
- 26.Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, Egashira K, Kaibuchi K, Takeshita A. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20:2351–2358. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- 27.Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- 28.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 29.Sandu OA, Ragolia L, Begum N. Diabetes in the Goto-Kakizaki rat is accompanied by impaired insulin-mediated myosin-bound phosphatase activation and vascular smooth muscle cell relaxation. Diabetes. 2000;49:2178–2189. doi: 10.2337/diabetes.49.12.2178. [DOI] [PubMed] [Google Scholar]
- 30.Essler M, Retzer M, Bauer M, Heemskerk JW, Aepfelbacher M, Siess W. Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. J Biol Chem. 1999;274:30361–30364. doi: 10.1074/jbc.274.43.30361. [DOI] [PubMed] [Google Scholar]
- 31.Hiroki J, Shimokawa H, Mukai Y, Ichiki T, Takeshita A. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;326:154–159. doi: 10.1016/j.bbrc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corretti MC, Plotnick GD, Vogel RA. The effects of age and gender on brachial artery endothelium-dependent vasoactivity are stimulus-dependent. Clin Cardiol. 1995;18:471–476. doi: 10.1002/clc.4960180810. [DOI] [PubMed] [Google Scholar]
- 34.Hornig B, Landmesser U, Kohler C, Ahlersmann D, Spiekermann S, Christoph A, Tatge H, Drexler H. Comparative effect of ace inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation. 2001;103:799–805. doi: 10.1161/01.cir.103.6.799. [DOI] [PubMed] [Google Scholar]
- 35.Noma K, Higashi Y, Jitsuiki D, Hara K, Kimura M, Nakagawa K, Goto C, Oshima T, Yoshizumi M, Chayama K. Smoking activates rho-kinase in smooth muscle cells of forearm vasculature in humans. Hypertension. 2003;41:1102–1105. doi: 10.1161/01.HYP.0000067062.92836.9E. [DOI] [PubMed] [Google Scholar]
- 36.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 37.Laufs U, Endres M, Custodis F, Gertz K, Nickenig G, Liao JK, Bohm M. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102:3104–3110. doi: 10.1161/01.cir.102.25.3104. [DOI] [PubMed] [Google Scholar]
- 38.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 39.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kishi T, Hirooka Y, Masumoto A, Ito K, Kimura Y, Inokuchi K, Tagawa T, Shimokawa H, Takeshita A, Sunagawa K. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005;111:2741–2747. doi: 10.1161/CIRCULATIONAHA.104.510248. [DOI] [PubMed] [Google Scholar]
- 41.Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2002;39:319–327. doi: 10.1097/00005344-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]