Abstract
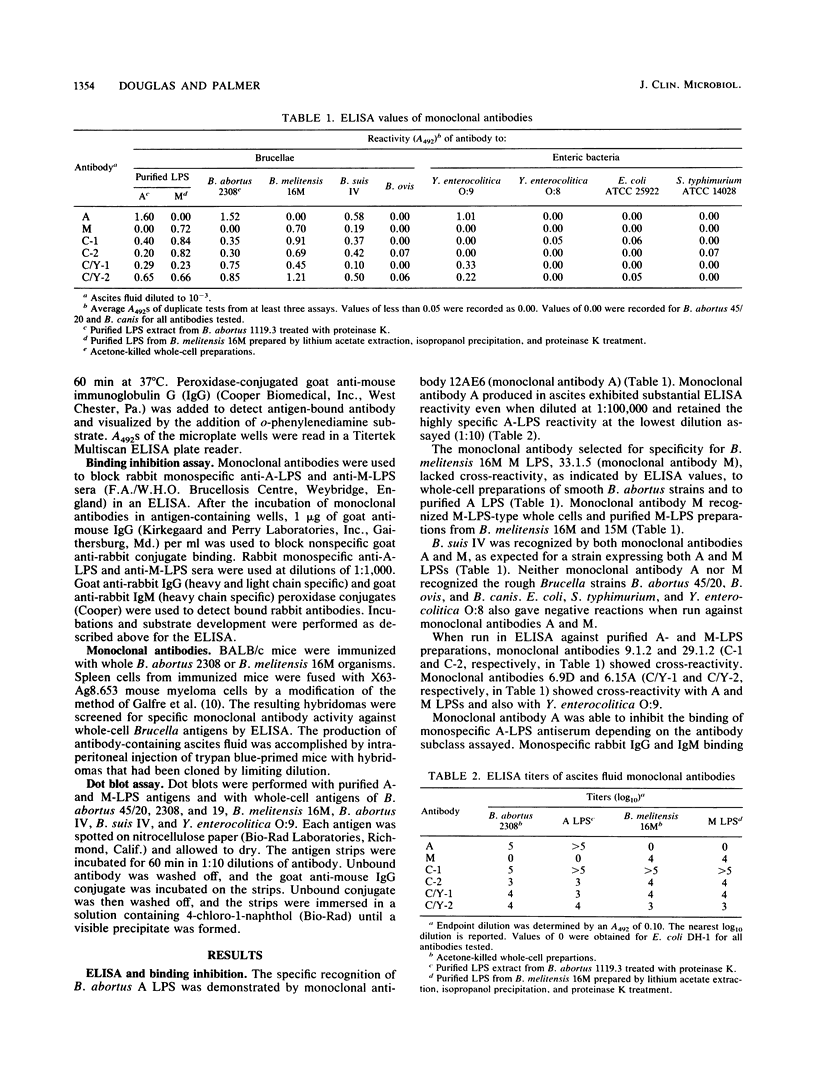
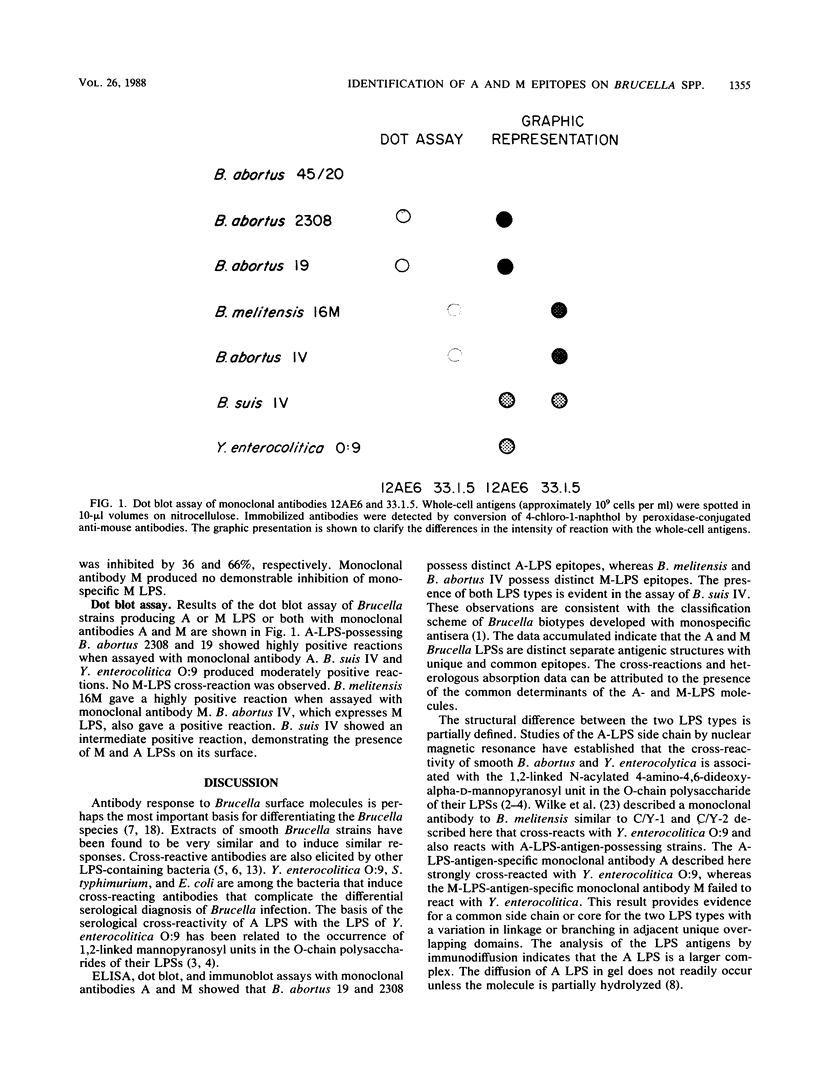
The smooth types of Brucella species express two lipopolysaccharides (LPSs) (A and M) which are antigenically distinct. Their existence as cross-reactive antigenic complexes makes definitive serology difficult. Murine hybridomas were produced and selected for their ability to produce monoclonal antibodies to the specific A- or M-LPS epitopes. The specificity of the monoclonal antibodies was determined by microplate enzyme-linked immunoassay, binding inhibition assay, microplate agglutination, and dot blot assay. Monoclonal antibody 12AE6 was specific for an epitope on the A LPS of Brucella spp., which was also expressed on Yersinia enterocolitica O:9. A unique epitope of M LPS was detected by monoclonal antibody 33.1.5. The two monoclonal antibodies did not exhibit cross-reactions when assayed with whole Brucella cells or purified M- and A-LPS preparations. Cross-reactive serology with polyvalent sera can be attributed to the presence of common antigenic determinants on both molecules. The use of A- and M-LPS-specific monoclonal antibodies has the potential to replace the more laborious methods involved in the production of monospecific sera.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundle D. R., Cherwonogrodzky J. W., Caroff M., Perry M. B. The lipopolysaccharides of Brucella abortus and B. melitensis. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):92–98. doi: 10.1016/0769-2609(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur J Biochem. 1984 Feb 15;139(1):195–200. doi: 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Chukwu C. C. Differentiation of Brucella abortus and Yersinia enterocolitica serotype 09 infections: use of lymphocyte transformation test. Int J Zoonoses. 1985 Jun;12(2):126–135. [PubMed] [Google Scholar]
- Corbell M. J. The serological relationship between Brucella spp., Yersinia enterocolitica serotype IX and Salmonella serotypes of Kauffmann-White group N. J Hyg (Lond) 1975 Aug;75(1):151–171. doi: 10.1017/s0022172400047173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Garatea P., Jones L. M., Moriyon I. Radial immunodiffusion test with a Brucella polysaccharide antigen for differentiating infected from vaccinated cattle. J Clin Microbiol. 1979 Jul;10(1):37–41. doi: 10.1128/jcm.10.1.37-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Leong D., Wilson J. B. Surface antigens of smooth brucellae. J Bacteriol. 1968 Oct;96(4):893–901. doi: 10.1128/jb.96.4.893-901.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. T., Naka S. O., Lee J. W. Development of an ELISA for detection of antibody in leprosy. Int J Lepr Other Mycobact Dis. 1984 Mar;52(1):19–25. [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Greiser-Wilke I., Moennig V., Thon D., Rauter K. Characterization of monoclonal antibodies against Brucella melitensis. Zentralbl Veterinarmed B. 1985 Sep;32(8):616–627. doi: 10.1111/j.1439-0450.1985.tb02002.x. [DOI] [PubMed] [Google Scholar]
- Kreutzer D. L., Robertson D. C. Surface macromolecules and virulence in intracellular parasitism: comparison of cell envelope components of smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):819–828. doi: 10.1128/iai.23.3.819-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Tizard I. R., Barnum D. A. Serological cross-reactions between Brucella abortus and Yersinia enterocolitica 0:9. Int J Zoonoses. 1985 Sep;12(3):219–227. [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T., Boettcher L. A. Biological activities of Brucella abortus lipopolysaccharides. Infect Immun. 1981 Jan;31(1):362–370. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Speth S. L., Jones L. M., Berman D. T. Immunochemical characterization of Brucella lipopolysaccharides and polysaccharides. Infect Immun. 1981 Jan;31(1):214–222. doi: 10.1128/iai.31.1.214-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn R., Campbell A. M., Phillips A. P. A monoclonal antibody specific for the A antigen of Brucella spp. J Gen Microbiol. 1984 Sep;130(9):2285–2289. doi: 10.1099/00221287-130-9-2285. [DOI] [PubMed] [Google Scholar]
- Raybould T. J., Chantler S. Serological differentiation between infected and vaccinated cattle by using purified soluble antigens from Brucella abortus in a hemagglutination system. Infect Immun. 1980 Aug;29(2):435–441. doi: 10.1128/iai.29.2.435-441.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Hammerberg C., Finkler B. R. Monoclonal antibodies to Brucella surface antigens associated with the smooth lipopolysaccharide complex. Am J Vet Res. 1984 May;45(5):967–971. [PubMed] [Google Scholar]
- Sutherland S. An enzyme-linked immunosorbent assay for detection of Brucella abortus in cattle using monoclonal antibodies. Aust Vet J. 1985 Aug;62(8):264–268. doi: 10.1111/j.1751-0813.1985.tb14248.x. [DOI] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L., Ritchie A. E. Isolation and characterization of toxic fractions from Brucella abortus. Infect Immun. 1979 Nov;26(2):668–679. doi: 10.1128/iai.26.2.668-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]




