Abstract
In this paper, we report on the ongoing work in our laboratories on the effect of lateralization produced by light exposure in the egg on social cognition in the domestic chick (Gallus gallus). The domestic chick possesses a lateralized visual system. This has effects on the chick's perception towards and interaction with its environment. This includes its ability to live successfully within a social group. We show that there is a tendency for right brain hemisphere dominance when performing social cognitive actions. As such, chicks show a left hemispatial bias for approaching a signalled target object, tend to perceive gaze and faces of human-like masks more effectively when using their left eye, are able to inhibit a pecking response more effectively when viewing a neighbour tasting a bitter substance with their left eye, and are better able to perform a transitive inference task when exposed to light in the egg and when forced to use their left eye only compared to dark-hatched or right eye chicks. Some of these effects were sex specific, with male chicks tending to show an increased effect of lateralization on their behaviours. These data are discussed in terms of overall social cognition in group living.
Keywords: chick, Gallus gallus, lateralization, social cognition, brain hemisphere
1. Introduction
It is now well known that brain asymmetries occur throughout the animal kingdom (Rogers & Andrew 2002; Vallortigara & Rogers 2005). What was once considered a uniquely human characteristic since being described by Broca (1865), brain lateralization and its behavioural effects has been found and studied in a wide range of species, including non-human primates (e.g. Fernandez-Carriba et al. 2002), birds (e.g. Rogers 1997), amphibians (Vallortigara et al. 1998), fishes (e.g. Sovrano et al. 1999) and invertebrates (Ades & Ramires 2002; Letzkus et al. 2007; Rogers & Vallortigara 2008).
This paper concerns itself with the research carried out in our laboratories on the domestic chick (Gallus gallus domesticus), specifically in relation to the effect of brain lateralization on its social cognition. By social cognition, we would suggest this to mean the way the chick interacts and perceives others, including non-conspecifics such as predators, in such a manner as to perform biologically relevant responses important for its survival within a group context.
The development and behavioural effects of brain lateralization have been extensively studied using the chick as a model (see Rogers 1995). As such, the domestic chick, as with other avian species studied, provides a highly malleable experimental model to study lateralization owing to the fact that the embryo develops outside the female's body, independently in an egg.
Lateralization of the chick's brain is triggered by the exposure of the embryo in the egg to light (Rogers & Sink 1988). During development, the embryo turns so that the right eye faces outward, towards the translucent egg shell and to any available light. At the same time, the left eye is turned towards the body mass and receives little or no light. While still in the egg, a visual pathway in the chick, known as the thalamofugal pathway, undergoes differentiation. During a critical period (from embryonic day 17 to 21; see Rogers 2008), exposure to light produces an asymmetrical stimulation of the two eyes such that there is an increase in forebrain projections from the left side of the thalamus (fed by the light-stimulated right eye) compared with the right side (Rogers & Deng 1999; Koshiba et al. 2003). It is thought that as little as 2 hours exposure to light prior to hatching is sufficient to induce these brain asymmetries (Rogers 1997). However, if the chick does not receive light during incubation, this lateralization is largely prevented (see Rogers & Bolden 1991). Nevertheless, there are some forms of lateralization in chicks that do not depend on light exposure of the embryo, including social recognition (Deng & Rogers 2002b), response to olfactory versus visual cues (Rogers et al. 1998) and components of object or spatial-specific cues (Chiandetti et al. 2005: also see Vallortigara & Rogers 2005). Factors such as the position of the nest, the threat of predation and the social status of the female may all help to determine the amount of light the chick embryo is exposed to. Also, the chick itself appears to regulate its light exposure from within the egg by manipulating the hen's behaviour: chicks call to the hen during the critical period before hatching to stimulate the hen to turn the egg and expose the embryo to light (Tuculescu & Griswold 1983).
The physical asymmetries of the thalamofugal pathway inevitably turn out to have behavioural consequences: experimentally induced changes in anatomical asymmetry are accompanied by changes in behavioural asymmetry. For example, research has shown that the left eye system (i.e. the left eye and its contralateral connections to the right hemisphere) plays a preferential role in spatial representations, specifically in the learning and memory for global, distally located spatial information (Rashid & Andrew 1989; Vallortigara 2000; Prior et al. 2002; Regolin et al. 2004). In addition, the right hemisphere tends to focus on broad attentional cues and also controls fear and escape responses and response to novelty (see Andrew 1991; Vallortigara & Andrew 1994). The right eye system (left hemisphere), on the other hand, is important for learning about the features of the goal but also for the representation of local, landmark cues to locate goals in space (Tommasi & Vallortigara 2001). In this way, the left hemisphere is able to discern cues that separate relevant stimuli from distracting stimuli (e.g. food from pebbles). Thus, it has been suggested that the left hemisphere works on the level of defining an item at a categorical level while the right hemisphere determines the more specific values of an object (see Rogers & Andrew 2002; Vauclair et al. 2006).
Anatomical lateralization and its behavioural correlates remain largely confined to each hemisphere in chicks because the avian brain does not have a corpus callosum and displays a virtual complete decussation of optic fibres at the optic chiasm (Csillag & Montagnese 2005). Methodologically speaking, this means that the visual input to the chick's brain can be restricted to one hemisphere without the need for invasive surgical procedures. Specifically, a simple patch over the eye can be used to discern hemisphere specializations (Rogers 1997; Gülbetekin et al. 2007). In addition, birds can use their eyes independently allowing, for example, the scanning for predators with one eye while categorizing food and non-food items with the other (Rogers 2000). This spontaneous eye use can also allow us to discern hemispheric specializations. Indeed, it appears that behavioural and analytical processes are generally carried out by the eye connected to the hemisphere most adapted for carrying out these different activities. These processes also include those required for social interactions. The domestic fowl derives from the red jungle fowl (Gallus gallus spadiceus), which is a highly social species living in flocks of between 4 and 30 adults in addition to the young birds (Mench & Keeling 2001). The chick, on hatching, will spend its lifetime interacting, initially, with its siblings and mother, then later on with other conspecifics within the group. The ability to interact successfully with its fellows requires an individual to possess skills in social cognition (Zuberbuhler & Byrne 2006). Social cognition implies that an individual not only understands other individuals within a group but also controls its own actions and controls processes that involve the interaction between itself and other members of the social group. In this way, the chick will be able to find food and shelter and avoid predators not only by itself but also by watching and learning from others. The ability to learn from the actions of others allows the individual to subsequently interact with its environment with greatly reduced fitness costs. Living in groups is also a predisposition for social facilitation and social learning behaviours. Indeed, fowl engage in social learning during foraging, dust bathing and preening (Lundberg 2002; Nicol 2004). When young, social learning is of an increased importance because chicks are apparently unable to recognize food types and have to learn to avoid items that are not worthwhile eating, to the extent that Hogan (1984) reported chicks will die owing to their preference for ingesting gravel to food if they are not shown otherwise.
There are a variety of mechanisms that an individual can employ in order to learn from the behaviour of others (see Nicol 1995): these include enhancement (local or social), in which another animal (known as the demonstrator) may increase a motivational component to enhance the individual's attention to an action or place coupled with a reward. Also, copying and emulating the demonstrator's actions in order to produce similar results (in terms of access to food; see Zentall 2003), known as imitation, may be employed. In these ways, an animal can learn to exploit new food types, determine food quality, the dominance status of others in its group, etc (see Heyes & Galef 1996). All these are important for the chick because it is a precocial animal which, upon hatching from its egg, must quickly imprint on its mother and conspecifics, while initiating feeding. Being a social species, it is important for the chicks to recognize their conspecifics and to be able to interpret the social interactions between them (from Queiroz & Cromberg 2006; also, Regolin et al. 1994). This becomes more apparent when agonistic activity begins and social hierarchies are formed.
All these types of social behaviour may be impinged upon by the anatomical, and subsequent behavioural, asymmetries in brain development, most notably of the visual system, caused by the light exposure in the egg. These asymmetries appear to be conserved throughout avian species. For example, the ability to recognize familiar from unfamiliar conspecifcs appears to be a right hemisphere process in the precocial domestic chick (Gallus gallus; Vallortigara 1992a; Andrew et al. 2004), as does predator detection and avoidance in the altricial Australian magpie (Gymnorhina tibicen; Koboroff et al. 2008) and later in life, courtship and copulatory behaviours (in this case in a precocial wader, the black-winged stilt Himantopus himantopus; Ventolini et al. 2005). The left hemisphere appears to be more involved in approach towards predators (Koboroff et al. 2008) and in prey detection (Ventolini et al. 2005).
In the following, we will examine work from our laboratories showing that the two hemispheres are differentially involved in social cognition tasks in the domestic chick G. gallus. This is apparent in initial pecking responses, both with regard to a model hen demonstrating what to peck and with regard to learning what not to peck by observing another conspecific, in the perception of human gaze and in face recognition and, when older, using a pecking response to demonstrate that a dominance hierarchy has been learnt.
2. Lateralization of response to ‘tidbitting’
In humans, it is well documented that there is an asymmetry of spatial attention (e.g. Bottini & Torraldo 2003). In the human brain, a form of pseudoneglect exists, with a left-hemispace bias in perception of length, size and numerosity being present (Orr & Nicholls 2005). A leftward attentional bias has also been reported in birds (Diekamp et al. 2005): a left-sided visuospatial bias was seen when birds were given a free choice to orient towards and peck at grains spread evenly over an area in front of them. In addition, Rugani et al. (2007) found that chicks, when identifying the position of a hole in a series, would start from the left end of the series, and not the right, in order to refer to the correct hole. This confirms Regolin's (2006) work describing a left bias in a line-bisection task. This propensity for a leftward bias of asymmetry is thought to be associated with the right brain hemisphere's superior ability to perform in spatial tasks compared to the left.
We investigated this visuospatial bias further based on the chick's response to an auditory and visual tidbitting signal, which the hen performs on encountering a suitable food item. This consists of the female hen emitting pulsatile food calls and at the same time performing a visual display, involving a repeated, rhythmic motion of the head and neck often picking up and dropping the food item chosen (Stokes & Williams 1972; Smith & Evans 2008). This behaviour allows a form of social learning in which the chick learns which foods are good to eat as signalled by its mother (Allen & Clarke 2005), since it attracts chicks to the area where the hen is (Moffatt & Hogan 1992). Chicks subsequently learn to emulate the hen's preferences in food choice; in this way, a form of social transmission of food choice may evolve (Gajdon 2001).
Previously, Suboski & Bartashunas (1984) successfully investigated the social transmission of pecking preferences in chicks using a specially designed model arm. An arrow operated to produce vertical ‘pecking’ movements was found to both elicit and to direct pecking in naïve newly hatched chicks. We decided to carry out a similar experiment to see whether there would be an attentional bias, presumably to the left hemispace.
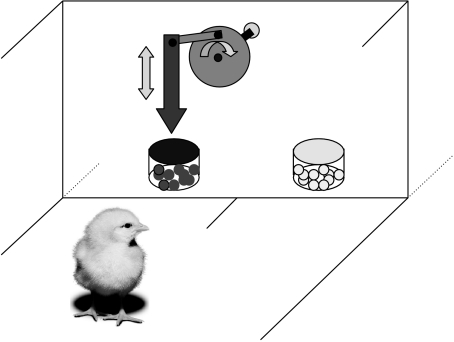
In order to investigate this phenomenon we used day-old chicks of both sexes, which had no previous experience of, or exposure to, any food items. Chicks were placed in an arena consisting of a wooden box with one side made of a see-through glass ‘window’ through which a mechanical arm and bead set-up could be seen (similar to Suboski & Bartashunas 1984): two translucent glass pots containing coloured plastic beads (3 mm in diameter; one with blue beads and one with yellow beads), one on the left and one on the right side of the apparatus (figure 1). The arrow was made to oscillate vertically (visual signal) and produced a tapping sound on the downstroke when encountering the stimulus (auditory signal): one of two pots. The arrow therefore emulated the female hen's tidbitting display, providing both visual and auditory components.
Figure 1.
A diagrammatic representation of the chick in the tidbitting apparatus. The motor arm was made to oscillate above one of two pots of coloured beads; in this case, the pot containing the blue (darker) beads, to signal it as the target.
A procedure involving an initial attraction of the chick to an object followed by a habituation was invoked. For this, chicks were attracted to one or the other of the two sets of beads (defined as the target) by the motion of the tidbitting arm pecking at the pot of beads in bursts of 10 s on and 10 s off. When the chick had approached the area near to the motor arm, the action became a continuous motion (enhancing subsequent habituation). Inevitably, the chick would lose interest in this motion action, because it was unable to interact directly with either the motor arm or the object of the arm's intent. According to the criterion employed, habituation was considered to have occurred when the chick looked away from the moving motor arm for at least 10 s.
Dependent measures considered during the test were: time required to attract chicks' attention to the target object and time that elapsed before losing chicks' attention (habituation time), signifying the end of the experiment.
A significant interaction of the sex of the chicks and the colour of the beads in the pot was found, which suggested that the two sexes were reacting differently according to the colour of target. Male chicks were found to have their attention drawn to the target differently according to an interaction between the target colour and the target's position: they were significantly faster to attend to the stimulus when the blue target was presented on the left side compared with the right. This was specific to the blue target because no such positional effect was present when the target was yellow for attention. Females showed no differences in either habituation or attention responses.
It appears that the chicks prefer (are quicker, at least) to go to their left when the target is blue, than to their right. This is a colour- (blue only) and sex-specific (male only) effect.
A leftward attentional bias has been reported in birds (Diekamp et al. 2005). Both pigeons (Columba livia) and chicks display a left-sided visuospatial bias. It is argued that neural circuits in the right hemisphere can attend to and represent both left and right sides, while the left hemisphere attends to the contralateral side only. Our results again demonstrate a left-sided bias. However, most interestingly, this bias was both object and sex specific. Only when the stimulus signalled was blue did the male chicks (but not females) show any differentiation in terms of directional bias in approaching the stimuli.
Colour preference has been observed in chicks (Mastrota & Mench 1995; Taylor et al. 1969 from Ham & Osorio 2007). Thus, predispositions are present in these young birds. Whether sex differences were present in previous colour choice experiments was not noted, however. Sex differences do exist, though, in food detection in a pebble-floor task (Rogers 1997). Females also appear to be more attentive to a primary target (Tommasi & Vallortigara 2004), although this appears not to be the case in our work. The sex differences found in this and other experiments will be discussed further in §7.
A strong selective feeding is shown when (female) chicks use both eyes, but there is less selectivity when they are in the monocular mode (Prior & Wilzeck 2008). There is some lateralization though, with the left hemisphere (right eye) chicks being more selective than the left eye chicks. However, irrespective of which hemisphere is being used, the brain of female chicks appears to require the coordinated activity of both left and right to fully discriminate. It is likely that male chicks would show less selectivity since this would be associated with the increased hemispatial bias we have demonstrated in the present work.
It appears that there is a left-sided bias in male chicks at least, though why this should be limited to one object type only (the blue bead jar) remains unknown.
3. Passive avoidance learning
As we have seen above, the chick is predisposed to peck at objects shortly after hatching. However, equally important may be the inhibition of this response: if the item was either a non-food item or, more saliently, an aversive or potentially poisonous item, it would be advantageous for the chick to refrain from pecking at such an item. Being able to learn from others about how edible a food type is would be of the utmost importance. Nevertheless, this ability does not always seem to be present in animal species (e.g. rats; Galef 1996). Sherwin et al. (2002) demonstrated that nine-week-old (adult) observer hens were not able to learn to avoid pecking at a coloured food that elicited a ‘disgust’ reaction from another hen, but they will avoid pecking at one kind of food if they see another hen standing near the food without pecking it.
Young chicks learning for the first time to classify particles as edible or inedible may be more sensitive to the consequences of the feeding behaviour of others owing to the importance of learning about palatable items as quickly as possible. This sensitivity, however, reduces as the chicks mature and consequences of ingestion become an important source of information about food palatability via individual associative learning (Nicol 2004).
Pecking avoidance can be investigated experimentally in a procedure (passive avoidance learning, PAL) in which a chick is presented with a bead covered in a bitter-tasting substance (usually methyl anthranilate, MeA). On pecking the bead, the chick exhibits a disgust response (shaking its head, wiping its bill on the ground and emitting distress calls) associated with the ingestion/taste of the MeA. The chick will subsequently not peck again when presented with a similar bead at a time point (minutes to hours) later. It has been shown that memory formation for the inhibition of the pecking response occurs over a time course of several hours. During this period, there is a range of biochemical, physiological and morphological changes that will lead to a permanent memory associated with different memory phases (e.g. short-term, intermediate-term and long-term memory; see Gibbs et al. 2003; Chiandetti et al. 2007). It appears that specific brain areas are involved during these memory phases (including the mesopallium and medial striatum) and that there is a lateralization in activity of these areas, which is also time dependent (Rose 2000). Thus, a ‘flow’ of memory has been described in which there is a transfer of memory from the left mesopallium to the right mesopallium and then later to the left and right medial striata (e.g. Patterson et al. 1990). Lesion studies indicate that the bilateral or left, but not right, pre-training mesopallium lesions interfere with the acquisition of this task (Patterson et al. 1990), and this is backed up by biochemical evidence showing that the memory appears to consolidate first in the left mesopallium and then in the right (Sandi et al. 1993; also see Rose 2000). The right mesopallium may also be necessary for transfer of information to the basal ganglia (Patterson et al. 1990).
In PAL, chicks are trained and tested in pairs (Ng et al. 1991). This allows the possibility of investigating the presence of social information transmission between the cage mates. It is likely that the first chick that pecks at the bitter-tasting bead attracts the attention of the other chick towards the bead and conveys some information about the aversive nature of the bead (Galef 1988). However, some of the first experiments conducted seemed to indicate that the behaviour of one chick does not influence the behaviour of its cage mate (Gibbs & Ng 1977; De Vaus et al. 1980). This contrasts with the evidence indicating the presence of social learning for pecking avoidance in day-old chicks (Johnston et al. 1998). Johnston et al. (1998) used pairs of chicks in which one of the pair (the ‘demonstrator’) was presented with a chrome bead dipped in either MeA or water. The second chick in each pair was termed the ‘observer’, and was prevented from pecking at the training bead by the presence of wire mesh dividing the cage into two parts. During the test phase, the demonstrator and observer chicks were presented, one at a time, with a dry chrome bead for 10 s at specific time points after training (0.5, 3 or 24 hours), followed, after a further 5 min delay, by a 10 s presentation of a dry white bead to determine whether the chicks' response was general or specific to the bead, i.e. whether the chick was able to inhibit pecking on a specific (discriminatory) basis or merely inhibit all responses.
They found that both demonstrator and observer chicks avoided pecking at the chrome bead at test up to 24 hours after the observer chick had seen its demonstrator pecking a similar, but bitter-tasting, bead and displaying a disgust response. Chicks continued to peck at the dry chrome bead if, during the training phase, the demonstrator had pecked a similar bead that was coated in water, and which did not elicit any disgust reaction. They also demonstrated that this social learning occurred during training, but not at testing.
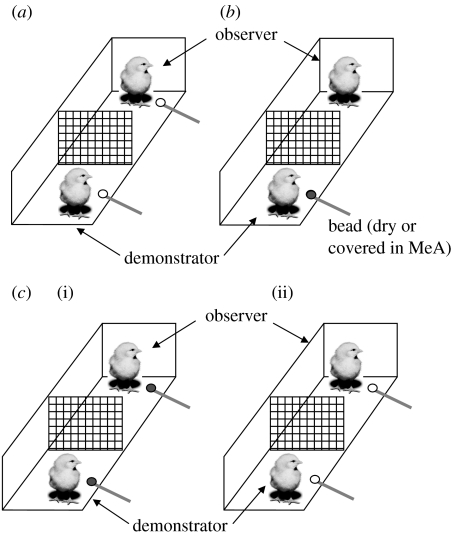
We have recently repeated this experimental procedure in order to investigate differential hemisphere use in the social learning version of this task (Daisley et al. 2007; Rosa Salva et al. in preparation). Chicks were maintained in pairs for 24 hours after hatching. Using the procedure described above, we were able to confirm the results of Johnston et al. (1998), in that observer chicks were able to successfully discriminate between two differently coloured beads. Specifically, following a pre-training in which chicks were presented with a dry white bead three times (figure 2a), half of the chicks (one from each pair; the demonstrator) were presented with an identical, but red, bead (figure 2b). The red bead was either dipped in 100 per cent solution of MeA (MeA-chicks) or was dry (dry-chicks). Thirty minutes after the end of training, the demonstrator (dem-chick) of each couple was presented with a dry red bead (figure 2c(i): lower chick). The observer (obs-chick) was then presented with a dry red bead (figure 2c(i): upper chick). Then, following a 5 min delay, dem- and obs-chicks were individually presented (in random order) with a dry white bead (figure 2c(ii)). The number of pecks during the test phase, to both red and white beads, was analysed.
Figure 2.
PAL. (a) The pre-training phase in which both birds are presented with a white bead three times, (b) the training phase in which one bird (the demonstrator) is presented with a red (darker) bead (either dry or covered with the bitter-tasting substance MeA) and (c) the testing phase in which both chicks are presented with (i) a dry red (darker) bead followed by (ii) a dry white bead.
Both observers and demonstrators learnt to avoid the bitter-tasting red bead and/or preferred to peck the white bead with respect to the red one. Observers appeared to master this task by direct observation of the demonstrators' behaviour, since there was a correlation between what the observer was doing in relation to its demonstrator for these MeA pairs.
Following this, we investigated whether there was a lateralization in the recall of this task in the observer chicks. We trained pairs of chicks in the same way as above: the observer chick was allowed to watch, using both eyes, the interaction of its demonstrator with the bead (coated in MeA or dry) at training. Directly afterwards, however, the observer was eye-patched such that either the right or the left eye was covered with a patch in order to obscure vision. After 30 min both chicks were tested, again as previously described. This meant that the observer chick was able to retrieve the memory for the bead from its contralateral hemisphere only. Thus, if chicks were largely using their left hemisphere to learn the task it may be that by patching the right eye they would no longer be able to successfully retrieve components of the memory necessary to produce the appropriate response.
Analysing the sexes and the eye used separately we found a significant difference between the left-eye system (LES) male MeA-obs-chicks and their controls (male LES-dry-obs-chicks) but not the right-eye system (RES) male chicks and their controls. Indeed, the LES observer males were significantly better at recalling the task than the RES males. Thus, there is again evidence for the differential involvement of the two cerebral hemispheres, this time in a social learning task. Males that used their left eye (right hemisphere) at test and both groups of monocular females were able to recall what they had learnt by observing their demonstrator during training, whereas males using their right eye (left hemisphere) were not, and consequently they did not prefer to peck the white bead with respect to the red one.
With regard to hemispheric lateralization associated with the task, there is a consensus that the memory for the interactive component of this task in its standard non-social version forms in the left hemisphere (Gibbs et al. 2003). This is suggested to be due to the left hemisphere involvement in the control of inhibition of the pecking response (Mench & Andrew 1986). As mentioned previously, the left hemisphere is also known to be necessary for distinguishing local specific cues associated with the target, in this case the bead, while the right hemisphere is generally concerned with spatial, topographical cues that are unlikely to be relevant for a successful discrimination as seen for the LES male chicks here. However, the results we have found suggest a successful discrimination based on the right hemisphere use, which would potentially point to a function of the right hemisphere in assessing the behavioural components of the demonstrator's interaction with the bead.
It should be noted that the task observers had to perform was somewhat different with respect to the standard PAL task, in that the observers did not have any direct contact with the stimulus whose properties chicks had to learn (the red bead). Indeed, learning could be achieved only by observing a familiar conspecific that interacted with the relevant stimulus.
Thus, one argument could be that this social learning version of the task may involve a mixture of components consisting of behavioural cues (from the demonstrator) associated with the bead's visual cues. In males, the fact that only the LES individuals were able to integrate these two components in order to produce the appropriate discriminatory response may suggest that for males, either the information necessary is held exclusively in the right hemisphere and/or that the left hemisphere requires information from the right (which, in the monocular RES context it is unable to do). For females, no such lateralization is seen, with chicks learning in either monocular condition, suggesting that females can access both hemispheres either directly or indirectly in order to produce the appropriate response.
It has been postulated that the memory for this task (and in general) may be distributed in ‘fragments’, involving both left and right brain structures, of differing informational content following initial exposure to the learning stimulus (see Gibbs et al. 2003). In addition, the two hemispheres undergo different patterns of cyclicity: the left has so-called ‘retrieval events’ in periods of 16 min (i.e. at 16, 32, 48 min, etc.) and the right at 25 min (i.e. 25, 50, 75 min, etc.; Andrew 1999). Testing 30 min after seeing the demonstrator's response may, therefore, still be a ‘right hemisphere event’, at least in males. The timing (30 min) also appears to coincide with the intermediate-term memory formation. According to Gibbs et al. (2003), information in the right hemisphere may be necessary to the left hemisphere during this phase. Our data would be in agreement with this and, in addition, it may also suggest that the timing for this memory formation or transfer between hemispheres is different in male and female birds, at least in relation to information retrieval for this social learning task.
4. Lateralization of fear responses (gaze cues)
Following the first few days after hatching, chicks start to show fear to novel stimuli. This is thought to be due to either the imprinting process per se (Bateson 1964) or the chick's running tendency (Hess 1959). In this way, the chick keeps in close contact with the hen and its siblings, thus receiving protection from predators (Rogers 1995).
An animal's ability to experience fear and to react to fearful events appropriately is essential for its survival. The perception of fear elicits a ‘fight or flight’ response in which the individual's heart rate and breathing are increased. In many animals, including gallinaceous birds, the fear response may manifest itself as ‘freezing’ in which the animal remains immobilized. This is thought to be adaptive by removing motion cues used by predators (Jones 1992).
Previously, it has been shown that human gaze directed at a chicken will increase the period of tonic freezing the bird experiences in comparison with an averted gaze (Gallup et al. 1971). Also, younger birds (3-day-old chicks) have been shown to have an increased latency to move in a novel environment when they are directly under the gaze of a dummy face (Vallortigara & Zanforlin 1988).
In chicks, functional asymmetries between the two hemispheres are also involved in the control of a fear-related response, suggesting the presence of a lateralized brain results adaptive also for a fear response. Dimond (1968) observed that chicks hatching from eggs incubated in the dark (‘non-lateralized’) showed a reduced fear response in comparison with light-hatched (‘lateralized’) chicks. This reduction in fear may be a critical factor in allowing the dark-hatched chicks of lower dominance rank to be able to compete more successfully for food with their more dominant siblings and conspecifics but may also ensure that they are at increased risk of predation (Rogers & Workman 1989; Queiroz & Cromberg 2006). This suggests to there being a lateralization of the fear response. Indeed, Phillips & Youngren (1986) demonstrated, using biochemical interventions, that the right hemisphere (specifically, the right archistriatum—now called the arcopallium following Reiner et al. (2004)) is involved in the control of avian fear behaviour. For example, it has been shown that the fear responses to predators are much quicker or more pronounced when the predator is detected by the left eye than by the right eye of domestic chicks (Andrew et al. 1982; Rogers 1997 for a review; Rogers 2000; Rogers et al. 2004). Indeed, the left eye is preferentially used to scan for a predator after advertisement of its presence (Evans et al. 1993) and, using the left eye system, chicks are quicker to detect the predator when engaged in a dual-task paradigm (selective feeding together with predator detection; Rogers et al. 2004).
We decided to investigate the effect of gaze perception in chicks using a human face-like mask and to determine whether fear responses associated with the predator's gaze were being differentially perceived/modulated by the two hemispheres (see Rosa Salva et al. 2007).
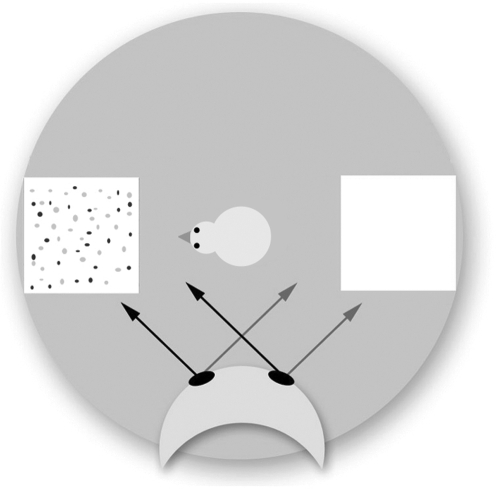
Chicks (8 days old) were given the choice of moving towards a pebble floor (a small area covered in small stone chips of similar size, shape and colour to the chicks' usual food together with food grains) or a clear floor in a novel testing arena. A mask with movable eyes was placed overlooking the arena. The eyes were placed so that they were either gazing towards the, more interesting, pebble floor or away from it (and at the clear floor; figure 3). The eye used by the chick to look at the mask was noted at all times together with the latency to approach the experimental surface. These chicks were quicker to approach the pebble floor when the mask's eyes were directed towards this surface. They also predominantly used their left eye (right hemisphere) to monitor the mask when it was gazing away from the pebble floor.
Figure 3.
Gaze perception in the chick. A schematic representation of the chick in the starting position inside the arena showing gaze of the mask's eyes towards either the pebble floor (black arrows) or the clear surface (grey arrows).
Therefore, it would seem that these chicks were used to having gaze directed towards them in a benign context by the experimenter, e.g. when the experimenter would provide food and water to the birds. Thus, no perception of fear was associated with gaze when the gaze was towards a biologically salient cue (the more interesting pebble floor). However, when the gaze was directed away from the pebble floor this produced an increased latency to approach. This result is unclear, but suggests either an increase in the chick's fear perception associated with the novel experience of a gaze looking away from the subject or an increased propensity to wish to follow the gaze (to the clear surface).
In order to investigate these responses more specifically, chicks completely naïve to human gaze were used. For this, great care was taken to ensure that the chicks were never exposed to human gaze throughout their raising period.
This time the chicks took longer to approach the pebble floor when the mask's eyes were directed towards it and had a tendency to use their left eye (right hemisphere) to look at the mask.
In this case, the mask's gaze appeared to be perceived as a fearful stimulus. These two results demonstrate that chicks have a predisposition to attend to potentially threatening stimuli with their left eye–right hemisphere system. These results also show that chicks have an innate ability to recognize eye shapes (i.e. gaze direction) and perceive them as being biologically active and relevant with regard to themselves. These results also support the view that the perception of fear is a right hemisphere event.
Overall, when a face representation is familiar (and has been learnt), chicks are no longer fearful of the presence of the gaze, but react to it, following what may be a biologically relevant cue (when the gaze is directed towards an area/stimulus of apparent interest). However, when naïve to the gaze's intent, chicks are still able to determine that the mask and its eyes are a relevant cue to which a fear response is required.
What components of the face or mask constitute a biologically relevant stimulus? Obviously, the gaze of the eyes is followed, but is it the face as a whole that is recognized and what constitutes a ‘face’ that can produce a response?
5. Lateralization of preference for face-like configurations
In addition to gaze perception, the right hemisphere is also thought to be involved in face perception. Data from human studies, including the data from neuroimaging and from brain disorder patients, show face perception (and emotional perception) as being a right hemisphere process, centred on the fusiform gyrus (De Renzi et al. 1994; Kanwisher et al. 1997; De Haan 2001). Also, the recognition of individual conspecifics is mainly processed using the right hemisphere, e.g. in humans (Sergent & Signoret 1992), sheep (Peirce et al. 2000) and chicks (Vallortigara & Andrew 1991, 1994).
We decided to investigate brain lateralization with regard to preference for faces and other top-heavy non-face-like stimuli (i.e. stimuli having more high-contrast elements in their upper part, as opposed to bottom-heavy configurations having more elements in their lower part) in chicks. Chicks have been shown to display a preference for face-like configurations with respect to other top-heavy stimuli (Rosa Salva et al. submitted). This makes ecological sense, since the hen bird and indeed the other nest mates will be characterized by a triangular face-like arrangement of features that will be biologically salient to the newly hatched chick (these cues should be followed and attended to in order to obtain food and other necessary resources).
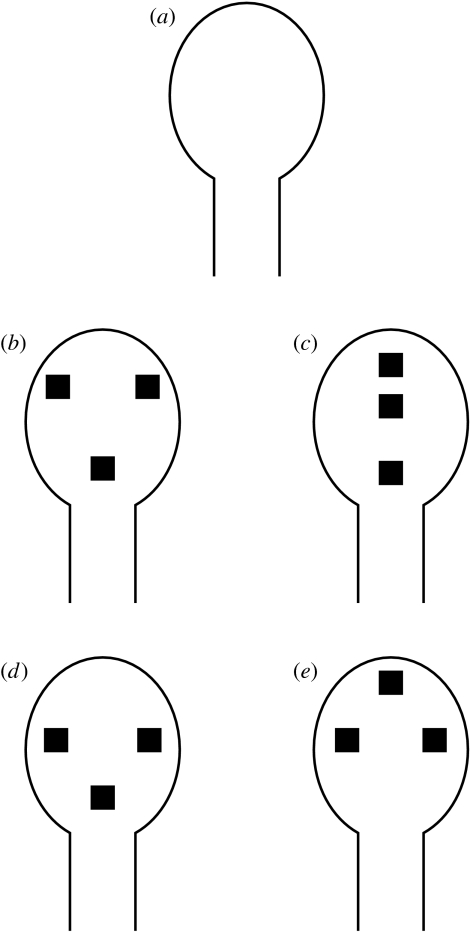
In a first experiment conducted, chicks were exposed to an imprinting stimulus directly following hatching. The imprinting stimulus consisted of a mask-like cut-out that was superimposed on one of the walls in each cage (figure 4a). The mask was empty (representing a ‘featureless face’), thus providing the chicks with no information regarding the internal features of a face. On test, each chick was allowed a free choice between two stimuli placed at the opposite ends of a runway. The two stimuli consisted of a face-like stimulus (figure 4b) and a non-face-like one (figure 4c; the face-like stimulus was defined as the ‘target stimulus’, for which a preference was expected). Test stimuli were identical to the imprinting object with the only difference being the presence of three square black blobs representing the stimuli's internal features. In the face-like stimulus, the blobs were arranged in a way that they represented the three main internal features of a face (the two eyes and the mouth/bill), whereas in the non-face-like one they were all aligned along the central vertical axis of the figure in a way that did not represent a face. Test stimuli were created by manipulating configurations that had been already employed in developmental studies on spontaneous preference for face-like displays in human newborns (see Morton & Johnson (1991) for a review). Chicks' behaviour was observed for a total of six consecutive minutes. The presence of lateralization effects was investigated by studying the effects of the eye used to view the face-like configuration at the beginning of the test on the time spent near the first stimulus approached. The expected effect of this manipulation was to increase or decrease (depending on the hemisphere processing the target stimulus) a general social facilitation effect (a facilitation to approach and stay near either test stimuli, which can be considered social objects also owing to their resemblance with the chicks' imprinting object). In this context, the social facilitation effect was defined as the longer time spent in the proximity of one stimulus when approaching it for the first time. Results of this first experiment showed that chicks having the face-like stimulus (i.e. the preferred stimulus) in their left monocular visual field (right hemisphere) at the beginning of the test, spent more time near the first stimulus approached (regardless of whether it was the face or non-face one). Therefore, there seemed to be a general facilitation effect on social behaviour determined by the presence of a face-like configuration in the visual field that is mainly processed by the right hemisphere, i.e. when a face-like configuration was presented to the right hemisphere, chicks made a more decided choice when approaching one of two stimuli that were both equally similar to their imprinting object.
Figure 4.
Face preference. (a) The imprinting object (representing a featureless face) employed in all the experiments. Test stimuli employed in the first (b,c) and second (d,e) experiments conducted, representing a top-heavy face-like stimulus (b), a bottom-heavy face-like stimulus (d) and top-heavy non-face-like stimuli (c,e).
In a second experiment, stimuli structure was manipulated so that in the face-like stimulus the blobs were arranged in a triangular bottom-heavy face-like configuration (figure 4d), whereas in the non-face-like one they were arranged according to the same configuration but with upside-down orientation (figure 4e). Test stimuli were created by manipulating configurations that had been already employed with newborns by Turati et al. (2002). The same lateralization effect, already demonstrated in the previous experiment, was also obtained in the present one: chicks having the face-like stimulus in their left monocular visual field (right hemisphere) at the beginning of the test spent more time near the first stimulus approached. Thus, here we confirmed the presence of a dominant role of the right hemisphere in face perception, regardless of whether stimuli employed were top-heavy or bottom-heavy configurations.
Overall, our results suggest that the mechanisms lateralized in favour of the right hemisphere could underlie chicks' preference for faces, independent from any—possible—preference for top-heavy configurations. In fact, a general social facilitation effect is determined by the presentation of a face-like stimulus to the right hemisphere, and this is true regardless of whether the face-like stimulus is a top-heavy (first experiment) or a bottom-heavy configuration (second experiment).
Moreover, it seems that in the presence of a face-like stimulus, the right hemisphere could play a predominant role in controlling chicks' social behaviour (first and second experiments).
We have seen in the previous session a tendency for chicks to use their left eye–right hemisphere to discern gaze initially, at least, in relation to a fear response. Once having determined that the gaze is benign, the chick will still preferentially use its left eye system to analyse the face as found by the stronger social facilitation when the face was placed to the left of the animal.
Thus, chicks react to gaze and are able to recognize the structural configuration of a face. These findings are reiterated by the fact that shortly after hatching, domestic chicks become capable of discriminating between individual conspecifics, at least to the level of the distinction between familiar and non-familiar individuals. Following approximately 12 hours of direct contact (Porter et al. 2005), chicks will prefer to remain near their cage mates (Riedstra & Groothuis 2002) and will have a tendency to peck more often at an unfamiliar partner (Rajecki et al. 1976). Indeed, the frequency of pecks directed at familiar and unfamiliar conspecifics appears to be the most sensitive measure of social discrimination in young birds (cf. Porter et al. 2006). It is proposed that pecking intensity may be a form of social exploration (Riedstra & Groothuis 2002) or aggression (Vallortigara 1992b). Either way, this interaction may subserve a mechanism that is involved in social hierarchy formation: testing the chick's relative level of dominance within a group.
6. Transitive inference learning
Dominance relationships are formed within the social groups. They can be followed by observations of overt aggressive and submissive behaviours when individuals confront one another (Craig 1986). In chickens, such dominance relationships may begin to form within the first week following hatching (Rogers & Astiningsih 1991), and are usually well developed by five or six weeks of age (McBride et al. 1969).
Animals have the ability to infer judgements on other individuals' ranks by observation only, and perhaps, therefore, to infer their own rank status: Paz-y-Miño et al. (2004) determined that Pinyon jays (Gymnorhinus cyanocephalus) can infer a judgement about their own dominance by observing strangers interacting with different known birds. This ability to make inferences means that the animal is able to predict the outcome of competition for resources (food, mating opportunities, etc.) and thus avoid unnecessary and potentially injurious fights with other conspecifics. Keeping track of the social position of others within the group requires a significant cognitive ability. Indeed, this process of inference, termed transitive inference (TI), has been described as a measure of logical reasoning ability (Piaget 1928).
Chickens have been shown to have the ability to emit a suitable social response on the judgement of interactions between dyads (Beaugrand et al. 1997). By watching another individual that was known to be dominant to them winning against an unknown bird, the bystander would behave as if it was of roughly equal status as the defeated newcomer. If the new bird defeated the bystanders' dominant bird, however, the bystander would be submissive to the new bird when in its presence.
That a lateralization in hemisphere use may be present in learning this task is implied by the data from Rogers & Workman (1989), in which they found that chicks which had been exposed to light in the egg formed more stable hierarchies when placed in a group together than did groups of dark-hatched (and therefore ‘less lateralized’) chicks. The lowest ranked light-hatched chicks tended to consistently receive less time at a feeding situation than did dark-hatched chicks. Thus, the social structure of dark-hatched chicks was more changeable and less rigid than in groups of chicks that had been exposed to light, and since the ability to perform TI may be linked to social complexity in animals, we considered it a likely possibility that there may be an improved ability of light-hatched (lateralized) chicks to perform TI successfully. Thus, we tested two groups of birds, one of which had received light for the last days of incubation, and would be assumed to have lateralized brains, the other dark-hatched and therefore non-lateralized (Daisley et al. in preparation).
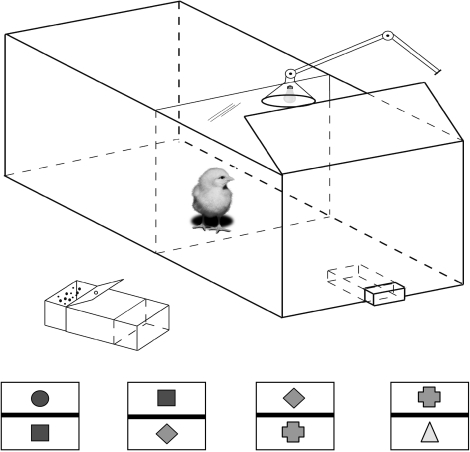
Following shaping, in which they learnt to peck at a stimulus (a small dot, 4 mm in diameter) to receive a food reward, chicks (12 days old) were confronted with paired presentations of stimuli in such a way as to learn a hierarchical order of the training stimuli: A>B>C>D>E. Each stimulus was of a different shape (star, circle, square, triangle and diamond) and colour (yellow, red, brown, blue and green) to aid discrimination (figure 5). The training stimuli were presented pairwise: one when pecked gave a food reward (+; i.e. the experimenter opened the box that held food) and the other did not (−; the box not containing food was opened). The stimulus pairs were presented in the order: A+B−, B+C−, C+D− and D+E−, ‘+’ indicating reinforcement, with each pair being presented until the chick had reached a criterion level.
Figure 5.
Diagram of the apparatus used to train and test chicks on a TI task. The four stimulus pairs, which the chick had to learn, are shown at the bottom of the figure together with the box from which the chick would receive a food reward when it pecked the correct stimulus (i.e. the stimulus that had been chosen as being the higher of the two in a hierarchical sequence).
Twenty-four hours after the end of the training session, chicks were again presented with the training pairs (AB, BC, etc.) but, in addition and interspersed within them, were presented the previously unseen pairs AE and BD (unrewarded) a total of 20 times across four testing sessions. The pair AE involved the ability to discriminate between a stimulus that had always been reinforced (A) and one that had never been reinforced (E) during the training; AE represented a non-transitive novel pairing. The pair BD represented the test of transitivity, because in order to discriminate successfully the pair BD (i.e. pecking B) chicks needed to remember the hierarchical arrangement of the stimuli (i.e. demonstrate TI learning), because stimuli B and D, in the training phase, were reinforced and not reinforced for the same number of trials.
Both groups were able to demonstrate the associative discrimination (AE). Also, both groups of chicks performed significantly above chance at test with the choice of B versus D as well. On comparing light- versus dark-hatched chicks, however, a difference emerged. As was predicted, those chicks hatched from eggs exposed to light during the sensitive period of incubation (days 19–21), and thus likely to have increased hemispheric lateralization, were performing better than those hatched in the dark.
This makes sense in the light of the data from Rogers & Workman (1989). If light-hatched chicks are forming more stable hierarchies than dark-hatched ones, this may be owing to these chicks being more able to determine individual differences and/or discern social interactions. Thus, it appears that TI performance may indeed be intertwined with social group formation and dominance hierarchies and is associated with brain lateralization. Is there a particular hemisphere necessary for TI performance?
We investigated this further by using another group of chicks, all light-hatched (lateralized). Chicks underwent the same training procedure as before. This time, however, a single box was used: the stimuli A+B−, etc. were now placed in the vertical plane as opposed to having two boxes with stimuli aligned horizontally. This was because at the test the chicks were in the monocular condition. One group of chicks performed the test with the right eye only (the left eye being covered by a patch of black cloth); the other group were tested using only the left eye, with the right eye being patched. Thus, after a suitable time in which the chicks were allowed to adapt to being eye-patched, they were tested in the same way as before: a total of 20 trials of being shown the pairs A and E, and B and D. The eye-patch itself did not hinder the performance of the task: both groups performed AE. However, only the chicks that were using their left eye (and hence, were accessing their right hemisphere) were successful in carrying out the TI discrimination (B versus D). The difference in performance between the two groups over the 20 trials was not significant, however. Does this mean that the difference is not real, though? Investigating further, we found that there was a significant difference between the two groups on the very first test, i.e. in the number of times B was chosen in the first set of five choices performed by the chicks. This may be owing to the fact that at test, whether choosing correctly (B) or incorrectly (D), the chicks received no feedback because the drawer was not opened to reveal whether the choice made was correct. Thus, the beginning of the test may be more pertinent to the testing of true TI learning since it is likely that the lack of feedback in further test trials may produce an additional learning. Indeed, when comparing the very first response peck made by the chicks at the very first presentation of BD, left-eye chicks were found to have chosen B more often than right-eye chicks.
It is thus clear that access to the right hemisphere is necessary for TI performance in these chicks. This also appears to be the case in humans where the right anterior hippocampus appears to be the area used in order to perform a TI task (Heckers et al. 2004). This is in general agreement with data showing that the hippocampus of mammals is involved in the organization of the representation of stimulus relationships (e.g. Dusek & Eichenbaum 1997). The initial learning and recognition of the sequences per se is carried out by other areas, though, most notably the parahippocampal gyrus. We cannot determine from our results which areas are involved either in learning the pairs or remembering the sequence. Previously, however, Strasser et al. (2004) found that even hippocampal-lesioned pigeons were able to perform a TI task. They suggested that a form of value transfer may have been used by the participants in other experiments and this may itself be hippocampal dependent. Furthermore, Frank et al. (2003) produced a model that would see the hippocampus being used during training, but not necessarily responding to the TI task.
Whatever the brain area involved, our chicks required the right hemisphere to retrieve the necessary information and/or to produce the appropriate response. Distinguishing between the different stimuli is likely to be a right hemisphere-specific process because the left eye system is known to respond to specific properties of the stimulus. It appears that distinguishing the different shapes associated with the stimuli may be more important than the colours intrinsic to those shapes (colour differentiation being more likely a left hemisphere specialization).
Linking TI performance to left eye–right hemisphere use is the fact that chicks using their left eye will perform better at distinguishing between familiar and unfamiliar conspecifics (Vallortigara 1992a; Deng & Rogers 2002a,b): the formation of social hierarchies is almost certainly dependent on the recognition of individual conspecifics. The left hemisphere may still be involved, since Deng & Rogers (2002a,b) showed that after being exposed for a time to a group of chicks, right-eye tested birds could now distinguish familiar individuals. Our results, assuming the TI test we perform is related to social hierarchy evaluation, would agree with their conclusion in that the right hemisphere may still be preferred when chicks are tested binocularly and may also be more efficient at carrying out this differentiation.
The ability to perform TI may be linked to the social complexity of the species studied: Bond et al. (2003) found that the less social scrub jay (Aphelocoma californica) although able to perform a TI task in which they had to learn a series of coloured keys, pecking at the higher ‘ranked’ key in order to receive a food reward, it was less capable than the Pinyon jay in performing a discriminative version of the task. Is this also related to individual experience? Can the left hemisphere be trained to perform TI? We are presently undertaking work to determine whether individuals kept in social groups during raising are more competent at a TI task, i.e. if the act of being exposed socially to other conspecifics allows the transfer of skills to stimulus premise pair recognition and subsequent unseen pair comparisons. Also, since prior experience of conspecifics means chicks could discriminate the familiarity of others with the left hemisphere, does this mean that the group-raised birds also show this property?
7. Discussion
(a) Right hemisphere advantage in social cognition
The right hemisphere seems to play an important role in many of the processes that we have examined. In fear responses to different directions of the gaze of masks, the right hemisphere is largely used. Further to this, having assessed the qualities associated with gaze direction and the face, chicks are able to recognize faces from other objects and to discern different individuals (Andrew et al. 2004). This appears also to be a right hemisphere process. Chicks also appear to use right hemisphere processes in order to evaluate other individuals in a group as discerned by the TI data. Recognizing not only other individuals, but also the exact qualities associated with stimuli presented (probably shape, but possibly also colour) seems to rely on right hemisphere processes too. Finally, the ability to learn to identify the right kind of food and assume the same from the experience of others is again a right hemisphere process.
Right hemisphere use in social cognition appears to be a phylogenetically conserved process. In humans (De Renzi et al. 1994), sheep (Kendrick 2006) and monkeys (Hauser 1993), the right hemisphere has been demonstrated to be involved in the perception of facial expression and in face recognition. In addition, fishes also use their left eye to examine a mirror reflection of themselves (Sovrano & Andrew 2006, also in Sovrano et al. 2001), again suggesting right hemisphere involvement in viewing conspecifics. We would suggest that this ability to recognize other individuals is a key factor in social cognition from which other abilities, such as social learning, learning inferences about others, etc., stem. Indeed, recent work in the realm of ‘self-related cognition’ (see Uddin et al. 2007) has identified the right hemisphere as playing a vital role in aspects of human and primate social cognition. Specifically, the right frontoparietal area of the human brain contains mirror neurons that are linked to imitative behaviour (Iacoboni 2005) and to social cognition (Iacoboni et al. 2005). These neurons are activated not only when performing certain goal-oriented actions but also when viewing them being performed by others.
In the domestic chick, it has been shown previously that there is a right hemisphere advantage for social recognition (see Vallortigara 1992a). This also holds true for another gallinaceous species, the quail (Coturnix sp.), which shows detour behaviour that differs in laterality according to whether the social target is a familiar (left eye used) or unfamiliar (right eye) bird (Zucca & Sovrano 2008). Vallortigara (1992a) suggested that the neural structures fed by the left eye (mainly located at the right hemisphere) are better at processing and/or storing of visual information that allows recognition of individual conspecifics. This may be part of a wider tendency to respond to small changes in any of a variety of intrinsic stimulus properties.
What does this leave for the left hemisphere in terms of social cognition? It seems that the left hemisphere may be more involved in the production of intentional social signals. In the human brain, the left hemisphere is dominant in the production of speech and signed language (Corballis 2002). In addition, Reynolds Losin et al. (2008) have shown that the motor control of facial movements associated with the production of learned sounds is lateralized to the left hemisphere. This coincides with work from Nottebohm (1999) who showed that the lesions to the left but not the right hypoglossal nerve resulted in significant deficits in song production in a song bird. Together, these suggest that the expression of a intentionally produced communicative action is a left hemisphere event (excluding the spontaneous expression of emotion; Stone et al. 1996), while the right hemisphere is required either for the emission of a simple event or social action (e.g. the pecking response in the left hemifield) or for the interpretation of a perceived event (e.g. from our data; the gaze of another, the interaction of a conspecific with a bitter-tasting bead or the dominance status of another).
(b) Lateralization of social behaviours
It has been argued that an advantage of having a lateralized brain is that it allows simultaneous processing of different information in the two hemispheres, with each hemisphere performing a function for which it is specialized (see Vallortigara & Rogers 2005). This is apparent in a dual task in which chicks have to simultaneously detect a predator (right hemisphere process) while controlling their pecking response (left hemisphere process; Rogers 2000). Those chicks exposed to light in the egg were able to detect the predator sooner than the dark-hatched chicks and also to learn to peck at grain and disregard the pebbles. It is thought that this ‘optimal’ lateralization of response may also have a consistent direction at the population level, specifically when individuals are required to interact with each other in social groups.
Lateralization at a population level may form an evolutionarily stable strategy if there are frequency-dependent costs and benefits associated with being lateralized in one or the other direction (Ghirlanda & Vallortigara 2004). It seems that there may be social constraints imposed on the asymmetrical individual when in a social group such that what is better for an (asymmetrical) individual to do may depend on what other (asymmetrical) individuals in the group are doing in order for a fitness advantage to accrue (from Vallortigara & Rogers 2005). It may favour an individual to be predictable in certain social situations (cooperative and coordinative interactions with other individuals) while in others (agonistic interactions, escape from predators) it may pay to be unpredictable (see also in this issue, Ghirlanda et al. (2009)). That hemispheric lateralization can be explained in terms of population-level interactions also points to lateralization as being important for social cognition throughout the chick's life, but undoubtedly, most relevant during its early life.
The experiments we have described previously have outlined some different investigations of these general, population-level lateralizations. However, a next step might be to open up the discussion with regard to individual lateralization(s) and its effect at the population level. Specifically, to determine the level and direction of the lateralization within the population. This would lead us to perform tests at the level of the individual. Once, having established the propensity of individual biases in laterality, we could ascertain its performance within the group context. Does it pay to be a less lateralized individual or an individual with the reverse lateralization compared to the group? Or, do the fitness consequences of producing differently lateralized young within a clutch (and that are raised together) outweigh the disadvantages possibly associated with having some chicks that are less socially viable? Either or both of these cases should be in the affirmative, according to Ghirlanda & Vallortigara (2004).
(c) Sex differences
In our work, we have found instances of there being sex differences in the expression of behavioural asymmetries. A prominent left visuospatial bias found in the tidbitting experiments is seen only with the male chicks (and, also, only when they are interacting with a specific target, a blue-coloured pot of beads). Also, where observation of another, same-sex, individual is involved in the PAL task, male chicks show a hemisphere bias but not females. It is argued that females seem to be able to use one hemisphere at a time to take overall control of attentional strategies; in other words, the hemisphere that is in control can impose its own attentional strategies to the partner, thus reducing the behavioural lateralization. On the contrary, this effect seems not to be present in males and therefore they show more pronounced asymmetries in the visual control of behaviour.
Why should this be the case? It points towards differences in the development of male and female brains in terms of the level of lateralization seen between the two hemispheres. Indeed, it has previously been shown that sex differences in brain lateralization exist in chicks and that this has an anatomical basis: the asymmetry of the thalamofugal pathway has been shown to be greater in males than in females (see Deng & Rogers (2002a) in Rogers & Andrew (2002)). The right hemisphere of males, therefore, receives a larger amount of binocular input than does the equivalent hemisphere of females.
The sex difference in lateralization also has implications for social behaviours and social cognition of the two sexes. Vallortigara & Andrew (1994) determined a difference in social attachment between the males and the females. In males, choice between a familiar and an unfamiliar chick was completed only when using the right hemisphere. Females also tended to use their right hemisphere; however, they were more adept at choosing their familiar partner. Females show a reduced latency to approach familiar birds rather than unfamiliar ones in approach response tests (Vallortigara 1992b). They also tend to choose to remain closer to their cage mates. Male chicks, on the other hand, tend to interact more aggressively with other conspecifics, e.g. by eliciting more pecks at social partners, than do females (Vallortigara 1992b) and they have a tendency to approach and stay with unfamiliar chicks more than females. Females run faster towards their cage mates whereas males run faster towards a food goal (Vallortigara et al. 1990).
(d) The effect of light and hormones on lateralization of social interactions
The sex differences in the expression of brain lateralization, potentially related to the greater level of asymmetry in male chick brains, leads to differences in sociality between the two sexes. According to the literature, females show greater social bonding and attachment while males show increased aggression (e.g. Vallortigara 1992b). This may lead, for example, to female adult birds being more competent (owing to increased opportunities) at social learning tasks. When required to perform the social tasks, we employed it is the male chicks that show more evidence of laterality than the females. This, we suppose, may be related to the increased bias in laterality shown in male chicks previously reported in the literature (see above) and also to the nature of the tasks used. Only in the PAL is there observation of another; all the other tasks, although suggested to require a social competence, do not involve the presence of another chick. Even in PAL there is only ever observation of a conspecific, not a direct interaction. It is worth noting that Nicol & Pope (1999) have investigated social learning effects in hens but not in cockerels. These sex differences may be due to interactions between hormone levels and light exposure (Rogers & Rajendra 1993). Halpern et al. (2005) go on to suggest that the interaction between the hormones and laterality may allow a role for the stress response of the hen to impinge upon and modulate the strength of asymmetry in her chicks.
In addition, it has been reported that chicks are able to influence maternal effects to light exposure by inducing egg turning (Tuculescu & Griswold 1983). However, the maternal influence on the developing embryo is likely to be greater. Considering the female chicken, it is quite possible that her choice of nesting site may be influenced by her rank status; this itself may influence not only the amount of light to which her eggs are exposed but also the amount of hormones they receive. Individual differences in phenotype that are produced by this differential hormone exposure are related to learning possibilities in geese (according to dominance: Pfeffer et al. 2002) and to fear responses in quail (Daisley et al. 2004). The addition of exogenous testosterone influences brain lateralization (Schwarz & Rogers 1992). Daisley et al. (2004) have shown that differences in sociality exist in quail following the application of exogenous testosterone to eggs: with treated birds, presumably those with increased lateralization, subject to increased stress response in the presence of others. The effect of social interactions early in life may also impinge upon the level of lateralization. In the rhesus monkey (Macaca mulatta), it has been shown that early rearing conditions play an important role in the development of lateralization: those monkeys raised not by their mother, but in a nursery with only same age individuals for companionship, showed a greater left-hand bias when reaching for a reward in a tube (Bennett et al. 2008). Also, rats, having been exposed to novel (potentially, more stressful) environments as pups, became less right-pawed as adults (Tang & Verstynen 2002). This shift appears to have an anatomical basis, with there being a commensurate increase in right hippocampal volume (Verstynen et al. 2001). Although the domestic chick is a precocial animal, it is still likely that some forms of lateralization may be shaped during early experiences out of the egg.
Therefore, it is likely that a hen experiencing social stress might end up nesting in thicker cover, leave the nest less often and deposit higher stress hormones in her eggs (according to Rogers 2008). This in turn could lead to reduced lateralization within her brood, which, although potentially increasing their survival in terms of food intake owing to a reduction in dominance hierarchy stability, may impinge upon her offspring's success within the context of the social group: according to our research, such individuals may not be able to successfully judge other's dominance in relation to them and may be at a disadvantage in learning.
8. Conclusions
It is likely that not all individuals are as lateralized as each other, whether through the different amounts of light received in the egg, the hormones deposited there by the female or even owing to the different levels of social interaction they received when shortly out of the egg. For example, it could be that differently ranked chicks in a group may have differences in social awareness (as judged by their performance in a TI task) and this could, in turn, be due to an increased lateralization found prior to hatching, or because of early experiences when out of the egg.
In addition, the work we have reported has relied upon implied social cognition, with the exception of the passive avoidance task where observation learning was used by the chicks. This has demonstrated a bias in lateralization for males. We would like to continue our investigations, but also to include more exacting social tests and tasks in which the naturally expressed lateralized behaviours can be related to direct social interactions.
Finally, it should be noted that many of the lateralization processes seen when the bird is young disappear when the bird reaches maturity (Rogers & Andrew 2002). This would suggest that the underlying anatomical asymmetries in the thalamofugal pathway are largely lost during later ontogeny (Rogers & Deng 1999). However, sexual behaviour, or at least the visual component of it, may still be guided by right/left differences: both for courtship and consummatory behaviour there is preferential right hemisphere use (Ventolini et al. 2005). Thus, there may be long-lasting effects of the initial asymmetries.
Acknowledgments
The experiments comply with the current Italian and European Community laws for the ethical treatment of animals.This research is a part of the project Evolution and Development of Cognitive, Behavioural and Neural Lateralisation, (EDCBNL 2006–2009), supported by the Commission of the European Communities within the framework of the specific research and technological development programme ‘Integrating and strengthening the European Research Area’ (initiative ‘What it means to be human’), through a financial grant to L.R. The authors would like to thank the following for their help with gathering the data and caring for the chicks: Adriana Berti, Consuelo Besazza, Diana Castelli, Federico Del Gallo, Alessio Fracasso, Tania Mattarello, Camilla Romor and Fabrizio Spinedi.
Footnotes
One contribution of 14 to a Theme Issue ‘Mechanisms and functions of brain and behavioural asymmetries’.
References
- Ades C., Ramires E.N. Asymmetry of leg use during prey handling in the spider Scytodes globula (Scytodidae) J. Insect Behav. 2002;15:563–570. doi:10.1023/A:1016337418472 [Google Scholar]
- Allen T.F., Clarke J.A. Social learning of food preferences by white-tailed ptarmigan chicks. Anim. Behav. 2005;70:305–310. doi:10.1016/j.anbehav.2004.10.022 [Google Scholar]
- Andrew R.J. The nature of behavioural lateralization in the chick. In: Andrew R.J., editor. Neural and behavioural plasticity: the use of the chick as a model. Oxford University Press; Oxford, UK: 1991. pp. 536–554. [Google Scholar]
- Andrew R.J. The differential roles of right and left sides of the brain in memory formation. Behav. Brain Res. 1999;98:289–295. doi: 10.1016/s0166-4328(98)00095-3. doi:10.1016/S0166-4328(98)00095-3 [DOI] [PubMed] [Google Scholar]
- Andrew R.J., Mench J., Rainey C. Right–left asymmetry of response to visual stimuli in the domestic chick. In: Ingle D.J., Goodale M.A., Mansfield R.J.W., editors. Analysis of the domestic chick as a model. Oxford University Press; Oxford, UK: 1982. pp. 166–177. [Google Scholar]
- Andrew R.J., Johnston A.N., Robins A., Rogers L.J. Light experience and the development of behavioural lateralisation in chicks. II. Choice of familiar versus unfamiliar model social partner. Behav. Brain Res. 2004;155:67–76. doi: 10.1016/j.bbr.2004.04.016. doi:10.1016/j.bbr.2004.04.016 [DOI] [PubMed] [Google Scholar]
- Bateson P.P.G. Changes in chicks' response to novel moving objects over the sensitive period of imprinting. Anim. Behav. 1964;12:479–489. doi:10.1016/0003-3472(64)90068-5 [Google Scholar]
- Beaugrand, J. P., Hogue, M. E. & Lague, P. C. 1997 Coherent use of information by hens assisting to the victory or defeat of their former dominant by a stranger: a case of transitive inference? In Processus cognitifs et ajustement écologique (ed. Société Française pour l'Étude du Comportement Animal), pp. 131–137. Toulouse, France: Presses de l'Université Paul Sabatier.
- Bennett A.J., Suomi S.J., Hopkins W.D. Effects of early adverse experiences on behavioural lateralisation in rhesus monkeys (Macaca mulatta) Laterality. 2008;13:282–292. doi: 10.1080/13576500802022216. doi:10.1080/13576500802022216 [DOI] [PubMed] [Google Scholar]
- Bond A.B., Kamil A.C., Balda R.P. Social complexity and transitive inference in corvids. Anim. Behav. 2003;65:479–487. doi:10.1006/anbe.2003.2101 [Google Scholar]
- Bottini G., Torraldo A. The influence of contralesional targets on the cancellation of ipsilateral targets in unilateral neglect. Brain Cogn. 2003;53:117–120. doi: 10.1016/s0278-2626(03)00091-5. doi:10.1016/S0278-2626(03)00091-5 [DOI] [PubMed] [Google Scholar]
- Broca P. Sur le siege de la Faculte du langage articule. Bull. Soc. Anthropol. (Paris) 1865;6:377–393. [Google Scholar]
- Chiandetti C., Regolin L., Rogers L.J., Vallortigara G. Effects of light stimulation of embryos on the use of position-specific and object-specific cues in binocular and monocular domestic chicks (Gallus gallus) Behav. Brain Res. 2005;163:10–17. doi: 10.1016/j.bbr.2005.03.024. doi:10.1016/j.bbr.2005.03.024 [DOI] [PubMed] [Google Scholar]
- Chiandetti C., Clara E., Regolin L., Tommasi L., Vallortigara G. Plasticity in the avian brain: from molecular to behavioral neurobiology. In: Canonaco M., Facciolo M., editors. Evolutionary molecular strategies and plasticity. Research Signpost; Kerala, India: 2007. pp. 187–214. [Google Scholar]
- Corballis M.C. Princeton University Press; Princeton, NJ: 2002. From hand to mouth: the origins of language. [Google Scholar]
- Craig J.V. Measuring social behaviour: social dominance. J. Anim. Sci. 1986;62:1120–1129. doi: 10.2527/jas1986.6241120x. [DOI] [PubMed] [Google Scholar]
- Csillag A., Montagnese C.M. Thalamotelencephalic organization in birds. Brain Res. Bull. 2005;66:303–310. doi: 10.1016/j.brainresbull.2005.03.020. doi:10.1016/j.brainresbull.2005.03.020 [DOI] [PubMed] [Google Scholar]
- Daisley J.N., Bromundt V., Möstl E., Kotrschal K. Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks Coturnix japonica. Horm. Behav. 2004;47:185–194. doi: 10.1016/j.yhbeh.2004.09.006. doi:10.1016/j.yhbeh.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Daisley, J. N., Rosa Salva, O., Vallortigara, G. & Regolin, L. 2007 Lateralization of social learning in the domestic chick (Gallus gallus): learning to avoid. Neural Plast 2007, 10. (doi:10.1155/2007/23250) [DOI] [PubMed]
- Daisley, J. N., Vallortigara, G. & Regolin, L. In preparation. Lateralization of hierarchy recall in the domestic chick (Gallus gallus).
- De Haan E.H.F. Face perception and recognition. In: Rapp B., editor. The handbook of cognitive neuropsychology: what deficits reveal about the human mind. Psychology Press; Philadelphia, PA: 2001. pp. 75–99. [Google Scholar]
- Deng C., Rogers L.J. Prehatching visual experience and lateralization in the visual Wulst of the chick. Behav. Brain Sci. 2002a;134:375–385. doi: 10.1016/s0166-4328(02)00050-5. doi:10.1016/S0166-4328(02)00050-5 [DOI] [PubMed] [Google Scholar]
- Deng C., Rogers L.J. Social recognition and approach in the chick: lateralization and effect of visual experience. Anim. Behav. 2002b;63:697–706. doi:10.1006/anbe.2001.1942 [Google Scholar]
- De Renzi E., Perani D., Carlesimo G.A., Silveri M.C., Fazio F. Prosopagnosia can be associated with damage confined to the right hemisphere: an MRI and PET study and a review of the literature. Neuropsychologia. 1994;32:893–902. doi: 10.1016/0028-3932(94)90041-8. doi:10.1016/0028-3932(94)90041-8 [DOI] [PubMed] [Google Scholar]
- De Vaus J.E., Gibbs M.E., Ng K.T. Effects of social isolation on memory formation. Behav. Neural Biol. 1980;29:473–480. doi: 10.1016/s0163-1047(80)92692-8. doi:10.1016/S0163-1047(80)92692-8 [DOI] [PubMed] [Google Scholar]
- Diekamp B., Regolin L., Gunturkun O., Vallortigara G. A left-sided visuospatial bias in birds. Curr. Biol. 2005;15:R372–R373. doi: 10.1016/j.cub.2005.05.017. doi:10.1016/j.cub.2005.05.017 [DOI] [PubMed] [Google Scholar]
- Dimond S.J. Effects of photic simulation before hatching on the development of fear in chicks. J. Comp. Physiol. Psychol. 1968;65:320–324. doi: 10.1037/h0025550. doi:10.1037/h0025550 [DOI] [PubMed] [Google Scholar]
- Dusek J.A., Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc. Natl Acad. Sci. USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. doi:10.1073/pnas.94.13.7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.S., Evans L., Marler P. On the meaning of alarm calls: functional reference in an avian vocal system. Anim. Behav. 1993;46:23–28. doi:10.1006/anbe.1993.1158 [Google Scholar]
- Fernandez-Carriba S.F., Loeches A., Morcillo A., Hopkins W.D. Functional asymmetry of emotions in primates: new findings in chimpanzees. Brain Res. Bull. 2002;57:561–564. doi: 10.1016/s0361-9230(01)00685-2. doi:10.1016/S0361-9230(01)00685-2 [DOI] [PubMed] [Google Scholar]
- Frank M.J., Rudy J.W., O'Reilly R.C. Transitivity, flexibility, conjunctive representations and the hippocampus. II. a computational analysis. Hippocampus. 2003;13:341–354. doi: 10.1002/hipo.10084. doi:10.1002/hipo.10084 [DOI] [PubMed] [Google Scholar]
- Gajdon, G. K. 2001 Social modification of early foraging in domestic chickens, Gallus gallus domesticus Dissertation, ETH Zürich, 14403.
- Galef B.G.J. Functions of social learning about food: a causal analysis of effects of diet novelty on preference transmission. Anim. Behav. 1988;46:257–265. doi:10.1006/anbe.1993.1187 [Google Scholar]
- Galef B.G.J. Social enhancement of food preferences in Norway rats: a brief review. In: Heyes C.M. Jr, Galef B.G., editors. Social learning in animals: the roots of culture. Academic Press; London, UK: 1996. pp. 49–64. [Google Scholar]
- Gallup G.G., Nash R.F., Ellison A. Tonic immobility as a reaction to predation: artificial eyes as a fear stimulus for chickens. Psychon. Sci. 1971;23:79–80. [Google Scholar]
- Ghirlanda S., Vallortigara G. The evolution of brain lateralization: a game-theoretical analysis of population structure. Proc. R. Soc. B. 2004;271:853–857. doi: 10.1098/rspb.2003.2669. doi:10.1098/rspb.2003.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlanda S., Frasnelli E., Vallortigara G. Intraspecific competition and coordination in the evolution of lateralization. Phil. Trans. R. Soc. B. 2009;364:861–866. doi: 10.1098/rstb.2008.0227. doi:10.1098/rstb.2008.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M.E., Ng K.T. Psychobiology of memory: towards a model of memory formation. Biobehav. Rev. 1977;1:113–136. doi:10.1016/0147-7552(77)90017-1 [Google Scholar]
- Gibbs M.E., Andrew R.J., Ng K.T. Hemispheric lateralization of memory stages for discriminated avoidance learning in the chick. Behav. Brain Res. 2003;139:157–165. doi: 10.1016/s0166-4328(02)00245-0. doi:10.1016/S0166-4328(02)00245-0 [DOI] [PubMed] [Google Scholar]
- Gülbetekin E., Güntürkün O., Dural S., Cetinkaya H. Asymmetry of visually guided sexual behaviour in adult Japanese quail (Coturnix japonica) Laterality. 2007;12:321–331. doi: 10.1080/13576500701307080. doi:10.1080/13576500701307080 [DOI] [PubMed] [Google Scholar]
- Halpern M.E., Güntürkün O., Hopkins W.D., Rogers L.J. Lateralization of the vertebrate brain: taking the side of model systems. J. Neurosci. 2005;25:10351–10357. doi: 10.1523/JNEUROSCI.3439-05.2005. doi:10.1523/JNEUROSCI.3439-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham A.D., Osorio D. Color preferences and color vision in poultry. Proc. R. Soc. B. 2007;274:1941–1948. doi: 10.1098/rspb.2007.0538. doi:10.1098/rspb.2007.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M.D. Right hemisphere dominance for the production of facial expression in monkeys. Science. 1993;261:475–477. doi: 10.1126/science.8332914. doi:10.1126/science.8332914 [DOI] [PubMed] [Google Scholar]
- Heckers S., Zalesak M., Weiss A.P., Ditman T., Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. doi:10.1002/hipo.10189 [DOI] [PubMed] [Google Scholar]
- Hess E.H. Imprinting. Science. 1959;130:133–141. doi: 10.1126/science.130.3368.133. doi:10.1126/science.130.3368.133 [DOI] [PubMed] [Google Scholar]
- Heyes C.M., Galef J.B.G. Academic Press; London, UK: 1996. Social learning in animals: the roots of culture. [Google Scholar]
- Hogan J.A. Pecking and feeding in chicks. Learn. Motiv. 1984;15:360–376. doi:10.1016/0023-9690(84)90003-1 [Google Scholar]
- Iacoboni M. Neural mechanisms of imitiation. Curr. Opin. Neurobiol. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. doi:10.1016/j.conb.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Molnar-Szakacs I., Gallese V., Buccino G., Mazziotta J.C., Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. doi:10.1371/journal.pbio.0030079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A.N.B., Burne T.H.J., Rose S.P.R. Observation learning in day-old chicks using a one-trial passive avoidance learning paradigm. Anim. Behav. 1998;56:1347–1353. doi: 10.1006/anbe.1998.0901. doi:10.1006/anbe.1998.0901 [DOI] [PubMed] [Google Scholar]
- Jones R.B. The nature of handling immediately prior to test affects tonic immobility fear reactions in laying hens and broilers. Appl. Anim. Behav. Sci. 1992;34:247–254. doi:10.1016/S0168-1591(05)80119-4 [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick K.M. Brain asymmetries for face recognition and emotion control in sheep. Cortex. 2006;42:96–98. doi: 10.1016/s0010-9452(08)70328-9. doi:10.1016/S0010-9452(08)70328-9 [DOI] [PubMed] [Google Scholar]
- Koboroff A., Kaplan G., Rogers L.J. Hemispheric specialization in Australian magpies (Gymnorhina tibicen) shown as eye preferences during response to a predator. Brain Res. Bull. 2008;76:304–306. doi: 10.1016/j.brainresbull.2008.02.015. doi:10.1016/j.brainresbull.2008.02.015 [DOI] [PubMed] [Google Scholar]
- Koshiba M., Nakamura S., Deng C., Rogers L.J. Light-dependent development of asymmetry in the ipsilateral and contralateral thalamofugal visual projections of the chick. Neurosci. Lett. 2003;336:81–84. doi: 10.1016/s0304-3940(02)01162-x. doi:10.1016/S0304-3940(02)01162-X [DOI] [PubMed] [Google Scholar]
- Letzkus P., Boeddeker N., Wood J.T., Zhang S.W., Srinivasan M.V. Lateralization of visual learning in the honeybee. Biol. Lett. 2007;4:16–18. doi: 10.1098/rsbl.2007.0466. doi:10.1098/rsbl.2007.0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg, A. 2002 Social influences on the behaviour of laying hens. Doctoral dissertation, Swedish University of Agricultural Sciences.
- Mastrota N.F., Mench J.A. Colour avoidance in northern bobwhites: effects of age, sex and previous experience. Anim. Behav. 1995;50:519–526. doi:10.1006/anbe.1995.0266 [Google Scholar]
- McBride G., Parer I.P., Foenander F. The social organisation of behaviour of the feral domestic fowl. Anim. Behav. Monogr. 1969;2:127–181. [Google Scholar]
- Mench J.A., Andrew R.J. Lateralisation of a food search task in the domestic chick. Behav. Neural Biol. 1986;46:107–114. doi: 10.1016/s0163-1047(86)90570-4. doi:10.1016/S0163-1047(86)90570-4 [DOI] [PubMed] [Google Scholar]
- Mench J., Keeling L.J. The social behaviour of domestic birds. In: Keeling L.J., Gonyou H.W., editors. Social behaviour in farm animals. CABI Publishing; Wallingford, UK: 2001. pp. 177–209. [Google Scholar]
- Moffatt C.A., Hogan J.A. Ontogeny of chick responses to maternal calls in Burmese red junglefowl (Gallus gallus spadiceus) J. Comp. Psychol. 1992;106:92–96. doi: 10.1037/0735-7036.106.1.92. doi:10.1037/0735-7036.106.1.92 [DOI] [PubMed] [Google Scholar]
- Morton J., Johnson M.H. CONSPEC and CONLERN: a two-process theory of infant face recognition. Psychol. Rev. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. doi:10.1037/0033-295X.98.2.164 [DOI] [PubMed] [Google Scholar]
- Ng K.T., et al. Molecular mechanisms of memory formation. Mol. Neurobiol. 1991;5:333–350. doi: 10.1007/BF02935556. doi:10.1007/BF02935556 [DOI] [PubMed] [Google Scholar]
- Nicol C.J. The social transmission of information and behaviour. Appl. Anim. Behav. Sci. 1995;44:79–98. doi:10.1016/0168-1591(95)00607-T [Google Scholar]
- Nicol C.J. Development, direction and damage limitation: social learning in domestic fowl. Learn. Behav. 2004;32:72–81. doi: 10.3758/bf03196008. [DOI] [PubMed] [Google Scholar]
- Nicol C.J., Pope S.J. The effects of demonstrator social status and prior foraging success on social learning in domestic hens. Anim. Behav. 1999;57:163–171. doi: 10.1006/anbe.1998.0920. doi:10.1006/anbe.1998.0920 [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The anatomy and timing of vocal learning in birds. In: Hauser M.D., Konishi M., editors. The design of animal communication. MIT Press; Cambridge, MA: 1999. pp. 63–110. [Google Scholar]
- Orr C.A., Nicholls M.E.R. The nature and contribution of space- and object-based attentional biases to free-viewing perceptual asymmetries. Exp. Brain Res. 2005;162:384–393. doi: 10.1007/s00221-004-2196-3. doi:10.1007/s00221-004-2196-3 [DOI] [PubMed] [Google Scholar]
- Patterson T.A., Gilbert D.B., Rose S.P.R. Pre- and post-training lesions of the intermediate medial hyperstriatum ventrale and passive avoidance learning in the chick. Exp. Brain Res. 1990;80:189–195. doi: 10.1007/BF00228860. doi:10.1007/BF00228860 [DOI] [PubMed] [Google Scholar]
- Paz-y-Miño C.G., Bond A.B., Kamil A.C., Balda R.P. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. doi:10.1038/nature02723 [DOI] [PubMed] [Google Scholar]
- Peirce J.W., Leigh A.E., daCosta A.P.C., Kendrick K.M. Configurational coding, familiarity and the right hemisphere advantage for face recognition in sheep. Neuropsychologia. 2000;38:475–483. doi: 10.1016/s0028-3932(99)00088-3. doi:10.1016/S0028-3932(99)00088-3 [DOI] [PubMed] [Google Scholar]
- Pfeffer K., Fritz J., Kotrschal K. Hormonal correlates of being an innovative greylag goose. Anim. Behav. 2002;63:687–695. doi:10.1006/anbe.2001.1949 [Google Scholar]
- Phillips R.E., Youngren O.M. Unilateral kainic acid lesions reveal dominance of right archistriatum in avian fear behaviour. Brain Res. 1986;377:216–220. doi: 10.1016/0006-8993(86)90861-9. doi:10.1016/0006-8993(86)90861-9 [DOI] [PubMed] [Google Scholar]
- Piaget J. Routledge and Kegan Paul; London, UK: 1928. Judgement and reasoning in the child. [Google Scholar]
- Porter R.H., Roelofsen R., Picard M., Arnould C. The temporal development and sensory mediation of social discrimination in domestic chicks. Anim. Behav. 2005;70:359–364. doi:10.1016/j.anbehav.2004.10.019 [Google Scholar]
- Porter R.H., Arnould C., Simac L., Hild S. Retention of individual recognition in chicks and the effects of social experience. Anim. Behav. 2006;72:707–712. doi:10.1016/j.anbehav.2006.01.021 [Google Scholar]
- Prior H., Wilzeck C. Selective feeding in birds depends on combined processing in the left and the right brain hemisphere. Neuropsychologia. 2008;46:233–240. doi: 10.1016/j.neuropsychologia.2007.07.014. doi:10.1016/j.neuropsychologia.2007.07.014 [DOI] [PubMed] [Google Scholar]
- Prior H., Lingenbauer F., Nitschke J., Guntrukun O. Orientation and lateralized cue use in pigeons navigating a large indoor environment. J. Exp. Biol. 2002;205:1795–1805. doi: 10.1242/jeb.205.12.1795. [DOI] [PubMed] [Google Scholar]
- Queiroz S.A., Cromberg V.U. Aggressive behavior in the genus Gallus sp. Rev. Bras. Cienc. Avic. 2006;8:1–14. doi:10.1590/S1516-635X2006000100001 [Google Scholar]
- Rajecki D.W., Ivins B., Rein J. Social discrimination and aggressive pecking in domestic chicks. J. Comp. Physiol. Psychol. 1976;90:442–452. doi:10.1037/h0077212 [Google Scholar]
- Rashid N., Andrew R.J. Right hemisphere advantage for topographical orientation in the domestic chick. Neuropsycholgia. 1989;27:937–948. doi: 10.1016/0028-3932(89)90069-9. doi:10.1016/0028-3932(89)90069-9 [DOI] [PubMed] [Google Scholar]
- Regolin L. The case of the line-bisection: when both humans and chickens wander left. Cortex. 2006;42:101–103. doi: 10.1016/s0010-9452(08)70330-7. doi:10.1016/S0010-9452(08)70330-7 [DOI] [PubMed] [Google Scholar]
- Regolin L., Vallortigara G., Zanforlin M. Perceptual and motivational aspects of detour behaviour in young chicks. Anim. Behav. 1994;47:123–131. doi:10.1006/anbe.1994.1014 [Google Scholar]
- Regolin L., Marconato F., Vallortigara G. Hemispheric differences in the recognition of partly occluded objects by newly hatched domestic chicks (Gallus gallus) Anim. Cogn. 2004;7:162–170. doi: 10.1007/s10071-004-0208-0. doi:10.1007/s10071-004-0208-0 [DOI] [PubMed] [Google Scholar]
- Reiner A., Perkel D.J., Mello C.V., Jarvis E.D. Songbirds and the revised avian brain nomenclature. Ann. NY Acad. Sci. 2004;1016:77–108. doi: 10.1196/annals.1298.013. doi:10.1196/annals.1298.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds Losin E.A., Russell J.A., Freeman H., Meguerditchian A., Hopkins W.D. Left hemisphere specialization for oro-facial movements of learned vocal signals by captive chimpanzees. PLoS ONE. 2008;3:e2529. doi: 10.1371/journal.pone.0002529. doi:10.1371/journal.pone.0002529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedstra B., Groothuis T.G.G. Early feather pecking as a form of social exploration: the effect of group stability on feather pecking and tonic immobility in domestic chicks. Appl. Anim. Behav. Sci. 2002;77:127–138. doi:10.1016/S0168-1591(02)00031-X [Google Scholar]
- Rogers L.J. CAB International; Wallingford, UK: 1995. The development of brain and behaviour in the chicken. [Google Scholar]
- Rogers L.J. Early experiential effects on laterality: research on chicks has relevance to other species. Laterality. 1997;2:199–219. doi: 10.1080/713754277. doi:10.1080/135765097397440 [DOI] [PubMed] [Google Scholar]
- Rogers L.J. Evolution of hemispheric specialization. Advantages and disadvantages. Brain Lang. 2000;73:236–253. doi: 10.1006/brln.2000.2305. doi:10.1006/brln.2000.2305 [DOI] [PubMed] [Google Scholar]
- Rogers L.J. Development and function of lateralization in the avian brain. Brain Res. Bull. 2008;76:235–244. doi: 10.1016/j.brainresbull.2008.02.001. doi:10.1016/j.brainresbull.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Andrew R.J. Cambridge University Press; Cambridge, UK: 2002. Comparative vertebrate lateralization. [Google Scholar]
- Rogers L.J., Astiningsih K. Social hierarchies in very young chicks. Br. Poult. Sci. 1991;32:47–56. doi: 10.1080/00071669108417326. doi:10.1080/00071669108417326 [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Bolden S.W. Light-dependent development and asymmetry of visual projections. Neurosci. Lett. 1991;121:63–67. doi: 10.1016/0304-3940(91)90650-i. doi:10.1016/0304-3940(91)90650-I [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Deng C. Light experience and lateralization of the two visual pathways in the chick. Behav. Brain Res. 1999;98:277–287. doi: 10.1016/s0166-4328(98)00094-1. doi:10.1016/S0166-4328(98)00094-1 [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Rajendra S. Modulation of the development of light-initiated asymmetry in chick thalamofugal visual projections by oestradiol. Exp. Brain Res. 1993;93:89–94. doi: 10.1007/BF00227783. doi:10.1007/BF00227783 [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Sink H.S. Transient asymmetry in the projections of the rostral thalamus to the visual hyperstriatum of the chicken, and reversal of its direction by light exposure. Exp. Brain Res. 1988;70:378–384. doi: 10.1007/BF00248362. doi:10.1007/BF00248362 [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Vallortigara G. From antenna to antenna: lateral shift of olfactory memory in honeybees. PLoS ONE. 2008;3:e2340. doi: 10.1371/journal.pone.0002340. doi:10.1371/journal.pone.0002340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.J., Workman L. Light exposure during incubation affects competitive behaviour in domestic chicks. Appl. Anim. Behav. Sci. 1989;23:187–198. doi:10.1016/0168-1591(89)90109-3 [Google Scholar]
- Rogers L.J., Andrew R.J., Burne T.H. Light exposure of the embryo and development of behavioural lateralisation in chicks. I. olfactory responses. Behav. Brain Res. 1998;97:195–200. doi: 10.1016/s0166-4328(98)00043-6. doi:10.1016/S0166-4328(98)00043-6 [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Zucca P., Vallortigara G. Advantages of having a lateralized brain. Proc. R. Soc. B. 2004;271:S420–S422. doi: 10.1098/rsbl.2004.0200. doi:10.1098/rsbl.2004.0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa Salva O., Regolin L., Vallortigara G. Chicks discriminate human gaze with their right hemisphere. Behav. Brain Res. 2007;177:15–21. doi: 10.1016/j.bbr.2006.11.020. doi:10.1016/j.bbr.2006.11.020 [DOI] [PubMed] [Google Scholar]
- Rosa Salva, O., Regolin, L. & Vallortigara, G. Submitted. Faces are special for newborn chicks: evidence for inborn domain-specific mechanisms underlying spontaneous preferences for face-like stimuli. [DOI] [PubMed]
- Rosa Salva, O., Daisley, J. N., Vallortigara, G. & Regolin, L. In preparation. Lateralization of social learning in the domestic chick (Gallus gallus): learning to avoid. [DOI] [PubMed]
- Rose S.P.R. God's own organism? The chick as a model system for memory studies. Learn. Mem. 2000;7:1–17. doi: 10.1101/lm.7.1.1. doi:10.1101/lm.7.1.1 [DOI] [PubMed] [Google Scholar]
- Rugani R., Regolin L., Vallortigara G. Rudimental numerical competence in 5-day-old domestic chicks: identification of ordinal position. J. Exp. Psychol. Anim. Behav. Process. 2007;33:21–31. doi: 10.1037/0097-7403.33.1.21. doi:10.1037/0097-7403.33.1.21 [DOI] [PubMed] [Google Scholar]
- Sandi C., Patterson T.A., Rose S.P.R. Visual input and lateralization of brain function in learning in the chick. Neuroscience. 1993;52:393–401. doi: 10.1016/0306-4522(93)90166-d. doi:10.1016/0306-4522(93)90166-D [DOI] [PubMed] [Google Scholar]
- Schwarz I.M., Rogers L.J. Testosterone: a role in the development of brain asymmetry in the chick. Neurosci. Lett. 1992;146:167–170. doi: 10.1016/0304-3940(92)90069-j. doi:10.1016/0304-3940(92)90069-J [DOI] [PubMed] [Google Scholar]
- Sergent J., Signoret J.L. Implicit access to knowledge derived from unrecognized faces in prosopagnosia. Cereb. Cortex. 1992;2:389–400. doi: 10.1093/cercor/2.5.389. doi:10.1093/cercor/2.5.389 [DOI] [PubMed] [Google Scholar]
- Sherwin C.M., Heyes C., Nicol J. Social learning influences the preferences of domestic hens for novel food. Anim. Behav. 2002;63:933–942. doi:10.1006/anbe.2002.2000 [Google Scholar]
- Smith C.L., Evans C.S. Multimodal signaling in fowl, Gallus gallus. J. Exp. Biol. 2008;211:2052–2057. doi: 10.1242/jeb.017194. doi:10.1242/jeb.017194 [DOI] [PubMed] [Google Scholar]
- Sovrano V.A., Andrew R.J. Eye use during viewing a reflection: behavioural lateralisation in zebrafish larvae. Behav. Brain Res. 2006;167:226–231. doi: 10.1016/j.bbr.2005.09.021. doi:10.1016/j.bbr.2005.09.021 [DOI] [PubMed] [Google Scholar]
- Sovrano V.A., Rainoldi C., Bisazza A., Vallortigara G. Roots of brain specializations: preferential left-eye use during mirror-image inspection in six species of teleost fish. Behav. Brain Res. 1999;106:175–180. doi: 10.1016/s0166-4328(99)00105-9. doi:10.1016/S0166-4328(99)00105-9 [DOI] [PubMed] [Google Scholar]
- Sovrano V.A., Bisazza A., Vallortigara G. Lateralization of response to social stimuli in fishes: a comparison between different methods and species. Phys. Behav. 2001;74:237–244. doi: 10.1016/s0031-9384(01)00552-2. doi:10.1016/S0031-9384(01)00552-2 [DOI] [PubMed] [Google Scholar]
- Stokes A.W., Williams D.H.W. Courtship feeding calls in gallinaceous birds. Auk. 1972;89:177–180. [Google Scholar]
- Stone V.E., Nisenson L., Eliassen J.C., Gazzaniga M.S. Left hemisphere representations of emotional facial expressions. Neuropsychologia. 1996;34:23–29. doi: 10.1016/0028-3932(95)00060-7. doi:10.1016/0028-3932(95)00060-7 [DOI] [PubMed] [Google Scholar]
- Strasser R., Ehrlinger J.M., Bingman V.P. Transitive behavior in hippocampal-lesioned pigeons. Brain Behav. Evol. 2004;63:181–188. doi: 10.1159/000076442. doi:10.1159/000076442 [DOI] [PubMed] [Google Scholar]
- Suboski M.D., Bartashunas C. Mechanisms for social transmission of pecking preferences to neonatal chicks. J. Exp. Psychol. Anim. B. 1984;10:182–194. doi:10.1037/0097-7403.10.2.182 [Google Scholar]
- Tang A.C., Verstynen T. Early life environment modulates ‘handedness’ in rats. Behav. Brain Res. 2002;131:1–7. doi: 10.1016/s0166-4328(01)00330-8. doi:10.1016/S0166-4328(01)00330-8 [DOI] [PubMed] [Google Scholar]
- Taylor A., Sluckin W., Hewitt R. Changing colour preferences of chicks. Anim. Behav. 1969;17:3–8. doi: 10.1016/0003-3472(69)90003-7. doi:10.1016/0003-3472(69)90105-5 [DOI] [PubMed] [Google Scholar]
- Tommasi L., Vallortigara G. Encoding of geometric and landmark information in the left and right hemispheres of the avian brain. Behav. Neurosci. 2001;115:602–613. doi:10.1037/0735-7044.115.3.602 [PubMed] [Google Scholar]
- Tommasi L., Vallortigara G. Hemispheric processing of landmark and geometric information in male and female domestic chicks (Gallus gallus) Behav. Brain Res. 2004;155:85–96. doi: 10.1016/j.bbr.2004.04.004. doi:10.1016/j.bbr.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Tuculescu R.A., Griswold J.G. Prehatching interactions in domestic chickens. Anim. Behav. 1983;31:1–10. doi:10.1016/S0003-3472(83)80168-7 [Google Scholar]
- Turati C., Simion F., Milani I., Umiltà C. Newborns' preference for faces: what is crucial? Dev. Psychol. 2002;6:875–882. doi:10.1037/0012-1649.38.6.875 [PubMed] [Google Scholar]
- Uddin L.Q., Iacoboni M., Lange C., Keenan J.P. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn. Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. doi:10.1016/j.tics.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Right hemisphere advantage for social recognition in the chick. Neuropsychologia. 1992a;9:761–768. doi: 10.1016/0028-3932(92)90080-6. doi:10.1016/0028-3932(92)90080-6 [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Affiliation and aggression as related to gender in domestic chicks (Gallus gallus) J. Comp. Psychol. 1992b;106:53–57. doi: 10.1037/0735-7036.106.1.53. doi:10.1037/0735-7036.106.1.53 [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Comparative neuropsychology of the dual brain: a stroll through left and right animals' perceptual worlds. Brain Lang. 2000;73:189–219. doi: 10.1006/brln.2000.2303. doi:10.1006/brln.2000.2303 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Andrew R.J. Lateralization in response to change in a model partner by chicks. Anim. Behav. 1991;41:187–194. doi:10.1016/S0003-3472(05)80470-1 [Google Scholar]
- Vallortigara G., Andrew R.J. Differential involvement of right and left hemisphere in individual recognition in the domestic chick. Behav. Process. 1994;33:41–58. doi: 10.1016/0376-6357(94)90059-0. doi:10.1016/0376-6357(94)90059-0 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L.J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. doi:10.1017/S0140525X05000105 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Zanforlin M. Open-field behavior of young chicks (Gallus gallus): antipredatory responses, social reinstatement motivation, and gender effects. Anim. Learn. Behav. 1988;16:359–362. [Google Scholar]
- Vallortigara G., Cailotto M., Zanforlin M. Sex differences in social reinstatement motivation of the domestic chick (Gallus gallus) revealed by runway tests with social and nonsocial reinforcement. J. Comp. Psychol. 1990;104:361–367. doi: 10.1037/0735-7036.104.4.361. doi:10.1037/0735-7036.104.4.361 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L.J., Bisazza A., Lippolis G., Robins A. Complementary right and left hemifield use for predatory and agonistic behaviour in toads. Neuroreport. 1998;9:3341–3344. doi: 10.1097/00001756-199810050-00035. doi:10.1097/00001756-199810050-00035 [DOI] [PubMed] [Google Scholar]
- Vauclair J., Yamazaki Y., Güntürkün O. The study of hemispheric specialization for categorical and coordinate spatial relations in animals. Neuropsychologia. 2006;44:1524–1534. doi: 10.1016/j.neuropsychologia.2006.01.021. doi:10.1016/j.neuropsychologia.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Ventolini N., Ferrero E.A., Sponza S., Della Chiesa A., Zucca P., Vallortigara G. Laterality in the wild: preferential hemifield use during predatory and sexual behaviour in the black-winged stilt. Anim. Behav. 2005;69:1077–1084. doi:10.1016/j.anbehav.2004.09.003 [Google Scholar]
- Verstynen T., Tierney R., Urbanski T., Tang A. Neonatal novelty exposure modulates hippocampal volumetric asymmetry in the rat. Neuroreport. 2001;12:3019–3022. doi: 10.1097/00001756-200110080-00008. doi:10.1097/00001756-200110080-00008 [DOI] [PubMed] [Google Scholar]
- Zentall T.R. Imitation by animals: how do they do it? Curr. Dir. Psychol. Sci. 2003;12:91–95. doi:10.1111/1467-8721.01237 [Google Scholar]
- Zuberbuhler K., Byrne R.W. Social cognition. Curr. Biol. 2006;16:R786–R790. doi: 10.1016/j.cub.2006.08.046. doi:10.1016/j.cub.2006.08.046 [DOI] [PubMed] [Google Scholar]
- Zucca P., Sovrano V.A. Animal lateralization and social recognition: quails use their left visual hemifield when approaching a companion and their right visual hemifield when approaching a stranger. Cortex. 2008;44:13–20. doi: 10.1016/j.cortex.2006.01.002. doi:10.1016/j.cortex.2006.01.002 [DOI] [PubMed] [Google Scholar]