Abstract
Lateralization of brain and behaviour has been the topic of research for many years in neuropsychology, but the factors guiding its development remain elusive. Based on sex differences in human lateralization, four hypotheses have been postulated that suggest a role for androgens, specifically testosterone. With the discovery that lateralization is a fundamental principle in the organization of brain and behaviour among vertebrates, it has now become possible to experimentally test such hypotheses in animal models. The use of different taxa, humans, other mammalian species and birds (with oestradiol and not testosterone involved in sexual differentiation in birds) facilitates to differentiate between the hypotheses. We used meta-analyses for analysing papers that provided sufficient information, and a semi-quantitative approach based on all relevant studies that we extracted from the literature. We tested the predictions of these hypotheses regarding strength and direction of lateralization for motor output, language and visuospatial cognition in these three taxa. We tested for sex differences and early organizational effects of testosterone (both correlative and experimental studies). We found sex differences in the direction of lateralization for non-human mammals (motor biases similar to humans) and in direction and strength in birds (visual cognitive tasks). However, the prediction that prenatal testosterone exposure affects the direction of lateralization was not supported for humans. In birds and non-human mammals, opposite trends were found, with the effect in non-human mammals being opposite to the expectation based on sex differences. None of the four hypotheses was sufficiently supported and more studies, testing a wider array of functions in different taxa while reporting the data more completely are needed.
Keywords: lateralization, testosterone, meta-analysis, hemispheric dominance, brain asymmetry, development
1. Introduction
Lateralization of brain and behaviour refers to the fact that the two hemispheres of the brain differ in their control of a wide array of functions, while they each predominantly affect the contralateral side of the body. This lateralization has long been thought to exist in humans only, and therefore been the domain of (neuro)psychologists. Although this human-oriented research has yielded many interesting hypotheses and elegant approaches, and revealed much interesting data, it has hampered the experimental testing of hypotheses about the nature, ontogeny and function of lateralization, due to the obvious limitation of especially physiological experimentation with humans and the lack of a comparative approach. One particular area of interest is the epigenesis of lateralization, concerning questions similar to its developmental plasticity, the extent of sensitivity to, and buffering against environmental influences, the interactions of the latter with the genetic make-up of the individual, and the relationship with health and disease. Now that it has become clear that lateralization is not restricted only to humans, but is a fundamental aspect of the organization of brain and behaviour in at least vertebrates (reviewed in Vallortigara 2000; Rogers 2002; Vallortigara & Rogers 2005), testing hypotheses in animal models has also fallen into the realm of biology. This paper aims at exploring the possibilities for such an approach by reviewing the literature on humans and other vertebrate species on one important aspect of the development of lateralization: the influence of steroid hormones.
Hypotheses about the influence of testosterone on lateralization were inspired by sex differences in lateralization of brain and behaviour. In humans, lateralized functions such as spatial orientation, language and hand preferences are thought to display sex differences, not only in performance but also in the direction and strength of lateralized control. The latter is of direct concern here. Males would show more left-handedness, and, in addition, a stronger dominance of the left hemisphere for language while females, although displaying lateralization in the same direction, are less strongly lateralized for language owing to a stronger involvement of the right hemisphere. Finally, although the right hemisphere is in both sexes dominant for visuospatial cognition, males would be stronger lateralized for this function. Although there is large overlap between the sexes in these laterality indices and the sex differences are minor and not always found, they have been confirmed in several studies, including large meta-analyses for language and cognitive functions (Voyer 1996) and a smaller meta-analysis for handedness (Sommer et al. 2008). However, two smaller scale meta-analyses did not confirm a sex difference in language function (Sommer et al. 2004, 2008) and the debate about sex differences in lateralization of brain and behaviour is still ongoing.
Since prenatal exposure to testosterone is well known to organize brain and behaviour, and in mammals, including humans, males are exposed to higher levels of prenatal androgens than females, several hypotheses for the potential influence on lateralization have been postulated. The first hypothesis stated that testosterone in males would decrease the information exchange between the two hemispheres by stimulating axonal pruning in the corpus callosum, leading to less information exchange between the hemispheres and therefore a stronger lateralization of functions (Witelson & Nowakowski 1991). This hypothesis is based on their finding that in males, but not in females, right-handed persons have a smaller corpus callosum than non-right-handers, the latter assumed to be less strongly lateralized for handedness and language. Since this relationship seems to be present only in males, and the formation of the corpus callosum, including cell death and axonal pruning would occur before birth, a role for prenatal exposure to testosterone is suggested. This hypothesis predicts that prenatal exposure to elevated levels of testosterone induces an increase in the strength of lateralization, but would not affect its direction (table 1). This mechanism may be specific for the male sex, but perhaps only so because males show higher levels and more variation of the hormone levels than females, so that elevated levels in females would induce similar effects as in males. This hypothesis is supported by two animal studies. Denenberg et al. (1991) reported in rats, that males have a smaller corpus callosum than females and that its size can be affected by prenatal hormones. Additionally, Rosen (1996) reported that rats with asymmetric brains have a smaller corpus callosum than rats with more symmetric brains.
Table 1.
Expected shifts in strength and hemispheric dominance in laterality when exposed to increasing testosterone levels, according to four different hypotheses. RH, right hemisphere; LH, left hemisphere.
| corpus callosum hypothesis | Geschwind and Galaburda hypothesis | sexual differentiation hypothesis | nonlinearity hypothesis | |||||
|---|---|---|---|---|---|---|---|---|
| direction | strength | direction | strength | direction | strength | direction | strength | |
| handedness | 0 | > | → RH | < | RH | < | RH, LH, RH | >, =, > |
| language | 0 | > | → RH | < | 0 | > | 0 | |
| visuospatial | 0 | > | 0 | > | 0 | > | 0 | |
The second hypothesis and most frequently cited one was proposed by Geschwind & Galaburda (1985). It hypothesizes among others that elevated prenatal exposure to testosterone inhibits the growth of the left hemisphere, inducing compensatory growth in corresponding regions of the right hemisphere. As a consequence, those functions that are dominated by the left hemisphere, such as handedness in right-handers (the majority of people, see Schaafsma et al. 2009) and language, would become either less strongly lateralized, or even dominated by the right hemisphere. This would explain the higher incidence of left-handedness and decreased language lateralization in males relative to females. Visuospatial functions that are dominated by the right hemisphere, would not change in direction, but become even more strongly lateralized by enhanced dominance of that hemisphere (see table 1 for a summary of these predictions). The theory also aims to explain by means of early exposure to androgens, the correlations between left-handedness, developmental disorders and immune diseases, which have received a lot of attention but is not our main concern in this paper.
The third hypothesis postulates that the sex differences in lateralization are due to being part of the process of sexual differentiation, which is in mammals under the influence of testosterone and would masculinize the direction and degree of lateralization (for a review see Grimshaw et al. (1993, 1995), see also Smith & Hines 2000). This hypothesis would yield the same predictions as above, except for the strength and direction of language lateralization. Since males would be more strongly lateralized than females for this function, early exposure to elevated testosterone levels would increase instead of decreasing the strength of language lateralization, and induce no change in its direction (see table 1).
Finally, more recently a fourth hypothesis has been put forward (Lauter 2007). Aiming to explain individual variation in behavioural and brain lateralization, it postulates an important role for individual variation in prenatal exposure to testosterone. It proposes, in contrast to the corpus callosum hypothesis (CCH), that variation in prenatal exposure to testosterone would induce individual variation in pruning of connectivity throughout the brain and in both sexes. This would have a differential effect on both hemispheres, as the left hemisphere develops relatively late and would be more vulnerable to the pruning effects of testosterone. Although laterality is mainly characterized in terms of skills, and not explicitly in terms of strength or direction, the author makes explicit predictions for handedness and fine motor control. Exposure to low levels of testosterone would allow full development and connectivity in both hemispheres, resulting in the full capacity of the left hemisphere, leading to strong right-handedness. Moderate exposure to prenatal testosterone would primarily affect the left hemisphere (note the similarity with the Geschwind and Galaburda hypothesis (GGH)), inducing more ambidexterity and left-handedness. A higher level of exposure to testosterone would also affect the right hemisphere, and in cases of severe overall pruning it would inhibit the supposed coordinating and ‘nurturing of the left brain’ function of the right hemisphere, releasing (over)growth of the left side. This would lead to strong right-handedness again and so the effect of prenatal testosterone exposure is not linear (see table 1).
As summarized in table 1, the four hypotheses make somewhat different predictions for the effect of testosterone on the direction and strength of lateralization of different functions. This can be tested in humans by measuring both direction and strength of lateralization of different functions in relation to testosterone levels, both in normals and in persons exposed to pathologically high or low levels of the hormone. Moreover, the effect of experimental manipulation of hormone levels, not possible in human early development, can be studied in animal models such as primates and other mammals. In addition, using birds as an animal model we have another strategy to compare the hypotheses. First, birds lack a corpus callosum (Cuenod 1974), so that any effect of testosterone on the strength of lateralization in birds cannot be attributed to its effect on a corpus callosum. Second, while sexual differentiation in mammals is primarily under the influence of testosterone, causing masculinization in males, in birds sexual differentiation is under the influence of oestradiol, inducing feminization of females (Schlinger 1998). Although testosterone can be converted to oestradiol by the enzyme aromatase, the enzyme is not very active in male birds, preventing feminization. Therefore, any effect of androgens on lateralization in birds cannot be explained by the sexual differentiation hypothesis (SDH) either.
The four hypotheses deal with the so-called organizational effects of testosterone. Such organizational effects are long-term effects inducing structural and irreversible changes in brain and behaviour during an early phase in development. However, scientists have, sometimes referring to one of the four hypotheses, also looked at the effect of hormones in adulthood. Such effects are not considered to be organizational, but activational, inducing highly reversible changes in brain and behaviour, with waning effects when hormone levels decrease. Although the distinction between organizational and activational effects is not absolute (Arnold & Breedlove 1985), the hypotheses mentioned here explicitly deal with prenatal exposure to testosterone, and therefore we only analysed studies dealing with organizational effects.
Although not always possible, due to limited numbers of studies, we used meta-analyses for reviewing the literature. To explore the scope for the influence of gonadal hormones, we start by presenting a meta-analysis of potential sex differences in lateralization of brain or behaviour. Since excellent reviews on this topic for humans have recently been published (Voyer 1996; Sommer et al. 2004, 2008), we only present the results for non-human mammals and bird species. Next, we present separate analyses for organizational effects of testosterone in humans, other mammalian species and bird species. A surprisingly large amount of published studies could not be used for proper meta-analyses, owing to incomplete statistical information. Therefore, we additionally calculated the number of studies that investigated the effects of sex or testosterone on either strength or direction of lateralization for motor, language and other cognitive functions. With a binomial test, we checked whether the number of studies showing positive or negative effects deviated from random expectation.
2. Material and methods
(a) Literature search: keywords and selection criteria
Literature for the different meta-analyses was searched via Web of Knowledge, with the keyword ‘lateralization’ OR ‘lateralization’, OR ‘asymmetry’ and additional different keywords for every topic. For sex differences: ‘sex difference’ OR ‘gender difference’; for hormone effects: ‘testosterone’ OR ‘hormone’, adding in a separate search ‘CAH’. In addition, we searched the reference lists in the literature found for relevant papers.
For testing sex differences, only those studies were included that tested lateralization of males and females against each other. For the effects of androgens only those studies were included in which the hormone levels were actually measured or manipulated early in ontogeny, plus two categories in the human literature. First, we included one study looking at same and opposite sex twins, assuming, based on extensive animal literature, that a female from opposite sex twins would be exposed to relatively high levels of testosterone, produced by her brother in utero. Second, we included studies on patients with congenital adrenal hyperplasia (CAH), which are exposed to abnormal high androgen levels due to enhanced prenatal production (see Mathews et al. 2004). We did not include patients prenatally exposed to elevated levels of oestrogens (offspring of mothers treated with diethylstilbestrol). Although such female offspring might show masculinization, we were specifically interested in the effects of testosterone and, furthermore, the involvement of oestrogens in human sexual differentiation is not clear. We did include studies on dihydrotestosterone, since this hormone has an even higher affinity to the androgen receptor than testosterone.
Owing to the low number of animal studies that looked at lateralized behaviour in the adult stage, we were forced to include data collected in younger stages. For the human studies we included only those studies that tested lateralization in older children or adults, when lateralization is expected to have become fixed. Furthermore, we only included studies that reported direct measurements of lateralization, such as hand preference, results from visual half-field or dichotic listening tasks, and indices for brain asymmetry. Especially in humans, indirect measurements of lateralization such as a better performance of spatial orientation or language function is often used as an indication of stronger lateralization, but the assumption that performance is directly related to lateralization is not valid (Friederici et al. 2008).
For the direction of lateralization we used, where possible, the standard laterality index (R−L)/(R+L), in which R and L stand for the frequency of right and left performance, respectively. In other cases we were forced to use a slightly different calculation, such as R−L, and this is specified in text, tables and figures. Some authors interpreted these formulae as an indication for strength. However, this is under the assumption that all subjects have the same direction of lateralization, so that a difference in the index is a difference in strength of lateralization. However, evidence for this assumption is not always given, and sometimes obviously not correctly based on the reported data. Therefore, we defined strength as the absolute value of the laterality index.
(b) Meta-analysis procedure
Meta-analyses were carried out using the program Comprehensive Meta Analysis v. 2. Effect sizes, expressed as the correlation coefficient r, were calculated from sample sizes, exact p-values and statistics (F- or t-values and d.f.) which were extracted from the papers or calculated by ourselves when possible. The Χ2 values (e.g. used for testing the proportion of left- against right-handedness) were transformed into phi-correlations (Fern & Monroe 1996; Nakagawa & Cuthill 2007). The sign of the correlation coefficient was assigned as follows: for sex differences in strength and direction, a positive value indicated that males show a higher degree in laterality than females and an increased right-hemispheric bias, respectively. In the analyses of organizational effects of testosterone, a positive value means that high testosterone levels are correlated with or induced increased right-hemispheric dominance.
Results from the three classes of domains presented in table 1 (handedness in humans or motor behaviour in other mammals; language lateralization in humans (e.g. measured by dichotic listening tasks) and on other (visuospatial) cognitive tasks in humans and other animals), were analysed separately because different hypotheses make different predictions for these functions. We analysed the results separately for taxonomic groups: humans, other mammals, and birds (other taxonomic groups did not yield more than one or two studies, but see below). Owing to the small number of studies in the analyses of organizational effects on strength of lateralization, only studies on direction were analysed in the meta-analyses.
In several studies more than one dependent variable was measured. In case these concerned the same function (motor biases, language or visuospatial domains), we used the weighted averages per study for the calculations of overall effect sizes. This is because these are not independent statistical units as they are derived from the same subjects and the same study. We do present them all, however, separately in the tables and graphs. We considered results within one study on males and females (when presented separately) as separate studies since they concern different subjects.
The program also analysed the homogeneity of the dataset to test whether the results can be considered replications of each other. Significant deviations from homogeneity can be due to the inclusion of different tests, or differences in study groups or animals and suggests that further partitioning might be necessary.
In order to control for the stability of overall effects, a fail-safe n-test was carried out for each meta-analysis. This test calculates the amount of studies needed to find overall no significant effect and indicates therefore how stable the overall effect size is.
(c) Additional analyses
A substantial number of studies reported the results in such a way that we could not extract the proper variables for the meta-analyses. This might lead to a bias in the meta-analyses, especially since non-significant results are relatively often incompletely reported. Therefore, we calculated over all studies, including those excluded from the meta-analyses, the number of studies that reported a significant positive or negative effect, or a non-significant effect of sex or hormone exposure, respectively. Similar to the meta-analyses this was calculated for each taxa and functional domain separately. The results of this semi-quantitative approach were tested with a binomial test, testing the number of studies that yielded a sex effect or hormonal effect in either a positive or negative (in the case of strength) or the left or right direction (direction). This was performed under the assumption that with a random distribution the chance of being in one of the two categories (strength: smaller or larger; direction: left or right bias) is 0.50. All studies that we used are listed in tables 2–5, results of the binomial tests are shown in table 6.
Table 2.
Summarizing table of literature search on sex differences of lateralization parameters for motor behaviour in non-human mammals. (Non-bold signs not used in the meta-analyses owing to lack of information; cells lacking a sign, data not statistically tested. L, left-side bias; R, right-side bias; 0, no side bias; if direction of lateralization was similar for both sexes: >, stronger side bias; <, less strong bias; =, equal bias.)
| strength of lateralization | direction of lateralization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| species | test | ♂♂ | ♀♀ | ♂♂ | ♀♀ | summary | authors | year | ||
| rats | T-maze | > | 0 | 0 | ♂♂ show higher strength in motor bias for left or right in T-maze without incentive | Alonso et al. | (1991) | |||
| rats (Purdue Wistar) | neonatal tail posture | L | < | L | males and females show a left side bias in neonatal tail posturing with females showing a stronger bias | Denenberg et al. | (1981) | |||
| horse | preferred forelegobstacle avoidance (o.a.)o.a. while riddenrolling | = | 0L0L | <>< | R0RR | ♂♂ show overall left motor bias (although not always significant), ♀♀ show overall right motor bias (although not always significant); no sex differences in strength (averaged over the four tests) | Murphy et al. | (2005) | ||
| dog | tape removal | L | > | 0 | ♂♂ preferably use the left paw, ♀♀ show a trend to use the right paw, data analysed for first paw used and overall paw use | Quaranta et al. | (2004) | |||
| rats (Purdue Wistar) | neonatal tail posture | L | < | L | males and females show a left side bias in neonatal tail posturing with females showing a stronger bias | Rosen et al. | (1983) | |||
| rats (Sprague–Dawley) | neonatal tail posture | 0 | < | R | females show a significant right side bias (neonatal tail posturing), whereas males show no significant difference between right and left tail posturing | Ross et al. | (1981) | |||
| dog | paw lifting blanket removal food retrieval | = | L | R | ♀♀ preferably use the right paw, ♂♂ the left paw, independent of the test; no sex difference in strength; effect size calculated for all experiments | Wells et al. | (2003) | |||
Table 3.
Summarizing table of literature search on sex differences of lateralization parameters in birds and fishes (for explanation see legend table 1). (C/I=proportion contralateral to ipsilateral projections from eye to brain. RES, right eye system, LES, left eye system.)
| strength of lateralization | direction of lateralization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| species | test | ♂♂ | ♀♀ | ♂♂ | ♀♀ | summary | authors | year | ||
| chicken | visual projections | > | R | 0 | in ♂♂, projections from RES show higher C/I ratio than from LES, no difference in ♀♀ | Adret & Rogers | (1989) | |||
| zebra finch | food discrimination | > | R | R | ♂♂ make less mistakes; both sexes perform better in binocular or RE condition | Alonso | (1998) | |||
| chicken | fear response | > | L | > | 0 | ♂♂ show higher response with LES, no difference in ♀♀ | Andrew & Brennan | (1984) | ||
| pigeon | visual projections | = | R | R | tectal asymmetry show larger cells projecting from RES, no sex differences | Güntürkün | (1997) | |||
| chicken | food discrimination | < | 0 | L | ♀♀ tested with LES made the fewest mistakes, no binocular condition | Mench & Andrew | (1986) | |||
| chicken | visual projections | > | R | R | in ♂♂, projections from RES show higher C/I ratio than from LES; same in ♀♀, but lesser degree | Rajendra & Rogers | (1993) | |||
| chicken | novel object | ≤ | L | L | ♀♀ tend to show stronger asymmetry than ♂♂ | Regolin & Vallortigara | (1996) | |||
| chicken | food discrimination (light incubation) | > | R | 0 | ♂♂ improve learning under binocular and RES condition, no difference between RES and LES in ♀♀ | Rogers | (1997) | |||
| food discrimination (dark incubation) | = | 0 | 0 | no differences between RES and LES in ♂♂ and ♀♀ | ||||||
| chicken | visual projections | > | R | > | R | ♂♂ show significantly stronger asymmetry in visual projections than ♀♀ | Schwarz & Rogers | (1992) | ||
| chicken | visual discrimination/position learning | R | = | R | ♂♂ and ♀♀ learned faster when good box was on their right side; no sex differences | Vallortigara et al. | (1996) | |||
| chicken | position learning | L | > | 0 | ♂♂ make less mistakes when box is placed on their left side; no difference in ♀♀ | Vallortigara et al. | (1988) | |||
| turning behaviour | 0 | 0 | without stimulus, naive males turn 50% left, 50% right, naive females 60% right, 40% left | |||||||
| chicken | food discrimination | > | R | 0 | ♂♂ improve learning under binocular and RES condition, no difference between RES and LES in ♀♀ | Zappia & Rogers | (1987) | |||
| fishes | social detour | L | > | 0 | ♂♂ show left side bias, ♀♀ show tendency towards left side | Brown et al. | (2007) | |||
| control detour | < | 0 | 0 | no side preference in ♂♂ and ♀♀, higher strength in ♀♀ | ||||||
Table 4.
Summarizing table of literature search on organizational effects of androgens on lateralization parameters in humans and other mammals. (CAH=subject with congenital adrenal hyperplasia; SDApc, sexually dimorphic area pars compacta; T, testosterone; DHTP, dihydrotestosterone propionate; TP, testosterone propionate; for further details see tables 1 and 2.)
| strength of lateralization | direction of lateralization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| species | test | high T | low T | high T | low T | summary | authors | year | ||
| human (♂♂ IHH patients) | handednessdichotic listeningsplit visual field | RRR | === | RRR | control group and IHH patients (congenital androgen deficiency) show no significant differences in handedness, dichotic listening or split visual field tasks | Cappa et al. | (1988) | |||
| human (twins) | dichotic listening | R | < | 0 | females from opposite sex twin pairs show right ear bias, females from same sex twin pairs show no side bias | Cohen-Behan et al. | (2004) | |||
| human (10 year old girls) | handednessdichotic listening (FDWT) | RR | >> | RR | testosterone levels in girls are associated with right-handedness and right ear advantage (REA) in a fused dichotic word task (FDWT). | Grimshaw et al. | (1995) | |||
| human (10 year old boys) | handednessdichotic listening (FDWT) | RR | == | RR | in boys, testosterone levels are not correlated with handedness or ear advantage in a dichotic listening task (FDWT) | |||||
| ♀♀ (CAH patients) | handednessdichotic listening task | RR | == | RR | no significant differences between CAH patient and matched controls | Helleday | (1994) | |||
| ♂♂ and ♀♀ (CAH patients) | handedness | R | < | R | CAH patients show a stronger left-handedness bias, when compared with controls, independent of sex | Kelso et al. | (1999, 2000) | |||
| ♂♂ and ♀♀ (CAH patients) | dichotic listening task | = | R | = | R | CAH and control subjects show a right ear bias, no differences in strength of lateralization | Mathews et al. | (2004) | ||
| ♂♂ and ♀♀ (CAH patients) | handedness | = | R | = | R | no differences in handedness between CAH patients and controls | ||||
| ♂♂ (CAH patients) | handedness | R | = | R | significantly more left-handed females in CAH group, no significant differences between control males and CAH patients | Nass et al. | (1987) | |||
| ♀♀ (CAH patients) | handedness | R | < | R | ||||||
| human (male neonates) | grasp reflex | R | > | 0 | right-handedness was positively correlated with testosterone levels in male and female neonates | Tan & Tan | (2001) | |||
| human (female neonates) | grasp reflex | R | > | 0 | ||||||
| ♂♂ and ♀♀ (CAH patients) | dichotic listening task | R | < | 0 | significantly decreased left ear recall resulting in a right side bias in CAH patients | Tirosh et al. | (1993) | |||
| gerbils (♀♀)gerbils (♂♂) | eye openingeye opening | => | RL | => | 00 | intrauterine position influences testosterone levels: offspring are exposed to high T levels between 2 brothers (2M), low T levels between 2 sisters (2F)); 2M males open significantly more often first their left eye than 2F males; 2M females exhibit more frequently primacy right eye opening, but difference is not significant | Clark et al. | (1993) | ||
| rhesus monkey | handedness | > | L | = | L | left-handedness bias in rhesus monkeys in both sexes; no differences between experimental group (elevated hormone levels), control group or group with suppressed hormone levels | Drea et al. | (1995) | ||
| gerbils (♀♀) | SDApc size | L | 0 | ♀♀ emitted after treatment as many ultrasonic sounds as males; left SDApc size increased (same pattern as found in sexually mature ♂♂) | Holman & Hutchinson | (1991) | ||||
| rats (♀♀, DHTP treatment)rats (♀♀, TP treatment)rats (♂♂, DHTP treatment)rats (♂♂, TP treatment) | neonatal tail postureneonatal tail postureneonatal tail postureneonatal tail posture | LRLL | =<== | LLLL | Purdue Wistar rats show a left side bias in neonatal tail posturing with females showing a stronger bias; DHTP treatment during gestation did not change direction of laterality, but TP in females caused a reversal in asymmetry; no effect of treatment in males | Rosen et al. | (1983) | |||
Table 5.
Summarizing table of literature search on organizational effects of gonadal hormones on lateralization parameters in birds. (For details see legend in tables 1 and 2.)
| strength of lateralization | direction of lateralization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| species | hormone | test | treatment | no treatment | treatment | no treatment | summary | authors | year | ||
| chicks (♂♂) | DHT | copulatory behaviour (bi-and monocular) | > | L | > | 0 | dihydrotestosterone (DHT) treated males show stronger asymmetry; than controls; more copulatory attempts with LES than RES, no difference in controls | Bullock & Rogers | (1992) | ||
| chicks (♂♂) | oestradiol | visual projections | 0 | < | R | treated males showed no asymmetries in comparison to control males | Rogers & Rajedra | (1993) | |||
| chicks (♂♂) | testosterone | visual projections | 0 | > | R | treated males showed marginal and reversed asymmetries in comparison to control males | Schwarz & Rogers | (1992) | |||
| chicks (♀♀) | 0 | = | R | treated females showed no asymmetries in comparison to control females | |||||||
| chicks (♂♂) | testosterone | food discrimination (bi- and monocular) | < | 0 | < | R | treated ♂♂ show weaker and reversed asymmetry, when compared to control males | Zappia & Rogers | (1987) | ||
| chicks (♀♀) | 0 | < | R | control and treated ♀♀ did not show superior learning with one eye in monocular test condition; however, treatment negatively effected binocular performance | |||||||
Table 6.
Overview of the results of the meta-analyses and the semi-quantitative approach concerning sex differences in strength and direction of lateralization, and the organizational (org.) effects of androgens, separated for functional domain and species. (Positive r-values for strength: males stronger lateralized than females. Positive r-values for direction: stronger right-hemispheric control in males versus females, or with higher prenatal exposure to testosterone. Semi-quantitative analysis (binomial test) sex differences strength: minus, males less lateralized than females; zero, no difference; plus, males stronger lateralized than females. Sex difference in direction: minus, males show stronger left hemisphere bias than females; zero, no difference; plus, males show stronger right hemisphere bias than females. Organizational effects of hormones: minus, high androgen levels correlated with left hemisphere dominance; zero, no correlation; plus, high androgen levels correlated with right hemisphere dominance. *p<0.10, *p<0.05, **p<0.01, ***p<0.001; ns, not significant.)
| semi-quantitative effect in all studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| meta-analyses | number of studies (n) | effect size (r) | test of significance (Z) | fail safe n-test (fixed factor) | heterogeneity (Q) | − | 0 | + | binomial test |
| sex differences strength | 3 | ||||||||
| mammals motor domain | 3 | 0.252 | 0.882 | 2 | 18.494*** | 0 | 2 | 1 | ns |
| birds cognitive domain | 0 | 1 | 3 | 7 | * | ||||
| sex differences direction | 11 | ||||||||
| mammals motor domain | 7 | 0.160 | 2.078* | 8 | 49.29*** | 2 | 1 | 4 | ns |
| birds cognitive domain | 4 | 0.097 | 2.012* | 6 | 5.61 | 5 | 7 | 2 | ns |
| org. effects of androgens | 22 | ||||||||
| humans, language direction | 6 | −0.070 | −0.940 | 0 | 8.106 | 3 | 4 | 0 | ns |
| humans, handedness direction | 9 | 0.098 | 1.155 | 0 | 18.610* | 3 | 5 | 0 | ns |
| other mammals, motor domain | 5 | −0.102 | −2.549 | 17 | 10.19* | 2 | 4 | 2 | ns |
| birds, cognitive domain | 2 | 0 | 1 | 4 | (*) | ||||
3. Results
(a) Sex differences
(i) Strength of lateralization
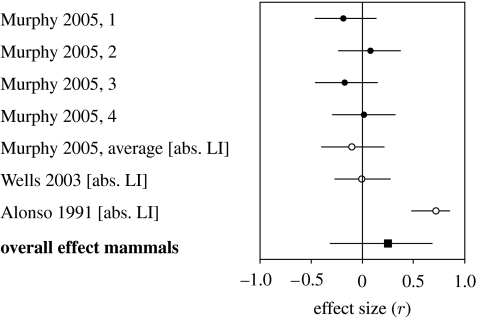
The meta-analysis for sex differences in non-human mammals could only include three studies and concerned motor biases. It showed no significant effects for strength (r=0.251, n=3, p=0.378; figure 1) whereas heterogeneity was significant (p<0.001). Numbers were insufficient for testing the results of the semi-quantitative approach (table 2). For birds, no studies could be used for the meta-analysis. However, 11 studies could be used for the semi-quantitative approach (table 3). These concerned the involvement of either eye (in most studies by occlusion of one eye) in the performance of a variety of tasks, often discrimination learning, and were therefore classified under the cognition domain. The results indicate that males show greater strength than females (table 6; p=0.035).
Figure 1.
Sex differences in the strength of lateralization in mammals. Plotted are effect sizes r±95% CI. Filled circles show results for separate dependent values within one experiment; open circles show averaged weighted effect sizes (r) for each study. Square, overall effect size (r). Positive values indicate higher strength in males than in females. Dependent variables are given in brackets; abs. LI=absolute value of laterality index (R−L)/(R+L).
(ii) Direction of lateralization
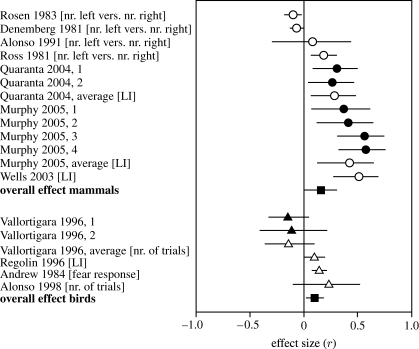
For non-human mammals, the overall sex effect in direction of motor biases is significantly different between males and females (r=0.160, n=7, p=0.038, seven studies, figure 2; table 2). The fail-safe n-test indicated that eight studies with non-significant results must be added to result in an overall p>0.05. Homogeneity of data was however not achieved (p<0.001). The finding that the right hemisphere is more dominant in males than females was not confirmed in the semi-quantitative approach (table 6). However, both approaches used the same studies and the meta-analyses, being more powerful, is more accurate.
Figure 2.
Sex differences in direction of lateralization for mammals (upper part) and birds (lower part). Birds were tested for right or left eye involvement in certain tasks, mostly by occlusion of either eye. Positive values indicate a stronger right-hemispheric bias in males, negative values indicate a stronger left-hemispheric bias in males. Circles indicate motor behaviour and triangles indicate cognitive tasks. For further details see legend to figure 1. [LI]=laterality index: (R−L)/(R+L); [nr. left vers. nr. right]=number of animals with left-side bias were tested against number of animals with right-side bias; [nr. of trials]=number of trials males and females needed to learn a task; [fear response]=number of fear responses.
For birds, the meta-analysis of sex differences in direction for more cognitive related tasks (see above) also revealed a significant difference (r=0.097, n=4, p=0.044; figure 2; table 3). The data show no significant heterogeneity (p=0.132). However, using all, and therefore many more studies in the semi-quantitative approach, binomial testing showed no significant effects (table 6).
(b) Organizational effects of testosterone
(i) Humans
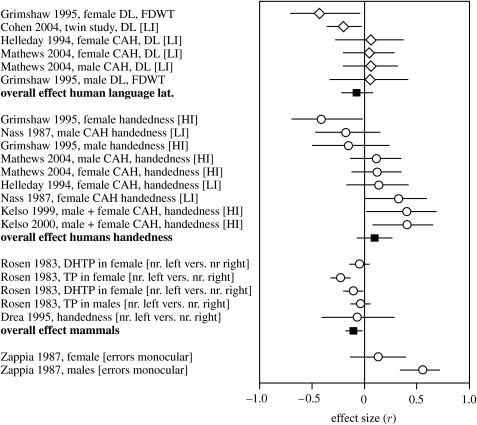
The meta-analysis for the effects of androgens on language lateralization in humans showed no significance (r=−0.070, p=0.347, n=6; figure 3; table 4). Although the two studies on normal subjects showed a tendency towards a stronger involvement of the left hemisphere and studies on CAH patients showed a right-hemispheric bias, the data did not show significant heterogeneity (p=0.150). Using 10 studies, the semi-quantitative approach did not yield a significant effect either, and if at all a trend towards a stronger involvement of the left hemisphere (table 6).
Figure 3.
Organizational effects of gonadal hormones on language lateralization in humans (CAH patients and healthy individuals) (first part); on handedness in humans (CAH patients and healthy individuals) (second part); motor behaviour in other mammals (rhesus monkeys and rats) (third part); and birds (fourth part). Positive values indicate a right-hemispheric dominance (for handedness, motor behaviour and language) with higher levels of testosterone. Circles indicate motor behaviour and/or handedness, diamonds indicate language lateralization (DL, dichotic listening; FDWT, fused dichotic words test). HI, handedness index measured by questionnaires or activity tasks. For further details see legend to figure 1.
The meta-analysis for the effects of androgens on handedness in humans also showed no significant effect (r=0.098, n=9, p=0.248; figure 3; table 4). There was a significant indication for heterogeneity (p=0.017), with most CAH studies showing a trend towards right hemispheric bias, and only one CAH study and both studies on normal subjects towards a left-hemispheric bias. In support of the meta-analysis the semi-quantitative approach did not yield a significant result either.
For the analysis on organizational effects of androgens in non-human mammals, five studies on motor lateralization could be used. The data indicate that in non-human mammals, in contrast to humans, high prenatal androgen exposure is associated with stronger left-hemispheric dominance (figure 3; table 4; r=−0.102, n=5, p=0.011). However, heterogeneity was significant (p=0.037) and the semi-quantitative approach including three more studies did not support the meta-analysis.
For birds, only two values from one study (males and females) could be used (figure 3). Owing to the small sample size, no meta-analysis was conducted. For the semi-quantitative approach we could use six studies, yielding a significant effect of prenatal testosterone on right-hemispheric dominance (table 6).
4. Discussion
The epigenesis of lateralization of brain and behaviour is still far from clear. Several genetic models for the explanation of patterns of inheritance of lateralization of language and hand preference or hand skill in humans have been put forward. However, their explanatory power is limited and scope for an important role for especially perinatal environmental factors exists (reviewed by Schaafsma et al. 2009). For at least two decades, there has been much speculation in the literature about the potential effects of androgens on the development of lateralization of brain and behaviour. Based on meta-analyses of a selection of papers and a semi-quantitative approach based on all relevant studies we extracted from the literature, we tested four specific hypotheses that have been put forward concerning the potential role of androgens on lateralization. We summarized the predictions of each of these hypotheses concerning the influence of prenatal exposure to testosterone on strength and direction of lateralization for motor behaviour, language and (other) cognitive functions (see §1 and table 1). The results are summarized in table 6. Based on this, we will discuss the evidence supporting or undermining the four hypotheses.
The CCH predicts no effect of sex or androgen exposure on direction, but an increase in strength of lateralization for all three domains investigated. Our data, and data from earlier meta-analyses do not support this. We could not find evidence for an effect of sex on strength of lateralization in non-human mammals. However, sample size was very small and only concerns motor biases. There was a significant effect of sex on the strength of cognitive functions in birds in a total of 11 studies. However, this finding does not differentiate between the four hypotheses since three of them have a similar prediction for the degree of lateralization in visuospatial functions. Furthermore, birds lack a corpus callosum, and this result therefore cannot be in support of the CCH. Moreover, our findings that sex and testosterone affect the direction of lateralization were not predicted by the CCH (see below).
Several studies claim to demonstrate an effect on strength whereas they actually do not report direct evidence for this. For example, higher scores for right-handedness might be an indication to a stronger degree but only under the assumption that no left-handers were present in the population. Since such data on direction is not always reported, we could not reliably use such claims, reducing our sample size.
The GGH predicts, in contrast to the CCH, a decrease in strength of lateralization for handedness and language and an increase in strength for visuospatial functions with increasing prenatal testosterone exposure (table 1). Only the latter is partly supported by the sex differences in birds, but, as mentioned before, sample sizes do not allow reliable conclusions.
The number of studies analysing direction of lateralization allow a more reliable test. Both in humans (Voyer 1996; Sommer et al. 2008) and mammals (this study, but only in the meta-analysis and not in the semi-quantitative approach), males display a stronger shift to right-hemispheric dominance than females for handedness. This is consistent with the predictions of the GGH. However, in contrast to this, our meta-analyses do not show that prenatal testosterone enhances this direction of lateralization in humans, neither for handedness nor for language. Moreover, and surprisingly, the meta-analysis for motor lateralization in non-human mammals even showed a significant effect of prenatal testosterone in the other direction than that found for humans. Although this finding is not supported by the semi-quantitative approach, it is intriguing and not consistent with the GGH. However, all these studies concern only one specific rat strain and one primate species, in which control animals already show a right-hemispheric dominance (a left bias for motor behaviour) in contrast to left-hemispheric dominance in motor biases than in other species including humans.
A second finding also undermines the GGH. In the semi-quantitative approach birds show a consistent and almost significant effect of perinatal testosterone exposure on the direction of lateralization opposite to what the hypothesis predicted. But again this result should be interpreted with caution. The tests on laterality concern the preferred use of the left or right eye in a variety of tests and their homology with visuospatial tasks in humans is not always clear. Moreover, the experimental treatment with androgens concern dosages above the normal physiological range of the species (see endogenous embryonic production as reported in Woods et al. 1975; Tanabe et al. 1979; Woods & Brazzil 1981).
The SDH predicts effects of sex and androgens on strength of lateralization (table 1) for which we have, as mentioned above, no sufficient data. It also predicts a stronger right-hemispheric dominance for handedness in males relative to females, and a stronger right-hemispheric dominance for handedness due to prenatal exposure to testosterone (table 1). As described above, we did not find this. Moreover, we found an effect of prenatal exposure to testosterone on motor biases in mammals in the direction opposite expectation (see above, table 6). Furthermore, we found a significant effect of sex and an almost significant effect of testosterone on cognitive functions in birds. Since in birds the sexual differentiation is under the influence of oestrogens and not testosterone itself (Schlinger 1998), this undermines the hypothesis.
The hypothesis by Lauter (2007) that we termed the nonlinearity hypothesis is more difficult to test. First, many hemispheric functions are labelled differently in other literature and explicit predictions about the influence of prenatal testosterone are made for handedness only. Second, the hypothesis postulates a dose-dependent effect of the hormone, which is not analysed in the studies that we used. Assuming that CAH females are exposed to supraphysiological levels of prenatal testosterone, we would expect a differential effect of testosterone in this group versus CAH males or normals. However, there is no indication for this in our dataset (figure 3).
In conclusion, we found some evidence for effects of sex and prenatal testosterone exposure on lateralization in humans, other mammals, and bird species. However, none of the hypotheses were convincingly supported. It can be questioned to what extent motor biases in animals such as paw preference are similar to fine motor control in humans. It is obviously even more debatable whether the tests concerning eye use or visual projections in birds should be classified in the same domain as visuospatial functions in humans. Our study clearly reveals a lack of coordination between the different fields of research working on different taxa. For example, birds are excellent models for studying effects of prenatal exposure to hormones on lateralization since the embryos develop outside the mother's body, facilitating measurement and manipulation of this exposure. However, the effect of this in the adult stage, on motor behaviour, on complex vocalizations and vocal imitation (song birds and parrots), facilitating comparison with humans, has not yet been studied. Comparison between humans and other animals may also be facilitated by measuring lateralization of emotions in relation to sex or hormones, since emotion is a trait that is in evolutionary terms very old, but so far we have only come across one such animal study (on sex differences in lateralization of fear in the domestic chick, Andrew & Brennan 1984). In addition, in all experimental studies care must be taken to manipulate hormone levels within the physiological range. Moreover, a surprising amount of studies did not contain proper data for separating strength and direction of lateralization, nor presented the proper statistical values for using these studies in meta-analyses; severely hampering an adequate overview over the field. We hope that this study will therefore stimulate new studies in this field of research, facilitating a better understanding of the effects of early exposure to androgens on the development of lateralization in humans and other vertebrates.
Acknowledgements
We thank Bernd Riedstra and Sara Schaafsma for valuable discussions, Reint Geuze and two anonymous reviewers for comments on the manuscript, and Jelle Boonekamp for help with the meta-analyses.
Footnotes
One contribution of 14 to a Theme Issue ‘Mechanisms and functions of brain and behavioural asymmetries’.
References
- Adret P., Rogers L.J. Sex difference in the visual projections of young chicks: a quantitative study of the thalamofugal pathway. Brain Res. 1989;478:59–73. doi: 10.1016/0006-8993(89)91477-7. doi:10.1016/0006-8993(89)91477-7 [DOI] [PubMed] [Google Scholar]
- Alonso Y. Lateralization of visual guided behaviour during feeding in zebra finches (Taeniopygia guttata) Behav. Process. 1998;43:257–263. doi: 10.1016/s0376-6357(98)00015-1. doi:10.1016/S0376-6357(98)00015-1 [DOI] [PubMed] [Google Scholar]
- Alonso J., Castellano M.A., Rodriguez M. Behavioral lateralization in rats: prenatal stress effects on sex-differences. Brain Res. 1991;539:45–50. doi: 10.1016/0006-8993(91)90684-n. doi:10.1016/0006-8993(91)90684-N [DOI] [PubMed] [Google Scholar]
- Andrew R.J., Brennan A. Sex-differences in lateralization in the domestic chick. Neuropsychologia. 1984;22:503–509. doi: 10.1016/0028-3932(84)90045-9. doi:10.1016/0028-3932(84)90045-9 [DOI] [PubMed] [Google Scholar]
- Arnold A.P., Breedlove S.M. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm. Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. doi:10.1016/0018-506X(85)90042-X [DOI] [PubMed] [Google Scholar]
- Brown C., Western J., Braithwaite V.A. The influence of early experience on, and inheritance of, cerebral lateralization. Anim. Behav. 2007;74:231–238. doi:10.1016/j.anbehav.2006.08.014 [Google Scholar]
- Bullock S.P., Rogers L.J. Hemispheric-specialization for the control of copulation in the young chick and effects of 5-alpha-dihydrotestosterone and 17-beta-estradiol. Behav. Brain Res. 1992;48:9–14. doi: 10.1016/s0166-4328(05)80133-0. doi:10.1016/S0166-4328(05)80133-0 [DOI] [PubMed] [Google Scholar]
- Cappa S.F., Guariglia C., Papagno C., Pizzamiglio L., Vallar G., Zoccolotti P., Ambrosi B., Santiemma V. Patterns of lateralization and performance levels for verbal and spatial tasks in congenital androgen deficiency. Behav. Brain Res. 1988;31:177–183. doi: 10.1016/0166-4328(88)90021-6. doi:10.1016/0166-4328(88)90021-6 [DOI] [PubMed] [Google Scholar]
- Clark M.M., Robertson R.K., Galef B.G. Intrauterine position effects on sexually dimorphic asymmetries of mongolian gerbils: testosterone, eye opening, and paw preference. Dev. Psychobiol. 1993;26:185–194. doi: 10.1002/dev.420260402. doi:10.1002/dev.420260402 [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan C.C.C., Buitelaar J.K., Van Goozen S.M.H., Cohen-Kettenis P.T. Prenatal exposure to testosterone and functional cerebral lateralization: a study in same-sex and opposite-sex twin girls. Psychoneuroendocrinology. 2004;29:911–916. doi: 10.1016/j.psyneuen.2003.07.001. doi:10.1016/j.psyneuen.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Cuenod M. Commissural pathways in interhemispheric transfer of visual information in the pigeon. In: Schmitt F.O., Worden F.G., editors. The neurosciences: third study program. The MIT Press; Cambridge, MA: 1974. pp. 21–29. [Google Scholar]
- Denenberg V.H., Rosen G.D., Hofmann M., Gall J., Stockler J., Yutzey D.A. Neonatal postural asymmetry and sex-differences in the rat. Dev. Brain Res. 1981;2:417–419. doi: 10.1016/0165-3806(81)90048-1. doi:10.1016/0165-3806(81)90048-1 [DOI] [PubMed] [Google Scholar]
- Denenberg V.H., Fitch R.H., Schrott L.M., Cowell P.E., Waters N.S. Corpus-callosum: interactive effects of infantile handling and testosterone in the rat. Behav. Neurosci. 1991;105:562–566. doi: 10.1037//0735-7044.105.4.562. doi:10.1037/0735-7044.105.4.562 [DOI] [PubMed] [Google Scholar]
- Drea C.M., Wallen K., Akinbami M.A., Mann D.R. Neonatal testosterone and handedness in yearling rhesus monkeys (Macaca mulatta) Physiol. Behav. 1995;58:1257–1262. doi: 10.1016/0031-9384(95)02026-8. doi:10.1016/0031-9384(95)02026-8 [DOI] [PubMed] [Google Scholar]
- Fern E.F., Monroe K.B. Effect size estimates: issues and problems in interpretation. J. Consum. Res. 1996;23:89–105. doi:10.1086/209469 [Google Scholar]
- Friederici A.D., Pannekamp A., Partsch C.J., Ulmen U., Oehler K., Schmutzler R., Hesse V. Sex hormone testosterone affects language organization in the infant brain. Neuroreport. 2008;19:283–286. doi: 10.1097/WNR.0b013e3282f5105a. [DOI] [PubMed] [Google Scholar]
- Geschwind N., Galaburda A.M. Cerebral lateralization: biological mechanisms, associations, and pathology .3. A hypothesis and a program for research. Arch. Neuro-Chicago. 1985;42:634–654. doi: 10.1001/archneur.1985.04060070024012. [DOI] [PubMed] [Google Scholar]
- Grimshaw G.M., Bryden M.P., Finegan J.K. Relations between prenatal testosterone and cerebral lateralization at age 10. J. Clin. Exp. Neuropsychol. 1993;15:39–40. [Google Scholar]
- Grimshaw G.M., Bryden M.P., Finegan J.A.K. Relations between prenatal testosterone and cerebral lateralization in children. Neuropsychology. 1995;9:68–79. doi:10.1037/0894-4105.9.1.68 [Google Scholar]
- Gunturkun O. Morphological asymmetries of the tectum opticum in the pigeon. Exp. Brain Res. 1997;116:561–566. doi: 10.1007/pl00005785. doi:10.1007/PL00005785 [DOI] [PubMed] [Google Scholar]
- Helleday J., Siwers B., Ritzen E.M., Hugdahl K. Normal lateralization for handedness and ear advantage in a verbal dichotic listening task in women with congenital adrenal hyperplasia (CAH) Neuropsychologia. 1994;32:875–880. doi: 10.1016/0028-3932(94)90024-8. doi:10.1016/0028-3932(94)90024-8 [DOI] [PubMed] [Google Scholar]
- Holman S.D., Hutchison J.B. Lateralized action of androgen on development of behavior and brain sex-differences. Brain Res. Bull. 1991;27:261–265. doi: 10.1016/0361-9230(91)90079-y. doi:10.1016/0361-9230(91)90079-Y [DOI] [PubMed] [Google Scholar]
- Kelso W.M., Nicholls M.E.R., Warne G.L. Effects of prenatal androgen exposure on cerebral lateralization in patients with congenital adrenal hyperplasia (CAH) Brain Cogn. 1999;40:153–156. [Google Scholar]
- Kelso W.M., Nicholls M.E.R., Warne G.L., Zacharin M. Cerebral lateralization and cognitive functioning in patients with congenital adrenal hyperplasia. Neuropsychology. 2000;14:370–378. doi: 10.1037//0894-4105.14.3.370. doi:10.1037/0894-4105.14.3.370 [DOI] [PubMed] [Google Scholar]
- Lauter J.L. The EPIC model of functional asymmetries: implications for research on laterality in the auditory and other systems. Front. Biosci. 2007;12:3734–3756. doi: 10.2741/2348. doi:10.2741/2348 [DOI] [PubMed] [Google Scholar]
- Mathews G.A., Fane B.A., Pasterski V.L., Conway G.S., Brook C., Hines M. Androgenic influences on neural asymmetry: handedness and language lateralization in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2004;29:810–822. doi: 10.1016/S0306-4530(03)00145-8. doi:10.1016/S0306-4530(03)00145-8 [DOI] [PubMed] [Google Scholar]
- Mench J.A., Andrew R.J. Lateralization of a food search task in the domestic chick. Behav. Neural. Biol. 1986;46:107–114. doi: 10.1016/s0163-1047(86)90570-4. doi:10.1016/S0163-1047(86)90570-4 [DOI] [PubMed] [Google Scholar]
- Murphy J., Sutherland A., Arkins S. Idiosyncratic motor laterality in the horse. Appl. Anim. Behav. Sci. 2005;91:297–310. doi:10.1016/j.applanim.2004.11.001 [Google Scholar]
- Nakagawa S., Cuthill I.C. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. doi:10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- Nass R., Baker S., Speiser P., Virdis R., Balsamo A., Cacciari E., Loche A., Dumic M.D., New M. Hormones and handedness: left handedness in female congenital adrenal hyperplasia patients. Neurology. 1987;37:711–715. doi: 10.1212/wnl.37.4.711. [DOI] [PubMed] [Google Scholar]
- Quaranta A., Siniscalchi M., Frate A., Vallortigara G. Paw preference in dogs: relations between lateralised behaviour and immunity. Behav. Brain Res. 2004;153:521–525. doi: 10.1016/j.bbr.2004.01.009. doi:10.1016/j.bbr.2004.01.009 [DOI] [PubMed] [Google Scholar]
- Rajendra S., Rogers L.J. Asymmetry is present in the thalamofugal visual projections of female chicks. Exp. Brain Res. 1993;92:542–544. doi: 10.1007/BF00229044. doi:10.1007/BF00229044 [DOI] [PubMed] [Google Scholar]
- Regolin L., Vallortigara G. Lateral asymmetries during responses to novel-coloured objects in the domestic chick: a developmental study. Behav. Process. 1996;37:67–74. doi: 10.1016/0376-6357(95)00076-3. doi:10.1016/0376-6357(95)00076-3 [DOI] [PubMed] [Google Scholar]
- Rogers L.J. Early experimental effects on laterality: research on chicks has relevance to other species. Laterality. 1997;2:199–219. doi: 10.1080/713754277. doi:10.1080/135765097397440 [DOI] [PubMed] [Google Scholar]
- Rogers L.J. Advantages and disadvantages of lateralization. In: Rogers L.J., Andrew R.J., editors. Comparative vertebrate lateralization. Cambridge University Press; New York, NY: 2002. pp. 126–153. [Google Scholar]
- Rogers L.J., Rajendra S. Modulation of the development of light-initiated asymmetry in chick thalamofugal visual projections by estradiol. Exp. Brain Res. 1993;93:89–94. doi: 10.1007/BF00227783. doi:10.1007/BF00227783 [DOI] [PubMed] [Google Scholar]
- Rosen G.D. Cellular, morphometric, ontogenetic and connectional substrates of anatomical asymmetry. Neurosci. Biobehav. R. 1996;20:607–615. doi: 10.1016/0149-7634(95)00073-9. doi:10.1016/0149-7634(95)00073-9 [DOI] [PubMed] [Google Scholar]
- Rosen G.D., Berrebi A.S., Yutzey A., Denenberg V.H. Prenatal testosterone causes shift of asymmetry in neonatal tail posture of the rat. Dev. Brain Res. 1983;9:99–101. doi: 10.1016/0165-3806(83)90114-1. doi:10.1016/0165-3806(83)90114-1 [DOI] [PubMed] [Google Scholar]
- Ross D.A., Glick S.D., Meibach R.C. Sexually dimorphic brain and behavioural asymmetries in the neonatal rat. Proc. Natl Acad. Sci. USA. 1981;78:1958–1961. doi: 10.1073/pnas.78.3.1958. doi:10.1073/pnas.78.3.1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma S.M., Riedstra B.J., Pfannkuche K.A., Bouma A., Groothuis T.G.G. Epigenesis of behavioural lateralization in humans and other animals. Phil. Trans. R. Soc. B. 2009;364:915–927. doi: 10.1098/rstb.2008.0244. doi:10.1098/rstb.2008.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger B.A. Sexual differentiation of avian brain and behavior: current views on gonadal hormone-dependent and independent mechanisms. Annu. Rev. Physiol. 1998;60:407–429. doi: 10.1146/annurev.physiol.60.1.407. doi:10.1146/annurev.physiol.60.1.407 [DOI] [PubMed] [Google Scholar]
- Schwarz I.M., Rogers L.J. Testosterone: a role in the development of brain asymmetry in the chick. Neurosci. Lett. 1992;146:167–170. doi: 10.1016/0304-3940(92)90069-j. doi:10.1016/0304-3940(92)90069-J [DOI] [PubMed] [Google Scholar]
- Smith L.L., Hines M. Language lateralization and handedness in women prenatally exposed to diethylstilbestrol (DES) Psychoneuroendocrinology. 2000;25:497–512. doi: 10.1016/s0306-4530(00)00005-6. doi:10.1016/S0306-4530(00)00005-6 [DOI] [PubMed] [Google Scholar]
- Sommer I.E.C., Aleman A., Bouma A., Kahn R.S. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127:1845–1852. doi: 10.1093/brain/awh207. doi:10.1093/brain/awh207 [DOI] [PubMed] [Google Scholar]
- Sommer I.E., Aleman A., Somers M., Boks M.P., Kahn R.S. Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization. Brain Res. 2008;1206:76–88. doi: 10.1016/j.brainres.2008.01.003. doi:10.1016/j.brainres.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Tan U., Tan M. Testosterone and grasp-reflex differences in human neonates. Laterality. 2001;6:181–192. doi: 10.1080/713754405. doi:10.1080/13576500042000151 [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Nakamura T., Fujioka K., Doi O. Production and secretion of sex steroid-hormones by the testes, the ovary, and the adrenal-glands of embryonic and young chickens (Gallus domesticus) Gen. Comp. Endocr. 1979;39:26–33. doi: 10.1016/0016-6480(79)90189-8. doi:10.1016/0016-6480(79)90189-8 [DOI] [PubMed] [Google Scholar]
- Tirosh E., Rod R., Cohen A., Hochberg Z. Congenital adrenal-hyperplasia and cerebral lateralizations. Pediatr. Neurol. 1993;9:198–201. doi: 10.1016/0887-8994(93)90084-p. doi:10.1016/0887-8994(93)90084-P [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Comparative neuropsychology of the dual brain: a stroll through animals' left and right perceptual worlds. Brain Lang. 2000;73:189–219. doi: 10.1006/brln.2000.2303. doi:10.1006/brln.2000.2303 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L.J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. doi:10.1017/S0140525x05000105 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Zanforlin M., Cailotto M. Right left asymmetry in position learning of male chicks. Behav. Brain Res. 1988;27:189–191. doi: 10.1016/0166-4328(88)90044-7. doi:10.1016/0166-4328(88)90044-7 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Regolin L., Bortolomiol G., Tommasi L. Lateral asymmetries due to preferences in eye use during visual discrimination learning in chicks. Behav. Brain Res. 1996;74:135–143. doi: 10.1016/0166-4328(95)00037-2. doi:10.1016/0166-4328(95)00037-2 [DOI] [PubMed] [Google Scholar]
- Voyer D. On the magnitude of laterality effects and sex differences in functional lateralities. Laterality. 1996;1:51–83. doi: 10.1080/713754209. doi:10.1080/135765096397874 [DOI] [PubMed] [Google Scholar]
- Wells D.L. Lateralised behaviour in the domestic dog, Canis familiaris. Behav. Process. 2003;61:27–35. doi: 10.1016/s0376-6357(02)00161-4. doi:10.1016/S0376-6357(02)00161-4 [DOI] [PubMed] [Google Scholar]
- Witelson S.F., Nowakowski R.S. Left out axons make men right: a hypothesis for the origin of handedness and functional asymmetry. Neuropsychologia. 1991;29:327–333. doi: 10.1016/0028-3932(91)90046-b. doi:10.1016/0028-3932(91)90046-B [DOI] [PubMed] [Google Scholar]
- Woods J.E., Brazzill D.M. Plasma 17-beta-estradiol levels in the chick-embryo. Gen. Comp. Endocr. 1981;44:37–43. doi: 10.1016/0016-6480(81)90353-1. doi:10.1016/0016-6480(81)90353-1 [DOI] [PubMed] [Google Scholar]
- Woods J.E., Simpson R.M., Moore P.L. Plasma testosterone levels in chick-embryo. Gen. Comp. Endocr. 1975;27:543–547. doi: 10.1016/0016-6480(75)90076-3. doi:10.1016/0016-6480(75)90076-3 [DOI] [PubMed] [Google Scholar]
- Zappia J.V., Rogers L.J. Sex-differences and reversal of brain asymmetry by testosterone in chickens. Behav. Brain Res. 1987;23:261–267. doi: 10.1016/0166-4328(87)90026-x. doi:10.1016/0166-4328(87)90026-X [DOI] [PubMed] [Google Scholar]