Abstract
Recent studies have revealed a variety of left–right asymmetries among vertebrates and invertebrates. In many species, left- and right-lateralized individuals coexist, but in unequal numbers (‘population-level’ lateralization). It has been argued that brain lateralization increases individual efficiency (e.g. avoiding unnecessary duplication of neural circuitry and reducing interference between functions), thus counteracting the ecological disadvantages of lateral biases in behaviour (making individual behaviour more predictable to other organisms). However, individual efficiency does not require a definite proportion of left- and right-lateralized individuals. Thus, such arguments do not explain population-level lateralization. We have previously shown that, in the context of prey–predator interactions, population-level lateralization can arise as an evolutionarily stable strategy when individually asymmetrical organisms must coordinate their behaviour with that of other asymmetrical organisms. Here, we extend our model showing that populations consisting of left- and right-lateralized individuals in unequal numbers can be evolutionarily stable, based solely on strategic factors arising from the balance between antagonistic (competitive) and synergistic (cooperative) interactions.
Keywords: asymmetry, brain evolution, brain lateralization, evolutionarily stable strategy, laterality, lateralization of behaviour
1. Introduction
Left–right asymmetries in brain and behaviour, once believed to be uniquely human, have now been established in many vertebrates (Rogers & Andrew 2002; Vallortigara & Rogers 2005) and in invertebrates (Pascual et al. 2004; Letzkus et al. 2006, 2007; Rogers & Vallortigara 2008), suggesting that lateralization contributes significantly to biological fitness. Lateralized animals have been shown to outperform non-lateralized ones in many circumstances (Fabre-Thorpe et al. 1993; McGrew & Marchant 1999; Güntürkün et al. 2000; Rogers et al. 2004), and researchers agree that a lateralized brain may confer several advantages: sparing neural tissue by avoiding duplication of functions in the two hemispheres (Levy 1977); processing information in parallel (Rogers 2002; Rogers et al. 2004); and preventing the simultaneous initiation of incompatible responses by allowing one hemisphere to have control over actions (especially in animals with laterally placed sensory organs, Andrew 1991; Vallortigara 2000).
One intriguing aspect of lateralization, however, cannot be explained by arguing that lateralized brains are more efficient. The direction of lateralization, in fact, is usually aligned at the population level, with 60–90% of individuals showing the same direction of bias (depending on species and behaviour considered, see Previc (1991) and Vallortigara & Rogers (2005), for humans). Individual efficiency does not require an alignment of lateralization at the population level, and does not explain why a minority of individuals lateralized in the other direction almost always exists (e.g. hand use in humans). One could argue that population-level lateralization is a mere by-product of genetic expression, but it has been proved that selection for the strength of lateralization does not necessarily favour one direction of lateralization over the other (e.g. Collins 1985).
Two explanations (not mutually exclusive) have been proposed for the evolution of population-level asymmetries. Some genetic models of human handedness (McManus 1999; Annett 2002) posit one or more ‘directional’ (D) alleles that cause right-handedness, and one or more ‘chance’ (C) alleles that cause left- or right-handedness at random. A population with a majority of right-handers and a minority of left-handers can be maintained, in these models, if DC genotypes have higher fitness than CC and DD genotypes (heterozygotic advantage), for instance, if intermediate levels of brain asymmetry are superior to both extreme asymmetry and symmetry (Corballis 2006). Suggested disadvantages of CC and DD homozygotes include impairments in spatial, verbal and other cognitive abilities (Annett 2002; Barnett & Corballis 2002; McManus 2002).
The second suggested explanation is that the population structure of lateralization may reflect, not a balance between symmetry and asymmetry, but an evolutionarily stable strategy that can arise when individually asymmetrical organisms must coordinate their behaviour with that of other asymmetrical organisms (Vallortigara & Rogers 2005). This hypothesis recognizes that brain asymmetries manifest themselves in behaviour, and thus may have fitness consequences in interactions with other organisms. For instance, vigilance behaviour and escape responses elicited by predators often show lateral biases (Lippolis et al. 2002, 2005; Vallortigara & Rogers 2005). We have studied this idea in a game-theoretical model considering group-living prey subjected to predation (Ghirlanda & Vallortigara 2004; Vallortigara 2006). We assumed first that lateralization influences the direction of escape from predators. We then considered two contrasting selection pressures on lateralization. On one hand, individuals in large groups have a lesser risk of being targeted by predators (the so-called ‘dilution’ of predation risk, Foster & Treherne 1981). This favours individuals who tend to escape in the same direction as the majority, thus promoting the same direction of lateralization across the whole population. On the other hand, given that predators may learn to anticipate prey escape strategies, individuals who escape in a different direction from the majority may surprise predators and survive predation attempts more often. This tends to favour populations in which left- and right-lateralized individuals are equally common.
We showed that, in this model, population-level lateralization can emerge provided that none of the two selection pressures is much stronger than the other. According to this view, the evolution of brain lateralization would have occurred in two steps: first, individuals became lateralized because of advantages from increased brain efficiency (e.g. Rogers et al. 2004); and second, individually lateralized organisms aligned the direction of their asymmetries when they started to interact to each other in ways that made their asymmetry relevant to each other's behaviour (e.g. in fishes shoaling, Vallortigara & Bisazza 2002). Here, we investigate whether a similar scenario could hold when selection pressures on lateralization arise purely from intraspecific interactions of competition and cooperation, rather than interspecific prey–predator interactions.
2. Model
We study the influence on lateralization of purely intraspecific interactions using a similar modelling strategy as Ghirlanda & Vallortigara (2004). We assume that individuals engage in both antagonistic (competitive) and synergistic (cooperative) interactions. An individual's pay-off depends on its success in interactions, which is a function of how common its lateralization is in the population. Synergistic activities tend to favour individuals with the same lateralization (they can, for instance, have an easier time coordinating physical activities, use efficiently the same tools, etc.). Antagonistic activities, on the other hand, tend to favour individuals different from the majority. The reason is similar to the one mentioned above for predation: minority-type individuals will be able to surprise opponents, adopting behaviours to which opponents are less accustomed. For example, it has been argued that human left-handers may hold an advantage in fighting, or in more recent times in certain sporting activities, but only so long as they remain in the minority (Raymond et al. 1996). Thus, if only synergistic interactions were present, the population would be composed entirely of individuals with the same lateralization. If only antagonistic interactions were present, the population would be composed of left- and right-lateralized individuals in proportion of one-half. We want to study whether, when both kinds of interactions exist, it is possible to maintain a population in which left- and right-lateralized individuals coexist in a proportion different from one-half, and how such a situation is influenced by model parameters.
Let x be the proportion of left-lateralized individuals in the population. We write the fitness f(x) of such an individual as the sum of a term accounting for antagonistic interactions (a) and one-term accounting for synergistic interactions (s)
| (2.1) |
where the parameter c weights the relative importance of the two kinds of interactions. We use the following forms for a(x) and s(x)
| (2.2) |
| (2.3) |
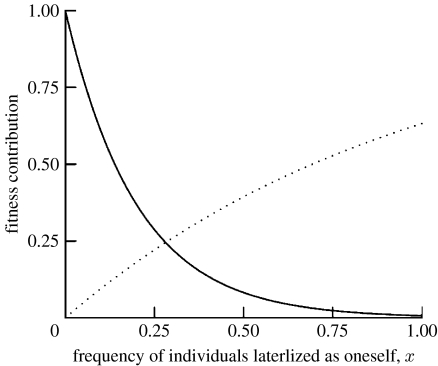
where ka and ks are positive parameters. In keeping with our assumptions, a(x) decreases with the proportion of individuals with the same lateralization, while s(x) increases (figure 1). We have chosen an exponential shape because it is often a good approximation to actual learning curves, that is, how performance on a given task increases as a function of increasing experience with that task (Mackintosh 1974; Pearce 1997). In figure 1, parameter values are chosen so that ka>ks (in particular ka=5, ks=1). This means that a(x) decreases more rapidly than s(x) increases; i.e. the fitness contribution of antagonistic interactions varies more quickly with strategy frequency than the fitness contribution of synergistic interactions.
Figure 1.
Graph of the functions a(x) and s(x) (equations (2.2) and (2.3)), which enter fitness (equation (2.1)). Parameter values: ka=5, ks=1. Since ka>ks=1, the fitness contribution of antagonistic interactions a(x) decays more quickly than the fitness contribution of synergistic interactions s(x) increases. Solid curve, antagonistic interactions, a(x); dotted curve, synergistic interactions, s(x).
The fraction of right-lateralized individuals in the population is 1−x. Since we are not assuming any intrinsic advantage of being left- or right- lateralized, the fitness of these individuals is
| (2.4) |
Evolutionary equilibria, x*, are derived by equating the fitness of left- and right-lateralized individuals
| (2.5) |
The evolutionary stability of an equilibrium x* is assessed by asking what happens if the proportion of left-handers deviates slightly from x*. If the equilibrium is stable, natural selection tends to restore the equilibrium proportion x*. Thus, an increase in the proportion of left-lateralized individuals from x* to x*+E should result in a situation in which their fitness falls below that of right-lateralized individuals. Formally
| (2.6) |
Likewise, a decrease in left-lateralized individuals should result in these individuals having a higher fitness
| (2.7) |
We show in the appendix that these conditions are equivalent to the following condition on the derivative f′(x) of f(x)
| (2.8) |
Combining this condition with the equilibrium condition (2.6) we can look for evolutionary equilibria and assess their stability. We also need to check whether populations composed entirely of left- or right-lateralized individuals are stable, corresponding to the conditions, respectively
| (2.9) |
| (2.10) |
We have performed this analysis by a mixture of analytical and numerical methods, as detailed in appendix A.
3. Results
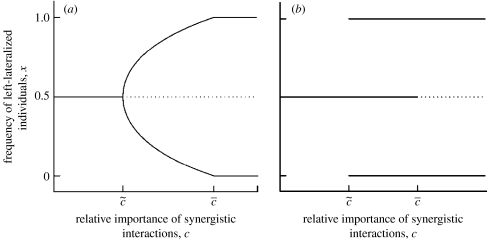
We study the model varying the relative importance, c, of synergistic and antagonistic interactions. The nature of the equilibria depends on the relationship between the parameters ka and ks. If ka>ks the situation is similar to that found by Ghirlanda & Vallortigara (2004) in interspecific prey–predator interactions (figure 2). There exists a value of c, , below which x*=1/2 is the only stable solution. In such a situation, synergistic interactions are too weak to cause a departure from the strategic equilibrium favoured by antagonistic interactions. Similarly, there exists a value of c, , above which x*=1/2 is unstable while x*=0 and x*=1 are both stable, corresponding to populations with only left- or right-lateralized individuals. Here synergistic interactions dominate, determining completely the population structure of lateralization. Finally, there is a range of c-values in between and where two values of x different from one-half are stable equilibria, corresponding to populations in which left- and right-lateralized individuals coexist but are not equally common. This is the situation we observe in humans and many other vertebrates. The range of c-values in which this situation occurs expands as ka increases with respect to ks.
Figure 2.
Equilibrium frequency of left-lateralized individuals x as a function of the relative importance of antagonistic and synergist interactions (parameter c in equation (2.1)). Solid lines represent stable equilibria; dotted lines unstable equilibria. (a) The case in which frequency dependence is stronger for antagonistic than synergistic interactions (ka>ks in equations (2.3) and (2.4)). For c<, x*=1/2 is the only stable solution; for c>, x*=1/2 is unstable while x*=0 and x*=1 (populations with only right- or left-lateralized individuals) are both stable. Between and , two values of x different from one-half are stable equilibria, corresponding to populations in which left- and right-lateralized individuals coexist but are not equally common. (b) The converse case (ks>ka). For c<, x*=1/2 is stable, for c>, x*=0 and x*=1 are stable, for intermediate c values x*=1/2, x*=0 and x*=1 are all stable, meaning that coexistence between left- and right-handers is not possible.
If ka≤ks, we still have that for small c, x*=1/2 is stable and that for large c, x*=0 and x*=1 are stable, but the situation for intermediate c-values is different. Coexistence between left- and right-handers is not possible; rather x*=1/2, x*=0 and x*=1 are all stable. In this case, synergistic interactions are strong enough to stabilize a monomorphic population, but not strong enough to destabilize a population with 50 per cent right- and left-handers. In summary, existence of lateralized populations requires in our model that ka>ks, meaning that the graph of a(x) must be steeper than the graph of s(x) (figure 1; see below for interpretations).
4. Discussion
Our model demonstrates that populations consisting of left- and right-type individuals in unequal numbers can be evolutionary stable based solely on strategic factors arising from intraspecific interactions. The model makes several testable predictions. An important prediction is that the frequency of the minority type depends on the balance between the fitness contributions of antagonistic versus synergistic interactions. When antagonistic interactions are more important for individuals' fitness, we expect the minority type to be more common. Likewise, when synergistic interactions are more important, we expect the minority type to be less common. To evaluate this prediction, we need data from populations that differ in the balance between antagonistic and synergistic interactions but are otherwise as similar as possible. One possibility is to compare different human groups. Faurie & Raymond (2005) provide data in agreement with our model, showing that the frequency of left-handers in eight traditional societies is strongly correlated with the rate of homicides, ranging approximately between 5 and 25 per cent as the adult homicide rate ranges between 0.01 to more than 1 per 1000 individuals per year. Another source of evidence may be comparative studies of related species. For instance, it is currently debated whether lateralization is more pronounced in humans than in non-human primates. This seems unlikely for cerebral lateralization in general (Vallortigara et al. 1999), but it could hold for certain forms of behavioural lateralization, such as handedness (Rogers 2007; and see Andrew et al. (2000) for the general issue of lateralization of non-bilateral effectors). Wild chimpanzees show population-level handedness for tool use (Lonsdorf & Hopkins 2005), but apparently not so strongly as humans do (Annett 2006). The fact that synergistic interactions are more important in humans (e.g. Jensen et al. 2007) may explain why we are more strongly lateralized at the population level.
Another prediction concerns the fact that the model allows for population-level lateralization only when the condition ka>ks is met (see §3 and appendix A). That is, when the frequency of majority- and minority-type individuals varies, the fitness contribution of antagonistic interactions should vary more quickly than the fitness contribution of synergistic interactions. From the point of view of selective pressures, this prediction means that minority-type individuals should lose their advantage in antagonistic interactions very quickly as they become more common, more quickly than they gain an advantage in synergistic interactions. From a behavioural point of view, this corresponds to the fact that individuals should learn quickly how to contrast minority-type individuals in antagonistic interactions, while they should learn more slowly how to cooperate with them in synergistic interactions. This prediction can be put to empirical test (perhaps in experimental populations in the laboratory), but presently, we are not aware of any direct evidence in favour or against it.
In conclusion, we have extended previous results on interspecific interactions to intraspecific interactions, reinforcing the view that strategic factors may have been a powerful force in the evolution of lateralization. We have considered a purely strategic model for simplicity, but future research should also consider how strategic factors interact with other potential determinants of lateralization, such as neurophysiological constraints, the genetic mechanisms of lateralization, and especially in humans, traditions and culture (Laland et al. 1995).
Appendix A.
A.1 Stability condition (equation (2.8))
A first order Taylor expansion of equation (2.6) yields
| (A1) |
Dropping terms of higher order in E and using the equilibrium condition f(x*)=f(1−x*) (equation (2.5)), we obtain equation (2.8). A similar argument shows that equation (2.7) is also equivalent to equation (2.8). Given that f(x)=a(x)+cs(x), equation (2.8) can be written as
| (A2) |
A.2 Stability of the equilibrium x*=1/2; (non-lateralized population)
The value x*=1/2 is always a solution of equation (2.5), hence it is always an equilibrium. Equation (A 2), evaluated for x*=1/2, implies that this equilibrium is stable if c is smaller than
| (A3) |
A.3 Stability of x*=0 and x*=1 (completely lateralized populations)
Now we consider the situation where the population is composed entirely of left- or right-lateralized individuals. These situations are stable if equations (2.9) and (2.10) hold, respectively. Using expressions (2.1) and (2.2) we see that both expressions hold if c is larger than
| (A4) |
A.4 Existence of partially lateralized populations
We have shown that a non-lateralized population (x*=1/2) is stable if c< and fully lateralized populations are stable only if c>. Thus, a lateralized population with 0<x*<1/2 or 1/2<x*<1 can be stable only if >. Using expressions (A 3) and (A 4), we see that the latter is equivalent to
| (A5) |
or
| (A6) |
which in turn is equivalent to
| (A7) |
because sinh(k)/k is monotonically increasing for k>0. When condition (A 7) holds, the interval of values between and can be explored numerically to calculate the equilibrium value x*. We performed this calculation, for instance, to build figure 2a. We used two methods to guard against numerical instability. The first method used the fsolve function of the Octave software (v. 2.9.9), designed to solve nonlinear equations. The second method looks for a solution by iterating the map
| (A8) |
obtained from equation (A 2) using equations (2.1) and (2.2). The fixed point theorem (Granas & Dugundji 2003) guarantees that a solution for this recursion exists. The two methods typically produced the same answer, but for some parameter values one or the other method would not converge to a solution. We then used the value obtained by the other method.
Footnotes
One contribution of 14 to a Theme Issue ‘Mechanisms and functions of brain and behavioural asymmetries’.
References
- Andrew, R. J. 1991 The nature of behavioral lateralization in the chick. In Neural and behavioral plasticity. The use of the chick as a model (ed. R. J. Andrew), pp. 536–554. Oxford, UK: Oxford University Press.
- Andrew R.J., Tommasi L., Ford N. Motor control by vision and the evolution of cerebral lateralisation. Brain Lang. 2000;73:220–235. doi: 10.1006/brln.2000.2304. doi:10.1006/brln.2000.2304 [DOI] [PubMed] [Google Scholar]
- Annett M. Psychology Press; Hove, UK: 2002. Handedness and brain asymmetry: the right shift theory. [Google Scholar]
- Annett M. The distribution of handedness in chimpanzees: estimating right shift in Hopkins' sample. Laterality. 2006;11:101–109. doi: 10.1080/13576500500376500. doi:10.1080/13576500500376500 [DOI] [PubMed] [Google Scholar]
- Barnett K.J., Corballis M.C. Ambidexterity and magical ideation. Laterality. 2002;7:75–84. doi: 10.1080/13576500143000131. doi:10.1080/13576500143000131 [DOI] [PubMed] [Google Scholar]
- Collins R.L. On the inheritance of direction and degree of asymmetry. In: Glick S.D., editor. Cerebral lateralization in nonhuman species. Academic Press; New York, NY: 1985. pp. 41–71. [Google Scholar]
- Corballis M.C. Cerebral asymmetry: a question of balance. Cortex. 2006;42:117–118. doi: 10.1016/s0010-9452(08)70335-6. doi:10.1016/S0010-9452(08)70335-6 [DOI] [PubMed] [Google Scholar]
- Fabre-Thorpe M., Fagot J., Lorincz E., Levesque F., Vauclair J. Laterality in cats: paw preference and performance in a visuomotor activity. Cortex. 1993;29:15–24. doi: 10.1016/s0010-9452(13)80208-0. [DOI] [PubMed] [Google Scholar]
- Faurie C., Raymond M. Handedness, homicide and negative frequency-dependent selection. Proc. R. Soc. B. 2005;272:25–28. doi: 10.1098/rspb.2004.2926. doi:10.1098/rspb.2004.2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster W.A., Treherne J.E. Evidence for the dilution effect in the selfish herd from fish predation of a marine insect. Nature. 1981;293:508–510. doi:10.1038/293466a0 [Google Scholar]
- Ghirlanda S., Vallortigara G. The evolution of brain lateralization: a game-theoretical analysis of population structure. Proc. R. Soc. B. 2004;271:853–857. doi: 10.1098/rspb.2003.2669. doi:10.1098/rspb.2003.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granas A., Dugundji J. Springer; New York, NY: 2003. Fixed point theory. [Google Scholar]
- Güntürkün O., Diekamp B., Manns M., Nottelmann F., Prior H., Schwarz A., Skiba M. Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr. Biol. 2000;10:1079–1081. doi: 10.1016/s0960-9822(00)00671-0. doi:10.1016/S0960-9822(00)00671-0 [DOI] [PubMed] [Google Scholar]
- Jensen K., Call J., Tomasello M. Chimpanzee are rational maximizers in an ultimatum game. Science. 2007;318:107–109. doi: 10.1126/science.1145850. doi:10.1126/science.1145850 [DOI] [PubMed] [Google Scholar]
- Laland K.N., Kumm J., Van Horn D., Feldman M. A gene-culture model of human handedness. Behav. Genet. 1995;25:433–445. doi: 10.1007/BF02253372. doi:10.1007/BF02253372 [DOI] [PubMed] [Google Scholar]
- Letzkus P., Ribi W.A., Wood J.T., Zhu H., Zhang S.W., Srinivasan M.V. Lateralization of olfactory learning in the honey bee Apis mellifera. Curr. Biol. 2006;16:1471–1476. doi: 10.1016/j.cub.2006.05.060. doi:10.1016/j.cub.2006.05.060 [DOI] [PubMed] [Google Scholar]
- Letzkus P., Boeddeker N., Wood J.T., Zhang S.-W., Srinivasan M.V. Lateralization of visual learning in the honeybee. Biol. Lett. 2007;4:16–18. doi: 10.1098/rsbl.2007.0466. doi:10.1098/rsbl.2007.0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. The mammalian brain and the adaptive advantage of cerebral asymmetry. Ann. NY Acad. Sci. 1977;299:264–272. doi: 10.1111/j.1749-6632.1977.tb41913.x. doi:10.1111/j.1749-6632.1977.tb41913.x [DOI] [PubMed] [Google Scholar]
- Lippolis G., Bisazza A., Rogers L.J., Vallortigara G. Lateralization of predator avoidance responses in three species of toads. Laterality. 2002;7:163–183. doi: 10.1080/13576500143000221. [DOI] [PubMed] [Google Scholar]
- Lippolis G., Westerman W., McAllan B.M., Rogers L.J. Lateralization of escape responses in the striped-face dunnart, Sminthopsis macroura (Dasyuridae: Marsupialia) Laterality. 2005;10:457–470. doi: 10.1080/13576500442000210. [DOI] [PubMed] [Google Scholar]
- Lonsdorf E.V., Hopkins W.D. Wild chimpanzees show population-level handedness for tool use. Proc. Natl Acad. Sci. USA. 2005;102:12 634–12 638. doi: 10.1073/pnas.0505806102. doi:10.1073/pnas.0505806102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh N. Academic Press; London, UK: 1974. The psychology of animal learning. [Google Scholar]
- McGrew W.C., Marchant L.F. Laterality of hand use pays off in foraging success for wild chimpanzees. Primates. 1999;40:509–513. doi:10.1007/BF02557586 [Google Scholar]
- McManus I.C. Handedness, cerebral lateralization, and the evolution of handedness. In: Corballis M.C., Lea S.E.G., editors. The descent of mind. Oxford University Press; Oxford, UK: 1999. pp. 194–217. [Google Scholar]
- McManus I.C. Widenfeld and Nicolson; London, UK: 2002. Right hand, left hand. [Google Scholar]
- Pascual A., Huang K.-L., Nevue J., Préat T. Brain asymmetry and long-term memory. Nature. 2004;427:605–606. doi: 10.1038/427605a. doi:10.1038/427605a [DOI] [PubMed] [Google Scholar]
- Pearce J.M. 2nd edn. Psychology Press; Hove, UK: 1997. Animal learning and cognition. [Google Scholar]
- Previc F. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol. Rev. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. doi:10.1037/0033-295X.98.3.299 [DOI] [PubMed] [Google Scholar]
- Raymond M., Pontier D., Dufour A., Moller A.P. Frequency-dependent maintenance of left handedness in humans. Proc. R. Soc. B. 1996;263:1627–1633. doi: 10.1098/rspb.1996.0238. doi:10.1098/rspb.1996.0238 [DOI] [PubMed] [Google Scholar]
- Rogers L.J. Advantages and disadvantages of lateralization. In: Rogers L.J., Andrew R.J., editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge, UK: 2002. pp. 126–153. [Google Scholar]
- Rogers L.J. Lateralization in its many forms, and its evolution and development. In: Hopkins W.D., editor. The evolution of hemispheric specialization in primates, special topics in primatology. vol. 5. Elsevier; Amsterdam, UK: 2007. pp. 23–56. [Google Scholar]
- Rogers L.J., Andrew R.J. Cambridge University Press; Cambridge, UK: 2002. Comparative vertebrate lateralization. [Google Scholar]
- Rogers L.J., Vallortigara G. From antenna to antenna: lateral shift of olfactory memory in honeybees. PLoS One. 2008;3:e2340. doi: 10.1371/journal.pone.0002340. doi:10.1371/journal.pone.0002340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.J., Zucca P., Vallortigara G. Advantage of having a lateralized brain. Proc. R. Soc. B. 2004;271:S420–S422. doi: 10.1098/rsbl.2004.0200. doi:10.1098/rsbl.2004.0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallortigara G. Comparative neuropsychology of the dual brain: a stroll through left and right animals' perceptual worlds. Brain Lang. 2000;73:189–219. doi: 10.1006/brln.2000.2303. doi:10.1006/brln.2000.2303 [DOI] [PubMed] [Google Scholar]
- Vallortigara G. The evolutionary psychology of left and right: costs and benefits of lateralization. Dev. Psychobiol. 2006;48:418–427. doi: 10.1002/dev.20166. doi:10.1002/dev.20166 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Bisazza A. How ancient is brain lateralization? In: Andrew R.J., Rogers L.J., editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge, UK: 2002. pp. 9–69. [Google Scholar]
- Vallortigara G., Rogers L.J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. doi:10.1017/S0140525X05000105 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L.J., Bisazza A. Possible evolutionary origins of cognitive brain lateralization. Brain Res. Rev. 1999;30:164–175. doi: 10.1016/s0165-0173(99)00012-0. doi:10.1016/S0165-0173(99)00012-0 [DOI] [PubMed] [Google Scholar]