Abstract
When food availability is restricted to a particular time each day, mammals exhibit food-anticipatory activity (FAA), a daily increase in locomotor activity preceding the presentation of food. Considerable historical evidence suggests that FAA is driven by a food-entrainable circadian clock distinct from the master clock of the suprachiasmatic nucleus. Multiple food-entrainable circadian clocks have been discovered in the brain and periphery, raising strong expectations that one or more underlie FAA. We report here that mutant mice lacking known circadian clock function in all tissues exhibit normal FAA both in a light–dark cycle and in constant darkness, regardless of whether the mutation disables the positive or negative limb of the clock feedback mechanism. FAA is thus independent of the known circadian clock. Our results indicate either that FAA is not the output of an oscillator or that it is the output of a circadian oscillator different from known circadian clocks.
Keywords: food-anticipatory activity, mouse genetics
When food availability is limited to a several-hour interval at a particular time each day, mammals quickly develop a new component of daily behavioral activity, a second period of arousal and increased locomotor activity that occurs shortly before the time of daily food presentation (1). This so-called food-anticipatory activity (FAA) is robust, stable over many daily cycles, and occurs even if the time of food availability lies within the light phase of the light–dark cycle, the resting period of the daily behavioral cycle for a nocturnal laboratory animal. Because feeding strategies are fundamental to survival, it is thought that FAA represents an evolutionary adaptation providing mammals with a highly flexible and efficient food-seeking program, one that is able, if necessary, to take advantage of food sources available at unusual times with respect to the animal's typical daily rest–activity cycle (2).
The mechanisms underlying FAA have been under investigation for nearly 30 years. The accumulated evidence suggests that FAA is more likely to be generated by a food-entrainable circadian oscillator than by plausible alternatives, such as a passive hourglass mechanism or an associative memory process (1). In general, an unequivocal demonstration that a rhythmic process is driven by an oscillator requires observation of the persistence of the rhythm over multiple cycles following removal of the entraining stimulus. In the case of daily rhythms entrained to a light–dark cycle, testing for a postulated underlying circadian oscillator simply requires switching off the lights and monitoring the daily rhythms for persistence in constant darkness. For example, daily rhythms of locomotor activity, feeding, and drinking in a light–dark cycle persist indefinitely after a transition to constant darkness, and were thus long ago demonstrated to be driven by a light-entrainable circadian oscillator (3). In contrast, a compelling demonstration that FAA relies on a self-sustained, food-entrainable oscillator has been difficult to obtain because laboratory animals cannot generally tolerate more than a few days of food deprivation. A further complication is that FAA is masked by a change from scheduled daily feeding to constant food availability (4), precluding demonstration of free-running rhythms of FAA under this condition as well.
To bypass these obstacles, a number of studies have examined the responses of FAA to acute shifts in the timing of food availability or alterations in its periodicity to probe the nature of the underlying processes. Although complex and in some ways puzzling, the results from these indirect paradigms make a reasonable circumstantial case that FAA is driven by a food-entrainable oscillator with a period in the circadian range (1). FAA is robust after a complete bilateral lesion of the suprachiasmatic nucleus (SCN) (5), indicating that it does not depend on the master clock driving circadian rhythms of behavioral activity under constant food availability. Thus was born the arduous search for the site of the postulated food-entrainable circadian clock underlying FAA (6–14), a clock that is privileged, like that of the SCN, to govern overt behavior, endowing it with particular importance.
The terms of this search were abruptly transformed by the discovery, made possible by the initial identification of molecular components of the clock, that circadian clocks are widely distributed in mammals, rather than being restricted to a few special sites, like the SCN and retina. Cultured cell lines, essentially all peripheral tissues, and multiple brain regions were found to have intrinsic circadian clocks capable of autonomous function (15–19). Especially striking was the finding that these distributed circadian clocks exhibit robust entrainment to scheduled daily feeding, with the notable exception being the SCN clock, which is readily entrained to light–dark cycles but has been proven to be impervious to manipulations of feeding time (17, 18, 20).
Thus, the field went suddenly from rags to an embarrassment of riches. Previously, it had seemed that identifying a hypothetical food-entrainable oscillator would be tantamount to discovering the circadian clock that drives FAA. Now, we know not only that food-entrainable circadian clocks exist but also that they are widespread in the brain and virtually ubiquitous in the periphery. In principle, any such clock could drive FAA.
As part of a broad study of the physiological functions of circadian clocks outside the SCN, we recently constructed a mouse line with a targeted conditional allele of the Bmal1 gene, allowing tissue-specific genetic ablation of circadian clock function (21, 22). One of our early plans for this system was to generate mice selectively lacking clock function in all CNS neurons, in all peripheral tissues, or in particular CNS structures or peripheral tissues to determine in an unbiased fashion which circadian clocks were important for FAA. Unexpected results in routine preliminary studies for this intended project led to the detailed investigations described below of FAA in mice with defective circadian clocks in all tissues. Contrary to widely held views, the results of our genetic studies indicate that FAA is entirely independent of the known circadian clock.
Results
Our planned conditional genetic analysis of FAA was based on the assumption that it is driven by at least one of the many food-entrainable circadian clocks discovered recently. To confirm this assumption, we performed a preliminary study of FAA in mice (N5, C57BL/6) lacking Bmal1 (Mop3), a core clock component acting in the positive limb of the circadian clock feedback loop (23) that is required for clock function (24). If correct, the assumption predicts that Bmal1−/− mice, lacking circadian clocks in all tissues, should fail to develop FAA when placed under temporal food restriction, whereas their wild-type littermates should show normal FAA. Because at about 14 weeks of age Bmal1−/− mice develop an arthropathy and begin in some cases to exhibit decreased locomotor mobility (25), we used mice aged 7–9 weeks at the start of the experiment. Initially, we used a paradigm for FAA in which animals were shifted acutely from constant food availability to a single, brief window of daily food availability (4); in this case, a 3-h window. To our surprise, Bmal1−/− mice, but not wild-type littermates, became sluggish, then moribund, and ultimately suffered a mortality rate of 80% during the first 7–10 days of the experiment. Subsequently, this outcome became less mysterious with the finding that Bmal1−/− mutants and mice with mutations in other circadian clock genes have defects in glucose homeostasis, lipid metabolism, and energy balance (22, 26, 27), providing a plausible context for the selective vulnerability of Bmal1−/− mice to a sudden reduction in the duration of food availability.
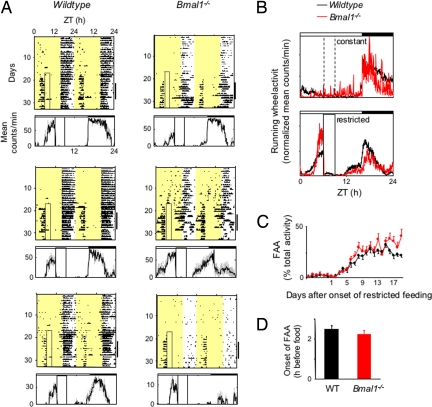
We then adopted a more gentle temporal food restriction paradigm, in which the duration of daily food availability was decreased gradually (28) from constant to a final 3-h window over a 4-day interval (Fig. S1). Under these conditions, both Bmal1−/− mice and wild-type littermates survived, appeared active and healthy, and exhibited robust behavior over the entire course of the experiments, which were 6–8 weeks in duration. Contrary to our expectations, Bmal1−/− mice exhibited robust and stable FAA, essentially identical to that of wild-type littermates (Fig. 1). Even in those several cases in which Bmal1−/− mice exhibited low baseline levels of running-wheel activity, FAA was nonetheless robust (Fig. 1A Lower Right).
Fig. 1.
Normal FAA in Bmal1−/− mice. (A) Representative double-plotted actograms of daily running-wheel activity of 3 Bmal1−/− mice and 3 wild-type littermates (as indicated) during constant food availability and under subsequent temporal restricted feeding. The boxed area toward the left side of each actogram indicates the daily interval of food availability under temporal food restriction, and yellow areas indicate time of lights-on (16:8 light–dark cycle in these experiments). Under a light–dark cycle, Bmal1−/− mice show a diurnal variation in locomotor activity because of the acute suppressive effect of light on the behavior (masking). For clarity, the 4-day gradual narrowing of the interval of food availability is not included in the boxed area (Fig. S1). The graphs below each plot show the profile of daily running-wheel activity for each animal, averaged across 7 days during temporal food restriction (marked by the black bar on the right of each actogram). Gray vertical lines represent ± SEM. A similar FAA was observed with Bmal1−/− mice in a 12:12 light–dark cycle. ZT, Zeitgeber time. (B) Mean locomotor activity profiles of Bmal1−/− mice and wild-type littermates (n = 10 for each genotype) under constant food availability (Upper) and after subsequent temporal food restriction (Lower). Individual mean 7-day profiles have been normalized by total daily activity so that each animal contributes equally to the shape of the profile; each data point represents normalized counts per minute averaged across a 6-min bin (mean ± SEM). Time of light–dark cycle is indicated by white (light) and black (dark) bars at the top of each panel. Broken vertical lines (Upper) indicate, for comparison, the daily interval corresponding to subsequent restricted food availability; solid vertical lines (Lower) indicate the daily interval of food availability under temporal food restriction. (C) Time course of the development of FAA in Bmal1−/− mice (n = 22) and wild-type littermates (n = 20). Shown is the daily percentage of running-wheel activity (mean ± SEM) allocated to a 3-h time interval, ZT3–6. After 4 days of a gradually narrowing window of food availability, the final temporal food restriction started on day 1 (food available from ZT6–9). (D) Number of hours by which FAA anticipated daily food availability in Bmal1−/− mice and wild-type littermates (n = 20 for each genotype). For each animal, the running-wheel activity profile was averaged over 7 consecutive days during stable temporal food restriction (as in B), and the average time of onset of FAA was defined as the time before food presentation at which the FAA peak rose to its half-maximum (mean ± SEM). The difference between genotypes is not statistically significant.
Detailed and quantitative aspects of FAA were preserved in mice lacking Bmal1. The normalized mean FAA profiles of the 2 populations after temporal food restriction were similar (Fig. 1B). The tendency of Bmal1−/− mice to allocate, on average, a slightly larger proportion of total activity to FAA than did wild-type littermates (Fig. 1B) resulted from the subset of Bmal1−/− mice with low baseline activity but strong FAA (as exemplified in Fig. 1A Lower Right). The time course of the appearance of FAA after initiation of temporal food restriction was essentially identical in Bmal1−/− mice and wild-type littermates, with FAA emerging at about 3 days and reaching a plateau at 8–9 days (Fig. 1C). The mean time by which FAA anticipated the daily onset of food availability (the phase angle with respect to food presentation) was slightly more than 2 h in both Bmal1−/− mice and wild-type littermates, statistically indistinguishable in the 2 genotypes (Fig. 1D).
These results indicate that the Bmal1 gene is not required for FAA, at least under the usual conditions of temporal food restriction in a light–dark cycle. Because Bmal1 is essential for all circadian clock functions documented to date, these findings strongly imply that the known circadian clock is not required for robust, quantitatively normal FAA. But, given the broad evidence suggesting that a circadian clock likely underlies FAA, we considered the possibility that a hypothetical circadian clock driving FAA could be an unusual exception that is based on the known mechanism but nonetheless not requiring Bmal1—perhaps, for example, because of a rarely expressed transcription factor that is redundant with Bmal1 in clock function. To examine this possibility further, we studied FAA in Per1−/−; Per2−/− double mutants. These mice lack circadian clock function as a consequence of targeted disruptions of 2 of the 3 Period (Per) genes (29), core clock components acting, in contrast to Bmal1, in the negative limb of the circadian clock feedback loop (30).
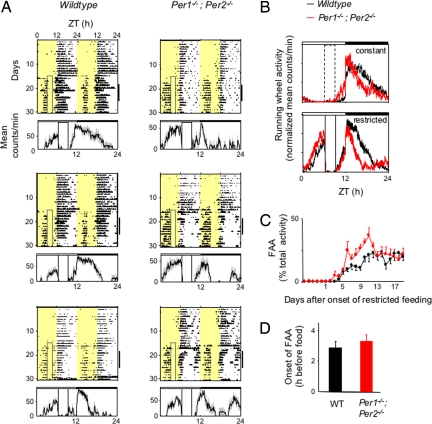
Using the gradual temporal food restriction protocol described above, we monitored FAA in Per1−/−; Per2−/− double mutants and wild-type mice derived from the same breeding colony (pure 129 background). The result was the same as that for Bmal1−/− mice: Per1−/−; Per2−/− mice exhibited robust, stable, and quantitatively normal FAA (Fig. 2). The normalized average FAA profiles of wild-type controls and Per1−/−; Per2−/− mice were nearly superimposable (Fig. 2B); the time course of the appearance of FAA after initiation of temporal food restriction was very similar in the 2 genotypes (Fig. 2C), and the mean time by which FAA anticipated the daily onset of food availability was statistically indistinguishable in the 2 genotypes—about 3 h in this genetic background (Fig. 2D). These results indicate that together, the Per1 and Per2 genes are not required for FAA under the usual conditions of temporal food restriction in a light–dark cycle. We observed the same full preservation of FAA in Per2−/− single-mutant mice (SI Text and Fig. S2). Thus, FAA is unaffected, regardless of whether the mutation disrupts the negative limb (Per1−/−; Per2−/−) or the positive limb (Bmal1−/−) of the circadian feedback loop, indicating that under these conditions, FAA is independent of the function of known circadian clocks.
Fig. 2.
Normal FAA in Per1−/−; Per2−/− double-mutant mice. (A) Representative double-plotted actograms of daily running-wheel activity of 3 wild-type and 3 Per1−/−; Per2−/− mice (as indicated) during constant food availability and under subsequent temporal food restriction. Data are displayed as in Fig. 1A. Under a light–dark cycle, Per1−/−; Per2−/− mice show a diurnal variation in locomotor activity because of the acute suppressive effect of light on behavior. (B) Mean locomotor activity profiles of wild-type (n = 9) and Per1−/−; Per2−/− mice (n = 8) under constant food availability (Upper) and after subsequent temporal food restriction (Lower). Data are displayed as in Fig. 1B. (C) Time course of the development of FAA in wild-type and Per1−/−; Per2−/− mice (n = 8 for each genotype). Data are displayed as in Fig. 1C. (D) Number of hours by which FAA anticipated daily food availability in wild-type and Per1−/−; Per2−/− mice (n = 8 for each genotype). Data are displayed as in Fig. 1D. The difference between genotypes is not statistically significant.
Because the results described above were obtained with mice under a light–dark cycle, standard conditions for studying FAA, they do not formally exclude the possibility that a circadian clock of the known type drives FAA. If a light–dark cycle drives oscillations of core components of a hypothetical FAA clock, thereby mimicking intact clock function, then light-driven molecular oscillations could, in principle, support entrainment to scheduled feeding, and thus drive FAA in circadian clock mutants. Because we observed quantitatively normal FAA in mice with mutations disabling different parts of the circadian feedback loop (Figs. 1 and 2), this scenario seemed improbable. However, if it were correct, then this hypothesis predicts that mutants lacking clock function would exhibit FAA in a light–dark cycle (as we observed) but would lack FAA in constant darkness. Alternatively, if FAA is fully independent of known circadian clocks, then robust FAA should persist in constant darkness in the mutants. To distinguish these alternatives, we performed studies of FAA in constant darkness in Bmal1−/− and Per1−/−; Per2−/− mice.
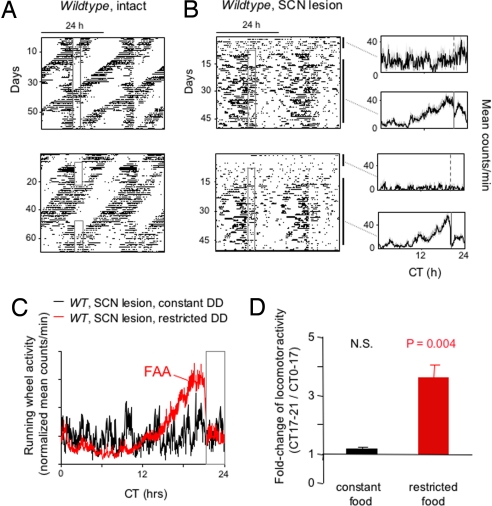
In constant darkness, mice lacking SCN circadian clock function, whether because of mutations that ablate clock function generally or because of an SCN lesion, display high-frequency (ultradian) bouts of locomotor activity that could potentially obscure FAA and make it difficult to recognize. As a guide to how FAA might look if present in constant darkness in Bmal1−/− or Per1−/−; Per2−/− mice, we performed control experiments in SCN-lesioned wild-type mice known to exhibit FAA (28). Despite the prominent ultradian activity, FAA was recognizable in the individual behavioral records of SCN-lesioned wild-type mice (Fig. 3B), and its presence was unequivocal in the averaged population profiles (Fig. 3C), which showed a specific and highly statistically significant increase in locomotor activity in the 4-h period immediately preceding the onset of food availability compared with other times of day (Fig. 3D).
Fig. 3.
Detection of FAA in constant darkness in behaviorally arrhythmic mice with prominent ultradian activity. (A) Representative double-plotted actograms of daily running-wheel activity of 2 wild-type mice (C57BL/6) during constant food availability and under temporal food restriction in constant darkness. Actograms are displayed as in Fig. 1A. (B) (Left) Representative double-plotted actograms of daily running-wheel activity of 2 SCN-lesioned wild-type mice (C57BL/6) during constant food availability and under subsequent temporal food restriction in constant darkness. Actograms are displayed as in Fig. 1A. (Right) Profile of daily running-wheel activity averaged across the days marked by the associated black bars to the right of each actogram, one during constant food availability and one during subsequent temporal food restriction. Gray vertical lines represent ± SEM. CT indicates circadian time (time of day during constant darkness conditions). (C) Mean locomotor activity profiles of SCN-lesioned wild-type mice (n = 7) during constant food availability (black) and after subsequent temporal food restriction (red), calculated as in Fig. 1B. Gray open box at right indicates the daily interval of food availability under temporal food restriction (CT21–24). (D) Quantification of FAA in SCN-lesioned mice in constant darkness (n = 7). Shown is the fold change of locomotor activity (running-wheel counts per minute; mean ± SEM) in each mouse for CT17–21 (when FAA would be expected under restricted food availability, see C) compared with CT0–17 (the rest of the day, except for CT21–24, the time when food was available under food restriction conditions). This latter interval was omitted from the computations because of the acute suppressive effect of food presentation on running-wheel activity (e.g., red trace in C). Under temporal food restriction, increased running-wheel activity (counts per minute) during CT17–21 compared with CT0–17 was highly significant (paired t test), but there was no significant difference under constant food access. N.S., not significant.
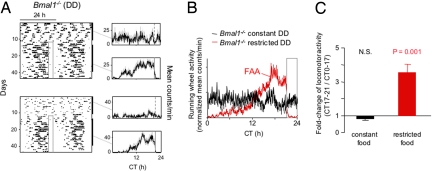
Thus informed of how FAA might look if present in the mutants, we next studied Bmal1−/− and Per1−/−; Per2−/− mice in constant darkness. Bmal1−/− mice exhibited an FAA in constant darkness that was essentially identical to that of the SCN-lesioned wild-type controls shown in Fig. 3. In individual records of Bmal1−/− mice, FAA was clearly recognizable, partially obscured by, but nonetheless standing out above, the baseline ultradian activity (Fig. 4A). The population activity profile indicated unambiguous FAA (Fig. 4B) that was highly statistically significant (Fig. 4C).
Fig. 4.
Normal FAA in Bmal1−/− mice in constant darkness. (A) Representative double-plotted actograms of daily running-wheel activity of 2 Bmal1−/− mice during constant food availability and under subsequent temporal food restriction in constant darkness. Data are displayed as in Fig. 3B. Note arrhythmic, ultradian activity before temporal food restriction. (B) Mean locomotor activity profiles of Bmal1−/− mice (n = 7) during constant food availability (black) and after subsequent temporal food restriction (red), displayed as in Fig. 3C. (C) Quantification of FAA in Bmal1−/− mice (n = 7) in constant darkness. Data are displayed as in Fig. 3D.
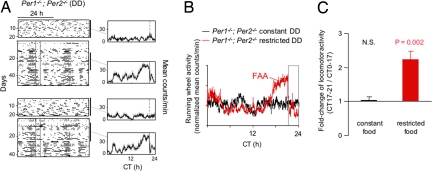
FAA was also fully preserved in Per1−/−; Per2−/− mice in constant darkness. FAA was apparent in individual activity records (Fig. 5A), and the population activity profile showed prominent FAA (Fig. 5B) that was highly statistically significant (Fig. 5C).
Fig. 5.
Normal FAA in Per1−/−; Per2−/− double-mutant mice in constant darkness. (A) Representative double-plotted actograms of daily running-wheel activity of 2 Per1−/−; Per2−/− mice during constant food availability and under temporal food restriction in constant darkness. Data are displayed as in Fig. 3B. Note arrhythmic, ultradian activity before temporal food restriction. (B) Mean locomotor activity profiles of Per1−/−; Per2−/− mice (n = 8) during constant food availability (black) and after subsequent temporal food restriction (red). Data are displayed as in Fig. 3C. (C) Quantification of FAA in Per1−/−; Per2−/− mice (n = 8) in constant darkness. Data are displayed as in Fig. 3D.
These results indicate that the Bmal1 or the Per1 and Per2 genes are not required for FAA in constant darkness. We conclude that FAA does not require the known circadian clock.
Discussion
It has long been argued that FAA is the behavioral output of a postulated food-entrainable circadian oscillator (1, 2). The recent discovery of widely distributed food-entrainable circadian clocks with the same molecular mechanism as that of the SCN clock (20) has evoked the widespread and reasonable expectation that FAA is driven by one or more of these known clocks (10, 13).
Previous studies of FAA in various circadian clock mutant genotypes have left open the question of the relationship of FAA to the known circadian clock mechanism. Clock mutant mice were reported to have intact FAA (31), but Clock mice have circadian clocks that are capable of free-running for a week or more, and they show normal behavioral entrainment to a light–dark cycle (32), so the persistence of FAA in this genotype could reflect the substantial residual clock function. Cryptochrome (Cry)-deficient mice were reported to exhibit FAA, but the study emphasized apparent alterations in the kinetics and stability of FAA in the mutants, arguing for a possible role of the clock in FAA (33).
Our results document that mutant mice lacking circadian clocks of the known kind in all tissues have FAA that is qualitatively and quantitatively indistinguishable from that in wild-type littermates in both a light–dark cycle and constant darkness, regardless of whether the mutation disables the positive or negative limb of the circadian clock feedback mechanism (Bmal1−/− or Per1−/−; Per2−/− mice, respectively). Thus, daily rhythms of FAA are independent of the function of the known circadian clock.
Two recent papers have offered a different conclusion. One found that FAA was lacking in mice with a mutation in Per2, specifically the Per2Brdm1 allele (34). We bred mice homozygous for a targeted disruption of Per2 (Per2−/− mice; ref. 29) in the same mixed genetic background as in the FAA study of Per2Brdm1 mice, and we found that Per2−/− mice exhibited normal FAA, indistinguishable from that of wild-type littermates (SI Text and Fig. S2). It is possible that the apparently discrepant findings result from the different Per2 alleles. In general, loss of a behavioral response in a mutant genotype is more likely due to nonspecific factors than is the persistence of a normal behavior. Given our additional findings of normal FAA in Per1−/−; Per2−/− double mutants and in Bmal1−/− mice, we suggest that Per2Brdm1 might not act as a pure loss-of-function allele. It is conceivable that Per2Brdm1, a small in-frame internal deletion in Per2 (35), has a deleterious effect on behavior independent of any defect in circadian clock function, particularly given evidence that Per2Brdm1 encodes a functional PER2 protein with abnormal properties (36).
The second paper reported a lack of FAA in Bmal1−/− mice (37). This study used a protocol with an abrupt shift from constant food availability to restricted food availability, like the initial protocol we used that caused Bmal1−/− mice to become lethargic and suffer a very high rate of mortality (see above). Notably, the study reported that under this acute food restriction, Bmal1−/− mice developed a torpor-like state, requiring manual stimulation to feed. On the basis of our similar initial food restriction experiments with the same mice, we suggest that the failure of Bmal1−/− mice to exhibit FAA under these conditions reflects a nonspecific effect of a severely compromised physiological or nutritional state, a point also raised recently by others (38).
Our results indicate that FAA does not require the function of the known circadian clock, necessitating fresh thinking about FAA, its underlying mechanism, and its anatomical substrates. Our findings are consistent with either an oscillatory or a nonoscillatory mechanism underlying FAA. If FAA is driven by an oscillatory mechanism, as suggested by multiple lines of evidence (1, 2), then there must be at least one class of circadian oscillator with a mechanistic basis different from that of known circadian clocks. This possibility is consistent with the finding that FAA to 24-h scheduled feedings in rats was unaffected after deuterium administration, whereas light-entrained behavior exhibited so strong a period-lengthening effect that it could no longer be entrained to a 24-h light–dark cycle (39).
The results reported here do not address the question of which neural or peripheral structures drive FAA or are necessary for its expression. However, they do remove the rationale for hypotheses about anatomical sites underlying FAA that are based on the presence of known circadian clocks at those sites. It appears that there is still much work to be done before the enduring mystery of FAA is solved.
Materials and Methods
Mice, Genotyping, and SCN Lesions.
Studies were performed in accordance with the protocol approved by the Harvard Medical School Standing Committee on Animals. For breeding, mice were housed in standard ventilated cages exposed to a 12:12 light–dark cycle. Genotyping was carried out as described (Bmal1−/−, ref. 24; Per1−/−; Per2−/− and Per2−/− mice, ref. 29). SCN lesions were performed as described previously (21) on 8-week-old C57BL/6J male mice.
Temporal Food Restriction.
Mice were transferred to individual cages equipped with running wheels at 7–9 weeks of age and were exposed to 12:12 or 16:8 light–dark cycles for 14 days with ad libitum access to regular chow pellets (LabDiet 5001; PMI Nutrition International). On the first day of the 4-day gradual food restriction period (Fig. S1), regular chow was removed at ZT (or CT) 18, and 12 h later, at ZT (or CT) 6, enriched food (LabDiet 5001 powdered chow mixed with canola oil; 4:1 wt/wt) was provided in a 40-mL glass jar (40). Each successive day, enriched food was removed 3 h earlier until food presentation was limited to 3 h, from ZT6 to ZT9 or CT6 to CT9 (Fig. S1). Under constant darkness, food removal and presentation and cage changes were carried out under infrared light with night-vision goggles.
Locomotor Activity Recordings and Data Analysis.
Running-wheel activity was recorded with ClockLab (Actimetrics). Animals were placed in individual cages equipped with running-wheels and housed in light-tight, ventilated boxes. During lights-on, mice were exposed to 150 lux of light. In the displayed actograms, single data points (tick marks) represent revolutions per minute per 6-min time bin. Individual mean daily activity profiles were computed by using ClockLab, and group average profiles were generated with Microsoft Excel.
Supplementary Material
Acknowledgments.
We thank Christopher Bradfield (University of Wisconsin, Madison, WI) for Bmal1−/− mice, David Weaver and Steve Reppert (University of Massachusetts Medical School, Worcester, MA) for Per1−/−; Per2−/− mice, Ming Liu and Xiao Ling Long for expert technical assistance, and Amanda Sadacca for helpful comments on the manuscript. This work was supported by a grant from the National Institutes of Health (to C.J.W.) and by a Deutsche Forschungsgemeinschaft Postdoctoral Fellowship (to K.-F.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902063106/DCSupplemental.
References
- 1.Mistlberger RE. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 2.Stephan FK. Food entrainable oscillators in mammals. In: Takahashi JS, Turek FW, Moore RY, editors. Circadian Clocks, Handbook of Behavioral Neurobiology. Vol 12. New York: Kluwer Academic/Plenum; 2001. pp. 223–246. [Google Scholar]
- 3.Pittendrigh CS. Circadian systems: General perspective. In: Aschoff J, editor. Biological Rhythms, Handbook of Behavioral Neurobiology. Vol 4. New York: Kluwer Academic/Plenum; 1981. pp. 57–80. [Google Scholar]
- 4.Coleman GJ, Harper S, Clarke JD Armstrong S. Evidence for a separate meal-associated oscillator in the rat. Physiol Behav. 1982;29:107–115. doi: 10.1016/0031-9384(82)90373-0. [DOI] [PubMed] [Google Scholar]
- 5.Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979;25:346–363. doi: 10.1016/s0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- 6.Inouye ST. Ventromedial hypothalamic lesions eliminate anticipatory activities of restricted daily feeding schedules in the rat. Brain Res. 1982;250:183–187. doi: 10.1016/0006-8993(82)90967-2. [DOI] [PubMed] [Google Scholar]
- 7.Mistlberger RE, Rechtschaffen A. Recovery of anticipatory activity to restricted feeding in rats with ventromedial hypothalamic lesions. Physiol Behav. 1984;33:227–235. doi: 10.1016/0031-9384(84)90104-5. [DOI] [PubMed] [Google Scholar]
- 8.Davidson AJ, Stephan FK. Feeding-entrained circadian rhythms in hypophysectomized rats with suprachiasmatic nucleus lesions. Am J Physiol. 1999;277:R1376–R1384. doi: 10.1152/ajpregu.1999.277.5.R1376. [DOI] [PubMed] [Google Scholar]
- 9.Davidson AJ, et al. Food-anticipatory activity persists after olfactory bulb ablation in the rat. Physiol Behav. 2001;72:231–235. doi: 10.1016/s0031-9384(00)00417-0. [DOI] [PubMed] [Google Scholar]
- 10.Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003;2:32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 11.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 12.Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- 13.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci USA. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22:467–478. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- 15.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in cultured Rat-1 fibroblasts. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 17.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 19.Abe M, et al. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: Time and food. J Biol Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 21.Storch KF, et al. Intrinsic circadian clock of the mammalian retina: Importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gekakis N, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 24.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunger MK, et al. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–132. doi: 10.1002/gene.20102. [DOI] [PubMed] [Google Scholar]
- 26.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchant EG, Mistlberger RE. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res. 1997;765:273–282. doi: 10.1016/s0006-8993(97)00571-4. [DOI] [PubMed] [Google Scholar]
- 29.Bae K, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 30.Sangoram AM, et al. Mammalian circadian autoregulatory loop: A Timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 31.Pitts S Perone E, Silver R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am J Physiol. 2003;285:R57–R67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vitaterna MH, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iijima M, et al. Altered food-anticipatory activity rhythm in Cryptochrome-deficient mice. Neurosci Res. 2005;52:166–173. doi: 10.1016/j.neures.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Feillet CA, et al. Lack of food anticipation in Per2 mutant mice. Curr Biol. 2006;16:2016–2022. doi: 10.1016/j.cub.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 35.Zheng B, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 36.Shearman LP, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 37.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mistlberger RE, et al. Comment on “Differential rescue of light- and food-entrainable circadian rhythms”. Science. 2008;322:675. doi: 10.1126/science.1161284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mistlberger RE, Marchant EG, Kippin TE. Food-entrained circadian rhythms in rats are insensitive to deuterium oxide. Brain Res. 2001;919:283–291. doi: 10.1016/s0006-8993(01)03042-6. [DOI] [PubMed] [Google Scholar]
- 40.Holmes MM, Mistlberger RE. Food anticipatory activity and photic entrainment in food-restricted BALB/c mice. Physiol Behav. 2000;68:655–666. doi: 10.1016/s0031-9384(99)00231-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.