Abstract
Although geneticists and archaeologists continue to make progress world-wide in documenting the time and place of the initial domestication of a growing number of plants and animals, far less is known regarding the critically important context of coalescence of various species into distinctive sets or complexes of domesticates in each of the world's 10 or more independent centers of agricultural origin. In this article, the initial emergence of a crop complex is described for one of the best-documented of these independent centers, eastern North America (ENA). Before 4000 B.P. there is no indication of a crop complex in ENA, only isolated evidence for single indigenous domesticate species. By 3800 B.P., however, at least 5 domesticated seed-bearing plants formed a coherent complex in the river valley corridors of ENA. Accelerator mass spectrometer radiocarbon dates and reanalysis of archaeobotanical assemblages from a short occupation of the Riverton Site in Illinois documents the contemporary cultivation at 3800 B.P. of domesticated bottle gourd (Lagenaria siceraria), marshelder (Iva annua var. macrocarpa), sunflower (Helianthus annuus var. macrocarpus), and 2 cultivated varieties of chenopod (Chenopodium berlandieri), as well as the possible cultivation of Cucurbita pepo squash and little barley (Hordeum pusillum). Rather than marking either an abrupt developmental break or a necessary response to population-packing or compressed resource catchments, the coalescence of an initial crop complex in ENA appears to reflect an integrated expansion and enhancement of preexisting hunting and gathering economies that took place within a context of stable long-term adaptation to resource-rich river valley settings.
Keywords: agriculture, archaeology, Chenopodium, domestication
Marking a major evolutionary episode in human history, the transition from hunting and gathering to agricultural economies spanned several millennia and occurred independently in 10 or more different world regions, including eastern North America (ENA) (1) (Fig. 1). In each of these independent centers, this long transition began with the initial domestication of a number of indigenous wild progenitor species. These different domesticates eventually were coalesced to form regionally distinctive complexes of domesticates and low-level food production economies. As a result of parallel and often cross-illuminating efforts by geneticists and archaeologists over the past several decades, we are gaining a much clearer idea of where and when domestication of different individual species of plants and animals occurred (3, 4). Much less is currently known, however, about the equally important process that led to numbers of different species being brought together to form coherent distinctive domesticate complexes in different world regions. When did such domesticate complexes initially develop? What was the identity and relative importance of each complex's different constituent species? What can be said regarding the environmental and cultural context of coalescence of these early domesticate complexes in different world regions, and what can be said about the societies that developed them? Combining extant information with new data, this article addresses these key questions and provides a clear picture of the initial emergence of a crop complex in one of the world's best-documented independent centers of domestication—the eastern woodlands of North America.
Fig. 1.
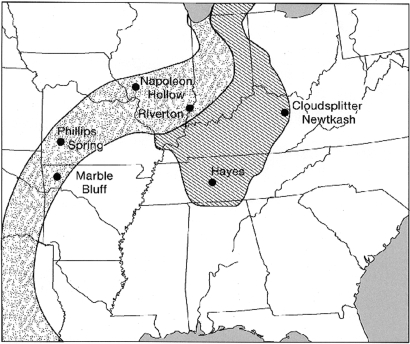
Locations of the late Archaic period archaeological sites discussed in the text. Oak-Savannah and Oak-Hickory Forest Regions at 5000 B.P. are based on ref. 2.
The Temporal and Spatial Context of Initial Plant Domestication in ENA
Based on several morphological changes associated with the adaptive syndrome of domestication that have been documented in seed specimens recovered from 4 Late Archaic period archaeological sites in the Oak-Savannah and Oak-Hickory forest regions of ENA (i.e., seed size increase and reduction in seed-coat thickness), at least 4 indigenous seed-bearing plants were brought under domestication in the region over a span of ≈1200 years from 5000 to 3800 B.P. These plants include squash (Cucurbita pepo ssp. ovifera), sunflower (Helianthus annuus var. macrocarpus), marshelder (Iva annua var. macrocarpa), and chenopod (Chenopodium berlandieri) (1). Maize (Zea mays), the first Mesoamerican domesticate to reach ENA, did not arrive for another 1,500 years, at ≈200 B.C. (see SI Text). In addition to these 4 species that exhibit morphological changes because of domestication, 3 other eastern seed plants that lack such changes have also been identified, based on their abundance in seed assemblages before 2000 B.P., as likely crops and as the subjects of deliberate planting and harvesting of stored seed stock. These plants include erect knotweed (Polygonum erectum), little barley (Hordeum pusillum), and maygrass (Phalaris caroliniana).
The 4 archaeological sites that have yielded the earliest evidence of indigenous domesticated plants in ENA—Phillips Spring, Hayes, Napoleon Hollow, and Riverton—offer a basic temporal and spatial starting point for closer consideration of the composition and nature of the region's initial crop complex (Fig. 1 and Table S1) (1). However, although 3 of these 4 sites provide the earliest evidence for 3 different domesticates, they have unfortunately yielded little additional information regarding other early crop plants or the societies that were cultivating them.
The earliest evidence for an eastern domesticate comes from the Phillips Spring site, which centers on a small artesian spring situated on the 1b terrace of the Pomme de Terre River in south-central Missouri. During excavation of the water-saturated anaerobic sediments adjacent to the spring, a “squash and gourd zone” (Unit K2) was uncovered that contained abundant wild plant remains (hickory, walnut, acorns, grape, elderberry, and ragweed) as well as bottle gourd rind fragments (see SI Text) and 125 uncarbonized C. pepo seeds and seed fragments (5–7). Based on the large size of these squash seeds and a direct date of 5025 calibrated calendar (cal) years B.P. (Table S1) (1), this assemblage provides the earliest evidence for the domestication of this species in ENA. The excavation of the Unit K2 squash and gourd zone, however, was very limited, and little information is available regarding the size or duration of what was the oldest of the 6 stratified living surfaces documented at Phillips Spring.
Similarly, evidence of only a single domesticated species was recovered from a Late Archaic context at the Napoleon Hollow site situated in the lower Illinois River Valley in west-central Illinois. This site is known primarily for its extensive and well-documented Middle Woodland occupations. A series of stratified Middle and Late Archaic period occupations, however, were encountered during excavation of the colluvial fan emanating from the bluff on the north side of Napoleon Hollow where it joins the Illinois Valley (8). One of the pit features associated with the Late Archaic Titterington phase (Feature 20) at Napolean Hollow contained abundant artifacts and plant remains, including Cucurbita rind fragments, chenopod, sunflower, and ragweed seeds, along with 44 carbonized achenes of domesticated marshelder. Based on their large size and a direct date of 4400 cal years B.P., this substantial I. annua var. macrocarpa assemblage provides the earliest evidence for the domestication of this species (Table S1) (1, 9). Given the number and variety of stone tools and the amount of debris recovered during excavation, the Titterington component at Napolean Hollow is considered to have been a seasonally occupied river valley base camp (8).
Like Phillips Spring and Napoleon Hollow, the Hayes site yielded evidence of only a single indigenous domesticate and little additional information. Situated on a T-1 terrace of the Duck River in west-central Tennessee, the Hayes site is a large (≈900 m2) midden with a 1.7-m thick Middle Archaic stratum of freshwater gastropod shells overlain by more than a meter of more recent alluvial deposits, including a Late Archaic shell-free habitation layer (10). A flotation sample recovered from Level 14 (130–140 cm below ground surface) in a small block excavation unit (10), contained 6 complete domesticate-size sunflower seeds, one of which yielded an accelerator mass spectrometer (AMS) radiocarbon date of 4840 cal years B.P. (Table S1) (1, 11). Although both Benton and Ledbetter projectile point/knives were also recovered from the same natural stratum as the sunflower seeds, a fuller description or characterization of the Late Archaic occupation of the Hayes site is not available.
Widely scattered in space and time and representing 3 isolated data points for 3 different indigenous domesticates, the Phillips Spring, Napoleon Hollow, and Hayes sites underscore the difficulties involved in obtaining a clear view of the Late Archaic societies that first combined crop plants into coherent crop complexes and low-level food production economies. At all 3 sites, horizontal exposure of the Late Archaic settlements was limited, and the documentation of individual domesticates was the result of fortuitous preservation of specimens through carbonization or anaerobic soil conditions combined with careful excavation, recovery, and analyses of archaeobotanical assemblages from a single feature or small excavation unit. The same variables of fortuitous preservation and careful recovery and analysis were also evident at the Riverton site located in the Wabash River Valley in southeastern Illinois (Fig. 1), but here the result was much different. At Riverton, a large block excavation unit exposed a substantial area of a settlement dating to 3800 cal years B.P., and unusual preservation contexts provided abundant information regarding utilization of a wide range of wild species of animals and plants as well as evidence for the contemporaneous cultivation of at least 5 (and perhaps as many as 7) different crop plants. These findings allow for a clear view of the composition and coalescence of an initial ENA crop complex.
The Riverton Site and an Initial Crop Complex
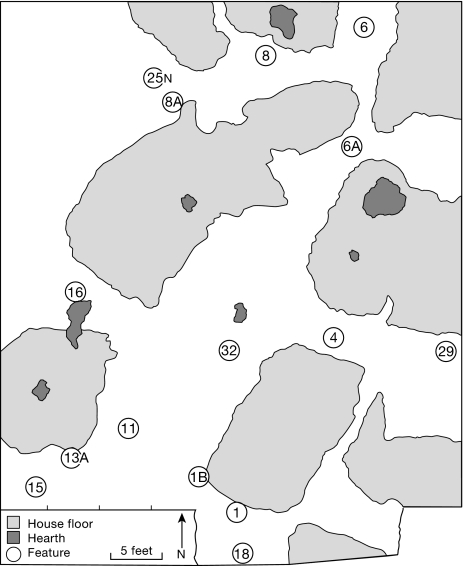
In his landmark study of the Late Archaic Riverton culture, Howard Winters excavated 3 large shell midden sites (Robeson Hills, Swan Island, and Riverton) located along a 20-mi stretch of the Wabash River in southeastern Illinois (12). The Riverton site was a l m deep midden extending over an area of ≈2 acres (≈470 × 220 ft) on a T-0 floodplain terrace. In 1961, five 5- × 5-ft excavation units in 3 separate locations exposed 44 in of cultural deposits, including abundant features and well-preserved material culture assemblages. In 1963, a large block excavation (Unit X) exposed a group of 10 clay floors just below the plow zone, along with associated pits and hearths, artifacts, and extensive midden deposits (Fig. 2).
Fig. 2.
Unit X block excavation at the Riverton site showing the location of house floors, hearths, and features. Species listed for each feature are from ref. 15 (seed counts listed, nut species listed if present). (Feature 1) Midden deposit: 100 “bony” chenopod, 5 carbonized chenopod, 1 cf. Hordeum sp., 4 elderberry, butternut, walnut, hickory, bitternut hickory, hazelnut, acorn. (Feature 1B) Nutshell concentration (3820 cal B.P.): 207 “bony” chenopod, 4 carbonized chenopod, 2 elderberry, butternut, walnut, hickory, hazelnut. (Feature 4) Midden deposit: 1 carbonized chenopod, walnut, hickory, bitternut hickory, hazelnut, acorn. (Feature 6) Midden deposit: 3 carbonized chenopod, walnut, hickory. (Feature 6A) Midden deposit: 200 “bony” chenopod. (Feature 8) Midden deposit: 1 walnut, hickory. (Feature 8A) Nutshell concentration (3690 cal B.P.): 23 carbonized chenopod, 1 sunflower seed, 11 polygonum, 1 persimmon, 2 Cucurbita rind fragments, butternut, walnut, hickory, bitternut hickory, acorn. (Feature 11) Oval storage pit: bitternut hickory. (Feature 13A) Small pit filled with charred nutshell (3810 cal B.P.): 30 “bony” chenopod, 3 Cucurbita rind fragments, butternut, walnut, hickory, bitternut hickory, acorn. (Feature 15) Burned sandstone concentration: 2 “bony” chenopod, 4 carbonized chenopod, bottle gourd rind fragment, butternut, walnut, hickory, bitternut hickory, hazelnut, acorn. (Feature 16) Midden deposit: 2 “bony” chenopod, butternut, walnut, hickory, bitternut hickory. (Feature 18) Burned sandstone concentration: hickory. (Feature 25N) Midden deposit: 4 carbonized chenopod, 1 persimmon, walnut, hickory, bitternut hickory, acorn. (Feature 29) Circular pit filled with fine gray ash (3620 cal B.P.): 8 carbonized chenopod, carbonized marshelder seeds, hickory, acorn. (Feature 32) Midden deposit: 3 carbonized chenopod, walnut, hickory, bitternut hickory, hazelnut, acorn.
Roughly rectangular in shape, the Riverton clay floors were ≈100–200 ft2 in size and from 4 to 6 inches in depth. Like similar features documented in Late Archaic period contexts across the eastern woodlands (13, 14), these clay floors are thought to be prepared house platforms. Surrounding the house floors, dense midden deposits “consisting of a number of lenses of organically stained soil and sterile sand or clay” (12) were initially assigned separate feature numbers but were frequently found to be linked on excavation. Winter's conclusion that the clay floors and associated midden deposits and other features in the Unit X block excavation at Riverton represented a short occupation was recently confirmed by a series of 4 AMS small-sample radiocarbon dates from different locations in Unit X, all of which cluster within a time span of 100–200 years at ≈3700 B.P. (Fig. 2 and Table 1).
Table 1.
Accelerator mass spectrometer radiocarbon dates from block excavation Unit X contexts at the Riverton site
| Feature | Age, AMS-calibrated calendar years B.P. |
Age, radiocarbon years B.P. | Laboratory sample no. | Material dated | |
|---|---|---|---|---|---|
| Intercept | 1σ age range | ||||
| 29 | 3,620 | 3,680–3,660 | |||
| 3,640–3,570 | 3,370 ± 40 | β253115 | Hickory hull | ||
| 8A | 3,690 | 3,810–3,800 | |||
| 3,720–3,640 | 3,440 ± 40 | β253117 | Walnut hull | ||
| 13A | 3,810 | ||||
| 3,800 | |||||
| 3,720 | 3,830–3,690 | 3,480 ± 40 | β253116 | Walnut hull | |
| 1B | 3,820 | ||||
| 3,800 | |||||
| 3,730 | 3,830–3,700 | 3,490 ± 40 | β253114 | Walnut hull | |
Calendar calibrations are based on the Pretoria Calibration Procedure and the Intcal 98 Calibration database.
R.A.Y. participated in the excavation of the Riverton site in August of 1963 and, in the process, obtained 50 soil samples from 24 separate provenience units, including 15 locations associated with the Unit X house floors (12, 15). With the exception of 2 burned sandstone concentrations and 3 pits, these soil samples were all recovered from general midden contexts around the clay platforms (Fig. 2). Subsequent laboratory flotation [and dry screening of a pit (feature 13A)] yielded 43 g of wood charcoal, 322 g of nutshell, 12 g of acorn meat, 635 seeds, and 6 rind fragments of squash/gourd (C. pepo) and bottle gourd (L. siceraria) (15).
In many respects, the Riverton plant assemblage reflects a general pattern of reliance on certain plant species and groups of species that extended over a broad geographical area during the Late Archaic period. Nut-bearing trees, for example, were an important food resource across much of the Oak-Savannah and Oak-Hickory forest regions during the Late Archaic period (9, 16–20), and the Riverton plant assemblage is dominated by nutshells from a variety of tree species, including walnut (Juglans nigra and Juglans cinerea), hickory (Carya sections Carya and Apocarya), hazelnut (Corylus cornuta and Corylus americana), and oak (Quercus sp.) (15).
Similarly, seed-bearing plants appear to play a much less important role than nuts in the East during the Late Archaic period (9, 16–20), and the Riverton plant assemblage contained 641 seeds and rind fragments from seed plants, with 605 of these representing a single species of chenopod (C. berlandieri) (15). Although few in number, the 36 other seeds and rind fragments initially identified in the Riverton assemblage represent 7 different species, 2 of which are clearly domesticated plants. A rind fragment of bottle gourd (L. siceraria) (see SI Text) was recovered from a concentration of burned sandstone (Feature 15), and a single carbonized sunflower kernel (H. annuus var. macrocarpus) was recovered from a concentration of nutshells (Feature 8A). Measuring 5.2 × 3.0 mm (Fig. S1), the sunflower kernel falls within the size range of domestication (20) and, with the exception of the Hayes specimens (Table S1) (1), is the oldest record for this domesticate in ENA.
Two other species initially identified in the seed assemblage may also represent cultivated plants. Five thin Cucurbita rind fragments recovered from a concentration of nutshells and a small pit containing charred nutshells (Fig. 2, Features 8A and 13A) represent either a wild gourd or a domesticate taxa of the same species. Although domesticated squash had already been present in the East for 1,200 years when Riverton was occupied, the large seeds of C. pepo recovered from the Phillips Spring site (Table S1) (1) suggest that the fruits of this domesticate retained a thin rind for many centuries, making it impossible to establish, based exclusively on the thin rind fragments recovered, whether the Riverton specimens represent a wild or domesticated plant (17–20). More tenuously, the single seed recovered from a midden deposit (Fig. 2, Feature 1), identified as likely Hordeum, may represent little barley H. pusillum, which is well-documented as a crop plant in subsequent Early and Middle Woodland period contexts in ENA (20).
In addition to the 2 definite domesticates (sunflower and bottle gourd) and the 2 possible cultivated species (squash and little barley) identified by Yarnell (15), recent reanalysis of the Riverton assemblage has also documented the presence of several additional domesticated seed plants: marshelder (I. annua var. macrocarpa) and chenopod (C. berlandieri). Two carbonized marshelder kernels from a circular pit filled with fine gray ash (Feature 29) fall well into the size range of domestication for the species (4.5 × 2.5 mm; 4.5 × 3.4 mm) (Figs. S2 and S3) and represent the second oldest record for this domesticate in ENA. The C. berlandieri specimens from Riverton, in contrast, represent the earliest record for this domesticate in the East, and 2 distinct cultivated varieties are present, along with a likely companion weed.
Domesticated C. berlanderi at the Riverton Site
Two distinct categories of chenopod seeds were identified during the initial analysis of the Riverton archaeobotanical assemblage. Fifty-five carbonized seeds with a diameter of 1.2–1.6 mm were recovered from 9 Unit X features, and 540 uncarbonized seeds measuring up to 2.4 mm in diameter and having the appearance of bone were recovered from six Unit X features, five of which were clustered in the southwest quadrant of the excavation (Features 1, 1B, 13A, 15, and 16) (15).
Recent reanalysis of the carbonized chenopod seeds revealed the presence of 2 different forms of seed coat or testa thickness. Ten seed-coat-thickness measurements taken on 5 specimens from a concentration of nutshells (Feature 8A) ranged from 12.6 to 15.2 μm, documenting the presence of a domesticated thin-testa cultivated variety (C. berlandieri ssp. jonesianum) in the Riverton seed assemblage (Fig. S4 A and B). In contrast, seeds from the circular pit containing gray ash (Fig. 2, Feature 29) exhibited the rounded margin, reticulate alveolate testa surface patterning, unpronounced beak, and seed-coat-thickness values characteristic of nondomesticated C. berlandieri (Fig. 4 C–E). Eighteen testa-thickness values obtained on 5 seeds from the circular pit (Fig. 2, Feature 29) ranged from 28.3 to 44.7 μm, spanning the 20- to 40-μm gap between range values for the domesticated thin-testa C. berlandieri ssp. jonesianum (<20 μm) and wild populations (>40 μm), indicating the presence of a nondomesticated form of C. berlandieri—either wild, or more likely, a companion weed (17, 18, 20–24).
In addition to the carbonized thin-testa domesticate and wild/companion weed chenopod specimens present in the Riverton assemblage, the 540 uncarbonized and bone-colored or “bony” seeds recovered from Unit X features represent a second cultivated variety of C. berlandieri. More than 400 of these “bony” seeds were found sealed, along with numerous small animal bones, in thin “mineralized” or “calcified” layers in a nutshell concentration and a midden deposit (Features 1B and 6A, respectively) (15). Like the 4 other Unit X Features that yielded uncarbonized cheonopod specimens, both Features 1B and 6A are located along the edge of clay house platforms (Fig. 2), and it is likely that sporadic redeposition of clay from house floors into adjacent midden areas (12) produced a rare and remarkable environment for the preservation of uncarbonized plant remains.
The distinctive color of the uncarbonized chenopod seeds recovered from Riverton reflects yet another change in seed-coat or testa thickness associated with domestication (as opposed to mineralization)—the complete loss of the hard, black outer epiderm. This loss leaves only the thin, translucent inner epiderm layer through which the white perisperm can be observed (25–30). Similar in morphology to a modern Mexican domesticated chenopod, C. berlandieri ssp. nuttalliae cv. “huauzontle,” this pale-seeded or “naked” chenopod was first identified in ENA in a dry rock shelter in Arkansas in contexts dating to ca. A.D. 1200 (25). Gayle Fritz (26–30) subsequently documented this pale-seed crop plant in more than a dozen additional sites in Arkansas, Illinois, and Oklahoma in contexts dating as early as ca. A.D. 300–500.
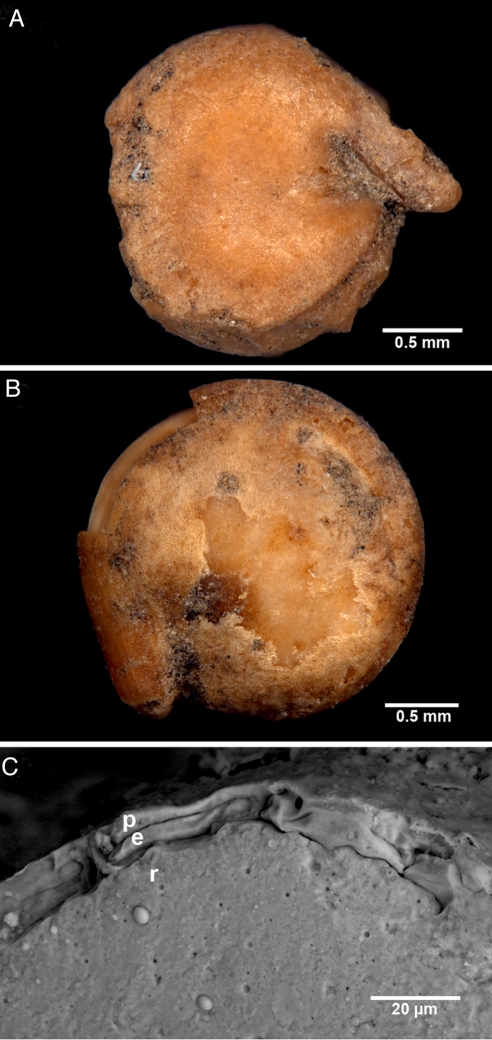
The 2 seeds shown in Fig. 3 A and B are representative of the Riverton pale-seeded specimens in their exhibition of the distinctive golden color of this huauzontle-like cultivated variety of C. berlandieri as well as the truncated seed margin and very prominent “beak” that together distinguish it from both the thin-testa domesticate and the wild and weedy forms of the species (31). Portions of intact pericarp can also be seen in the seeds shown in Fig. 3 A and B. Fig. 3C provides a cross-section of the margin of the Fig. 3B seed, showing the absence of an outer epiderm layer and the resultant surface pericarp directly overlying a thin inner epiderm layer.
Fig. 3.
Riverton Site (Unit X) pale C. berlandieri specimens exhibiting diagnostic domesticate characters: golden color, truncated seed margin, and very prominent “beak.” (A) Fruit from Fig. 2, Feature 13A. (B) Fruit from Fig. 2, Feature 1B. (C) Scanning electron micrograph of cross-section of seed coat structure of B showing pericarp (p), inner epiderm (e), and radicle (r).
The Riverton assemblage of >500 pale-seeded Chenopodium specimens extends the record of this cultivated variety back >1,000 years, to 3800 calendar years B.P., into close agreement with the archaeological record for its thin-testa counterpart, C. berlandieri ssp. jonesianum, and providing additional support for the independent domestication of this species in ENA. Although multiple cultivated varieties of chenopod were also independently developed in 2 other regions of the Americas (South America and Mexico), where they are still grown today, it is only in ENA that there is an extended archaeological record of cultivation of both a naked and thin-testa variety. Domesticated chenopod has yet to be recovered from any pre-Columbian contexts in Mexico (31), and, although considerable morphological variation has been recognized in the domesticated C. quinoa assemblages recovered from sites in the central and south-central Andes as early as 3500 B.P., distinct cultivated varieties have yet to be described (32).
The Developmental Context of an Initial Crop Complex
As a result of both an unusual context of preservation of uncarbonized plant remains and the careful recovery and analysis of multiple archaeobotanical samples (15), the Riverton site provides a rare view of the development of an initial crop complex in ENA. In contrast to earlier sites that yielded evidence of only a single indigenous domesticate from a single context (i.e., Phillips Spring, Napoleon Hollow, and Hayes), Riverton provides evidence for the contemporaneous cultivation of least 5 domesticated seed crops: thin-testa and pale-seeded chenopod, bottle gourd, marshelder, and sunflower. In addition, domesticated C. pepo squash and cultivated little barley may also have been present.
Based both on the overall abundance and relative ubiquity in different features at Riverton, it can also be argued that C. berlandieri played a central role in this initial ENA crop complex. Support for this suggestion is provided both by the extent to which wild C. berlandieri dominates earlier Titterington phase (4400 B.P.) seed assemblages from sites in Illinois (9) as well as the frequency with which domesticated chenopod dominates subsequent Late Archaic and Woodland period seed crop assemblages (17, 18, 20, 25, 26).
In addition to documenting the sustained importance of chenopod, several of these Late Archaic/Early Woodland period sites also provide evidence for the subsequent broad geographical extent and consistent species composition of the initial crop complex documented at Riverton. Excavation of the Marble Bluff Shelter, a small, seasonally occupied site in the Ozarks (Fig. 1), uncovered a rear-wall storage crevice containing twined bags of seeds of domesticated thin-testa chenopod, marshelder, sunflower, and squash dating to ≈3400 B.P (27, 29, 30). Similarly, a rock-lined storage pit (Feature 71) excavated at the Cloudsplitter Rockshelter in eastern Kentucky (Fig. 1) and dating to ≈2800 B.P. contained 7 L of seeds, primarily from domesticated crop plants: thin-testa and pale chenopod, marshelder, sunflower, and squash (28, 33, 34).
In addition to offering the earliest (3800 B.P.) clear evidence for the formation and composition of a chenopod-centered group of domesticates in ENA, the Riverton site also offers insights, at the scale of an individual society, into the larger environmental and cultural contexts within which an initial crop complex was created. Like other contemporary Late Archaic river valley settlements of the Oak-Savannah and Oak-Hickory forest regions of the East, Riverton was occupied by a small-scale society consisting of perhaps a half-dozen related extended family units. Situated along and tethered to first- through third-order tributary river valley corridors of the Mississippi River catchment, these societies followed an annual cycle that linked semipermanent to permanent river valley settlements in river valley locations like Riverton, with a range of other short-term multiple-family and single-family floodplain and upland occupations (13, 14, 19). Clay house floors like those at Riverton as well as post-mold patterns and spatially discrete feature clusters at similar settlements (35) provide occasional glimpses of the spatial structure and size of these settlements, which lack any indication of corporate organization above the level of extended-family domestic units. This picture of associated but largely autonomous extended-family units is also reflected in corporate mortuary sites (36), which, when present, indicate both sustained, long-term, shared “ownership” of a society's resource-catchment area and the absence of any within-group ascribed status differentiation.
The subsistence economies of these small-scale Late Archaic societies remained remarkably stable over several millennia, reflecting sustained and successful long-term adaptations to the resource-rich river valley corridors of the Oak-Savannah and Oak-Hickory Forest Regions of the East. Settlements like Riverton yield evidence of utilization of a wide range of aquatic resources including fish, bivalves, and snails, and the white-tailed deer (Odocoileus virginianus) consistently is the most important terrestrial prey, followed by lesser reliance on a suite of smaller species (e.g., turkey, raccoon, rabbits, and squirrels) (19, 37). The nuts of hickory, walnut, and oak species invariably dominate the plant assemblages of these river valley settlements as they do at Riverton (15), with low seed-to-nut ratios reflecting a lesser reliance on the seeds of wild annual seed-bearing plants (9, 16, 17–18).
When viewed in this larger regional context, the Riverton site represents an excellent society-scale case study example of general environmental and cultural contexts within which an initial crop complex was developed in ENA. The societies involved were small in size, exhibited little internal status differentiation, and maintained stable resource-catchment areas and semipermanent-to-permanent settlements tethered to resource-rich river valley environments. Although the number of river valley settlements increases in the Late Archaic and corporate cemeteries and deep midden deposits reflect the establishment of long-term human utilization and ownership of sections of river valley corridors and adjacent uplands (13, 19, 36), there does not appear to be much, if any, evidence that landscape-packing and resource competition played a causal role in either the initial domestication of eastern seed plants or their coalescence into an initial crop complex. There are many resource-rich river and stream valley settings in the region, for example, that did not witness substantial human occupation during the Late Archaic period (38). In areas where extensive surveys have documented the size and spacing of Late Archaic river valley settlements with a long history of occupation, resource-catchment zones are often quite substantial. The 3 large river valley settlements identified by Winters (12) in the Wabash Valley (Riverton, Swan Island, and Robeson Hills) are spaced at 10-mi intervals, and he estimates that the resource-catchment area on the west side of the river alone for all 3 settlements was 500 mi2.
Rather than provide support for explanatory frameworks that rely on external environmental stress, population growth, landscape-packing, constricted resource zones, and carrying-capacity imbalance in explaining the initial domestication of plants and animals and the subsequent coalescence of domesticate complexes, Riverton and ENA suggest an oppositional conclusion. The initial domestication of local seed plants and the subsequent formation of a crop complex does not appear to have occurred in response to any carrying-capacity challenges or seriously compressed and compromised resource-catchment areas. Rather, this domestication seems to have taken place within a context of stable, long-term adaptations to resource-rich environmental settings. In addition, the initial coalescence of these domesticate complexes and the associated emergence of low-level food production economies do not appear initially to have marked an abrupt developmental break; rather, they appear to have represented an integrated additive expansion and enhancement of preexisting hunting and gathering economies (17). As the temporal, environmental, and cultural contexts of the initial coalescence of domesticate complexes come into clearer focus in other regions of the world, it will be interesting to see to what extent this major transition parallels what we now know about ENA.
Supplementary Material
Acknowledgments.
We thank Maria Bruno, Gary Crawford, Gayle Fritz, Kristen Gremillion, and Joyce Marcus for reading and commenting on earlier versions of this article. Figs. 1 and 2 were drawn by Marcia Bakry. Photographs in Fig. 3. and Figs. S1–S4 were taken and compiled by Scott Whittaker of the Scanning Electron Microscopy Laboratory at the Smithsonian National Museum of Natural History (Washington, DC).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 6427.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901846106/DCSupplemental.
References
- 1.Smith BD. Eastern North America as an independent center of plant domestication. Proc Natl Acad Sci USA. 2006;103:12223–12228. doi: 10.1073/pnas.0604335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delcourt PA, Delcourt HR. In: Geobotany II. Romans R, editor. New York: Plenum; 1981. pp. 123–165. [Google Scholar]
- 3.Smith BD. Documenting plant domestication: The consilience of biological and archaeological approaches. Proc Natl Acad Sci USA. 2001;98:1324–1326. doi: 10.1073/pnas.98.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeder MA, Emshwiller E, Bradley DG, Smith BD. Documenting domestication: The intersection of genetics and archaeology. Trends Genet. 2006;22:139–155. doi: 10.1016/j.tig.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Kay M, King FB, Robinson K. Cucurbits from Phillips Spring: New evidence and interpretations. Am Antiq. 1980;45:807–822. [Google Scholar]
- 6.Kay M. In: Archaic Hunters and Gatherers in the American Midwest. Phillips JL, Brown JA, editors. Orlando, FL: Academic; 1983. pp. 41–70. [Google Scholar]
- 7.Kay M. In: Foraging, Collecting, and Harvesting: Archaic Period Subsistence and Settlement in the Eastern Woodlands. Neusius SW, editor. Carbondale, IL: Southern Illinois Univ; 1986. pp. 275–288. [Google Scholar]
- 8.Wiant MD, Farnsworth KB, Hajic ER. In: Archaic Societies: Diversity and Complexity Across the Midcontinent. Emerson TE, McElrath DL, Fortier AC, editors. Albany, NY: State University Press; 2009. pp. 229–285. [Google Scholar]
- 9.Asch D, Asch N. In: Prehistoric Food Production in North America. Ford RI, editor. Ann Arbor, MI: Univ of Michigan Museum of Anthropol; 1985. pp. 149–203. [Google Scholar]
- 10.Crites GD. Middle and late Holocene ethnobotany of the Hayes site. Midcon J Archaeol. 1987;12:3–32. [Google Scholar]
- 11.Crites GD. Domesticated sunflower in fifth millennium B.P. Temporal context: New evidence from middle Tennessee. Am Antiq. 1993;58:146–148. [Google Scholar]
- 12.Winters H. The Riverton Culture. Springfield, IL: Illinois State Museum; 1969. [Google Scholar]
- 13.Smith BD. In: Advances in World Archaeology. Wendorf F, Close A, editors. Vol 5. Orlando, FL: Academic; 1986. pp. 1–92. [Google Scholar]
- 14.Sassaman K, Ledbetter RJ. In: Archaeology of the Mid-Holocene Southeast. Sassaman K, Anderson D, editors. Gainesville, FL: Univ Press of Florida; 1996. pp. 75–96. [Google Scholar]
- 15.Yarnell RA. In: Aboriginal Ritual and Economy in the Eastern Woodlands. Cantwell AM, Conrad LA, Reyman JE, editors. Springfield, IL: Illinois State Museum; 2004. pp. 123–130. [Google Scholar]
- 16.Johannessen S. In: The Go Kart North Site. Fortier A, editor. Urbana, IL: Univ of Illinois; 1984. pp. 166–181. [Google Scholar]
- 17.Crawford GW. Late Archaic plant remains from west-central Kentucky: A summary. Midcont J Archaeol. 1982;7:205–224. [Google Scholar]
- 18.Crawford GW. In: Archaeology of the Middle Green River Region, Kentucky. Marquardt W, Watson PJ, editors. Gainesville, FL: Univ Press of Florida; 2005. pp. 181–212. [Google Scholar]
- 19.Marquardt W, Watson PJ, editors. Archaeology of the Middle Green River Region, Kentucky. Gainesville, FL: Univ Press of Florida; 2005. [Google Scholar]
- 20.Smith BD. Rivers of Change. 3rd Ed. Tuscaloosa, AL: Univ of Alabama; 2006. [Google Scholar]
- 21.Wilson HD, Heiser CH. The origin and evolutionary relationships of ‘huauzontle’ (Chenopodium nuttalliae Safford) Am J Bot. 1979;66:198–206. [Google Scholar]
- 22.Wilson HD. Quinua and relatives. Econ Bot. 1990;44:92–110. [Google Scholar]
- 23.Gremillion KJ. The evolution of seed morphology in domesticated chenopodium: An archaeological case study. J Ethnobiol. 1993;13:149–169. [Google Scholar]
- 24.Gremillion KJ. Crop and weed in prehistoric eastern North America: The Chenopodium example. Am Antiq. 1993;56:496–509. [Google Scholar]
- 25.Wilson H. Domesticated Chenopodium of the Ozark bluff dwellers. Econ Bot. 1981;35:233–239. [Google Scholar]
- 26.Fritz G. Identification of cultigen amaranth and chenopod from rockshelter sites in northwest Arkansas. Am Antiq. 1984;49:558–572. [Google Scholar]
- 27.Fritz G. PhD dissertation. Chapel Hill, NC: Univ of North Carolina; 1986. Prehistoric Ozark agriculture: The University of Arkansas rockshelter collections. [Google Scholar]
- 28.Fritz G. Multiple pathways to farming in precontact eastern North America. J World Prehist. 1990;4:387–435. [Google Scholar]
- 29.Fritz G. In: Agricultural Origins and Development in the Midcontinent. Green W, editor. Iowa City, IA: Univ of Iowa Office of the State Archaeol; 1994. pp. 105–126. [Google Scholar]
- 30.Fritz G. In: People, Plants, and Landscapes. Gremillion K, editor. Tuscaloosa, AL: Univ of Alabama; 1997. pp. 42–62. [Google Scholar]
- 31.Glore AG. PhD dissertation. St. Louis, MO: Washington Univ; 2006. Domesticated Chenopodium in North America: Comparing the past and the present. [Google Scholar]
- 32.Bruno M. In: Documenting Domestication: New Genetic and Archaeological Paradigms. Zeder MA, Emshwiller E, Bradley D, Smith BD, editors. Berkeley: Univ of California; 2006. pp. 32–45. [Google Scholar]
- 33.Cowan CW. PhD dissertation. Ann Arbor, MI: Univ of Michigan; 1984. From foraging to incipient food production. [Google Scholar]
- 34.Gremillion KJ. Changing roles of wild and cultivated plant resources among early farmers of eastern Kentucky. Southeastern Archaeo. 1998;17:140–157. [Google Scholar]
- 35.Fortier A. In: Archaic Hunters and Gatherers in the American Midwest. Phillips JL, Brown JA, editors. Orlando, FL: Academic; 1983. pp. 243–260. [Google Scholar]
- 36.Charles D, Buikstra J. In: Archaic Hunters and Gatherers in the American Midwest. Phillips JL, Brown JA, editors. Orlando, FL: Academic; 1983. pp. 117–145. [Google Scholar]
- 37.Parmalee P. In: The Riverton Culture. Winters H, editor. Springfield, IL: Illinois State Museum; 1969. pp. 139–144. [Google Scholar]
- 38.Claassen CP. In: Archaeology of the Mid-Holocene Southeast. Sassaman K, Anderson D, editors. Gainesville, FL: Univ Press of Florida; 1996. pp. 235–258. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.