Abstract
Purpose
The identification of nursing home residents who can continue to participate in advance care planning about end-of-life care is a critical clinical and bioethical issue. This study uses high quality observational research to identify correlates of advance care planning in nursing homes, including objective measurement of capacity.
Design and Methods
The authors used cross-sectional, cohort study between 1997 and 1999. Seventy-eight residents (M age = 83.97, = 8.2) and their proxies (M age = 59.23, SD = 11.77) were included across five nursing homes. The authors obtained data via chart review, proxy interviews, resident assessments, survey completion by certified nursing assistants, and direct observation of residents' daily behaviors.
Results
Capacity assessments revealed that most residents could state a simple treatment preference (82.4%), but a sizable number did not retain capacity to understand treatment alternatives or appreciate the consequences of their choice. Global cognitive ability (Mini-Mental State Examination score) was related to understanding and appreciation. When the authors removed the effects of global cognitive ability, understanding and appreciation were related to time spent by residents in verbal interaction with others. Residents were more likely to possess advance directives when proxies possessed advance directives, proxies were less religious, and residents were socially engaged.
Implications
Assessment of proxy beliefs and direct determination of residents' decisional capacity and social engagement may help nursing home staff identify families who may participate in advance planning for end-of-life medical care. Measures of global cognitive ability offer limited information about resident capacity for decision making. Decisional capacity assessments should enhance the verbal ability of individuals with dementia by reducing reliance on memory in the assessment process. Interventions to engage residents and families in structured discussions for end-of-life planning are needed.
Keywords: Advance planning, Nursing homes, Capacity, Observational research
In 1998, 1.5 million individuals older than the age of 65 years in the United States (5.8%) resided in nursing homes (American Health Care Association, 1999). Statistics describing nursing homes as the place of death are noteworthy. Nursing homes and hospitals account for at least 54% of all places of death (Gage & Dao, 2000). Approximately 76% of individuals who die in nursing homes are older than the age of 65 years (Blevins & Deason-Howell, 2002; Gabrel, 2000). The proportion of individuals of any age who die in nursing homes (30%) is rising (Field & Cassel, 1997), and 66% of persons of any age who live in nursing homes remain there until they die (Hanson, Henderson, & Rodgman, 1999).
Familial advance planning for the end of life consists of discussion of values and treatment preferences and, potentially, execution of advance directives (Allen & Shuster, 2002). These discussions may benefit both nursing home residents and their proxy decision makers. Most nursing home residents have incurable chronic diseases, and complaints of pain range in prevalence from 45% to 83% (Ferrell, 1995). Individuals with chronic physical illness often prefer palliative treatments emphasizing pain management and supportive care rather than the initiation of life-prolonging treatments (Zerzan, Stearns, & Hanson, 2000). Life-sustaining medical treatment preferences of cognitively intact nursing home residents have been found to be stable over 6 months, with treatment preference changes moving toward less intervention (Berger & Majerovitz, 1998). Examination of data from the Minimum Data Set has shown positive treatment outcomes for residents for whom palliative treatment options were executed (Miller, Gozalo, & Mor, 2001). Decedents who received hospice care had improved pain management, decreased hospitalization, and decreased use of feeding tubes relative to those residents who died receiving standard nursing home care (Miller et al., 2001).
In addition to providing better end-of-life care for residents, familial advance planning in nursing homes may decrease caregiving stress among proxy decision makers (Jacobson et al., 1996). Proxy plans for the medical care of cognitively impaired nursing home residents typically become the procedures implemented, and the stress of responsibility for life and death decisions can be compounded by the resident's degree of incapacity and the proxy's knowledge (or lack thereof) of what treatment option the resident would prefer. Hypothetical treatment preferences of health care proxies regarding treatment decisions for patients are poorly related to the individual's autonomous treatment preferences (Diamond, Jernigan, Moseley, Messina, & McKeown, 1989), even when proxies have access to the individual's formal advance directive (Ditto et al., 2001). In a study of nursing homes in Canada and the United States, only 48% of proxies who made the decision to place their resident on a feeding tube felt confident that their resident would have wanted the procedure (Mitchell, Berkowitz, Lawson, & Lipsitz, 2000). These proxies reported understanding the benefits (83%) but not the risks (49%) of tube feeding. Perhaps as a consequence of stressful medical decision making, surviving family members of recently deceased residents reported fewer positive experiences and numerous negative experiences associated with nursing home care in comparison with other treatments for their relative at the end-of-life (Hanson, Danis, & Garrett, 1997). Respondents voiced concerns about poorly trained nursing home staff, the remoteness of physicians in nursing homes, and quality of care issues that may have led to greater pain and suffering for the residents, or, potentially, to premature death. In light of these findings, familial advance planning for the end-of-life is a critical need in the nursing home.
Despite the potential benefits of advance planning in the nursing home, it is still relatively rare (Tilden, Nelson, Dunn, Donius, & Tolle, 2000). Since passage of the Patient Self-Determination Act (PSDA; Omnibus Budget Reconciliation Act, 1990), the number of nursing home residents with documented, formal advance directives has increased (Lynn et al., 1999; Mezey, Mitty, Bottrell, Ramsey, & Fisher, 2000). However, only 51% of all nursing home residents nationally have an advance directive (Mezey et al., 2000). Information provided by nursing homes in compliance with the PSDA is most frequently given to family members or other potential proxy decision makers (Bradley, Walker, Blechner, & Wetle, 1997), and it is unknown to what extent this information fosters spontaneous treatment preference discussions between proxies and their residents.
In practice, the participation of nursing home residents in their own end-of-life treatment planning is limited. Although a dementia diagnosis does not preclude a resident's participation in medical decision making (Smyer & Allen-Burge, 1999), one factor limiting their involvement is cognitive impairment (Bradley et al., 1997; Mezey, Mitty, Rappaport, & Ramsey, 1997). Nationally, 42% of residents have a diagnosis of dementia (American Health Care Association, 1999), and some researchers have reported that up to 74% of residents exhibit dementia symptoms (Rovner, Kafonek, Filipp, Lucas, & Folstein, 1986). However, even cognitively intact individuals may lack understanding and knowledge of their treatment options at the end of life (Mitchell et al., 2000; Silveira, DiPiero, Gerrity, & Feudtner, 2000). Thus, there is a growing need for consideration of decision making within a family context (Allen & Shuster, 2002; King, Kim, & Conwell, 2000) and for direct assessment of autonomous medical treatment decision-making capacity among older adults.
Most accepted models of informed consent rely on the ethical principals of beneficence and nonmaleficence on the part of health care professionals and autonomy on the part of the patient and require determinations of individual competency (Appelbaum & Grisso, 1995, 1998). Four aspects of treatment consent capacity have been identified: (a) the ability to communicate a treatment choice, (b) the ability to understand relevant information, (c) the ability to appreciate the consequences of the choice made, and (d) the ability to rationally manipulate the information provided and to give reasons for one's treatment decision (Appelbaum & Grisso, 1995; Marson, Chatterjee, Ingram, & Harrell, 1996; Marson, Ingram, Cody, & Harrell, 1995). In legal spheres, it has been generally agreed upon that the simple ability to state a treatment preference is too lenient for the determination of competence to give informed consent (Stanley, 1987). Instead, the most commonly used legal standard of decisional capacity requires that an individual be able to understand all the information that is presumed by law to be part of treatment decision making (Appelbaum & Grisso, 1995; Stanley, 1987).
The current study attempted to (a) quantitatively assess the capacity of nursing home residents to participate in treatment decisions regarding their end-of-life medical care and (b) identify correlates of residents' possession of advance directives, including previous familial advance planning, proxy beliefs, and resident behavior. We assumed that characteristics of both the resident and the proxy would be related to residents' possession of advance directives, on the basis of the finding that characteristics of patients and proxies impacted proxy end-of-life treatment decisions for a cognitively compromised older adult described in a vignette (Allen-Burge & Haley, 1997). Specifically, we assessed residents' capacity to make decisions about future placement of a feeding tube, proxy report of previous familial advance planning, proxy religiosity, and resident behavior within the nursing home. Our hypotheses were as follows. First, we proposed that our method of quantitatively assessing treatment consent capacity would be reliable and valid in comparison with a measure of global cognitive status (Mini-Mental State Examination [MMSE]; Folstein, Folstein, & McHugh, 1975). We proposed that residents who retained decisional capacity would be more actively engaged with their physical and social environments, assessed by computer-assisted behavioral observation. We proposed that proxies would be more likely to report that residents possessed advance directives already who were more ill (i.e., with more severe cognitive impairment and less behavioral engagement), and, potentially, closer to death. We did not offer a specific hypothesis regarding the relation of proxy religiosity to possession of an advance directive for the resident.
Methods
Participants
Participants (N = 78, Male = 20, Female = 58)Were enrolled in an intervention study designed to improve communication between nursing home residents and staff via communication skills training and use of external memory aides (Allen-Burge, Burgio, Bourgeois, Sims, & Nunnikhoven, 2001; Burgio et al., 2001). This report uses data from the initial 4-week study period. For entry into the study, residents completed a 5-min semistructured conversation assessing their capacity for spontaneous speech (Bourgeois, 1993) and, to be included in the study, residents had to demonstrate use of multiword phrases during the conversation. Additionally, residents had to have an identifiable proxy decision maker listed in their medical chart who was willing to participate in a 90-min interview regarding their resident's care. Only proxies with a personal connection with the resident were included (i.e., family members and fictive kin included; court-appointed guardians without personal ties to the resident excluded).
Ninety-two consenting residents (mostly by proxy) met full entry criteria and completed the initial 4-week study period. Seventy-eight of these residents had an identified familial proxy who agreed to the 90-min interview, reflecting an 85% response rate for this study. There were no differences in age, gender, or cognitive status between the 78 residents with a familial proxy and the 14 residents without a familial proxy. There was, however, a significant difference in race, χ2 (1, N = 92) 12.22, p .0005, with 50% of African American residents dropped because of lack of familial proxy in comparison with 11.54% of White residents.
The average age of residents was 83.97 years (SD = 8.20; range 61-105 years); 88% were White and 12% were African American. All residents demonstrated use of multiword phrases. Their average MMSE score was 14.04 (SD 6.50; range 1-29) and their average Functional Independence Measure score was 45.58 (SD = 22.90; range 13-89; Hamilton, Laughlin, Fielder, & Granger, 1994; Kidd et al., 1995), where high numbers indicate greater independence. Their average score on the Charlson Comorbidity Index was 2.10 (SD = 1.60; range 0-7; Charlson, Pompei, Ales, & MacKenzie, 1987), indicating a moderate degree of comorbid illness. Forty-eight percent of residents carried a diagnosis of dementia in their medical chart.
The individual identified as the sponsor of the nursing home resident in the resident's medical chart was operationally defined as the proxy decision maker. The average age of proxies was 59.23 years (SD = 11.77; range 31.42-88.38 years); 91% were White and 9% were African American. Their average education was 15 years (SD = 2.1; range 11-18 years). Proxies were predominantly (64%) adult sons (n = 21) and daughters (n = 29) of nursing home residents. Spouses (n = 5) accounted for only 6% of proxy decision makers. Thirty percent of proxies (n = 23) were classified as “other relatives,” including siblings, in-laws, grandchildren, nieces, and nephews.
Measures
We contacted proxies for each nursing home resident by phone and explained the purpose of the current study. If this individual agreed to participate, we scheduled an in-person semistructured interview with a licensed clinical psychologist at the proxy's convenience. We obtained informed consent in accordance with the standards of the Institutional Review Board of the University of Alabama at Birmingham.
Proxy Interviews
A semi-structured interview asked proxies about their end-of-life planning activities for the resident and for themselves. We coded items as follows: (a) possession of a living will or durable power of attorney for health care = formal planning, (b) discussing treatment preference wishes with others without these documents = informal planning, and (c) participating in neither activity = having no plans.
Religiosity
This 5-item measure was developed for the National Institutes of Health-funded Resources for Enhancing Alzheimer's Caregiver Health (REACH; Coon, Schultz, & Ory, 1999). By using this tool, we measured the proxy's attendance at religious services, the importance of faith in the proxy's life, and the frequency with which the proxy engaged in prayer and/or meditation. We also measured the perceived meaningfulness of attendance at religious services and prayer.
Staff Interviews
We used the Functional Independence Measure (FIM)—REACH Version (Hamilton et al., 1994) for staff interviews. This version was developed for the National Institutes of Healthfunded REACH (total score range: 13-91; Coon et al., 1999). Certified nursing assistants (CNAs) familiar with the resident's daily care provided information. Reliability data indicate an intraclass correlation coefficient of .96 for the motor domain, with unweighted Kappas ranging from .53 to .66 (Hamilton et al., 1994; Kidd et al., 1995).
Resident Interviews and Chart Review
We developed the Decisional Capacity Assessment— Advance Directives Version and scoring system on the basis of prior research (Marson et al., 1995; Marson et al., 1996; available from the authors upon request). In consultation with two board-certified geriatricians, we developed a vignette requiring a decision about a potential, future medical problem involving the placement of a feeding tube. Fifteen open-ended questions assessed the resident's capacity, and we audiotaped responses for transcription and analysis. We assessed four aspects of the capacity of nursing home residents to participate in treatment planning: (a) evidence of choice [possible score range 0, 1, 2], (b) understanding of the treatment situation [possible score range 0 to 30], (c) appreciation of the consequences of the choice [possible score range 0 to 5], and (d) ability to rationally manipulate the treatment information [possible score range 0 to 21].
MMSE (Folstein, et al., 1975)
The MMSE is a brief cognitive assessment tool that measures orientation, memory, attention, ability to name, ability to follow verbal and written commands, ability to write a sentence spontaneously, and ability to copy a figure. The maximum score on this test is 30, and it has been shown to differentiate reliably between individuals with and without global cognitive impairment (Kafonek et al., 1989). The test-retest and interevaluator reliabilities are .89 and .83, respectively.
Philadelphia Geriatric Center Pain Measure (Parmelee, Katz, & Lawton, 1991)
This instrument is an adaptation of the McGill Pain Questionnaire (Melzack, 1975) consisting of six questions regarding the residents' subjective experience of pain. Each response is scaled from “not at all” to “extremely” (1 to 5). This provides a composite measure of the experience of pain and has been shown to have high internal consistency (α = .87; Allen et al., 2003).
Documentation of Do-Not-Resuscitate (DNR) Orders
Residents' charts were reviewed for documented (yes/no) do-not-resuscitate orders.
Charlson Comorbidity Index (Charlson et al., 1987)
This scale was designed for use in obtaining medical diagnostic information from medical records; scale scores correlate with outcomes such as mortality, length of hospital stay, and discharge to nursing homes (Deyo, Cherkin, & Ciol, 1992).
Computer-Assisted Behavioral Observation System (CABOS)
We generated real-time CABOS data by using the software programs previously used by our research group (Burgio et al., 1994; Repp, Karsh, van Acker, Felce, & Harman, 1989) and adapted for this intervention study (Burgio et al., 2001). We sampled resident behaviors during half-hour periods two times per week between the hours of 12-2 p.m. and 5-7 p.m. for an average total of 4 hr (range 1.52- 4.55 hr) across the 4-week study period. These observation periods were chosen because prior research indicated residents spend more time in verbal interaction during these periods. One set of keys coded whether or not the resident was “engaged in activity” or “inactive.” Two sets of keys coded the residents' social behavior (a) “resident speech in the presence of others,” and (b) “verbal interaction from others directed to the resident.” We coded all targeted behaviors with the observed resident as the point of reference. We assessed interobserver agreement independently among four observers during 12.51% of the total observation time (359.42 hr) and calculated interobserver agreement through a secondby-second comparison of the observational files using Cohen's Kappa (Cohen, 1968). Average Kappa reliability across all categories was .78 (range .58-.98).
Statistical Analysis
We examined associations between study variables and reported them as significant at p = .05. We examined assessment of resident capacity for internal consistency and reliability. We calculated interrater reliability for each aspect of capacity by using oneway repeated measures analysis of variance (ANOVA) where the repeated factor was rater. This design can be viewed as a one-facet generalizability study to assess the dependability of the coding scheme across raters for each aspect of capacity. We treated both residents and raters as random effects. We assessed formality of plans initially at three levels for both residents and proxies: (a) those with advance directives, (b) those with informal plans via prior treatment preference discussions, and (c) those with no plans.
Results
Hypothesis One: Assessment of Resident Capacity
Reliability
We developed detailed and welloperationalized scoring criteria for each aspect of capacity. Internal consistency estimates were acceptable for three out of four aspects, with alpha = .99 for evidence of choice, alpha = .90 for understanding, alpha = .82 for appreciation of consequences, and alpha = .66 for provision of rational reasons for the treatment choice made.
We established interrater reliability by using 11 cases (14%, M MMSE = 17.45, SD = 6.55) scored from rsident transcripts by three trained raters. Estimates of interrater reliability revealed acceptable reliability for research purposes for three out of four aspects of capacity. Ratings of the evidence of choice showed good evidence for reliability, r = .98. Assessment of understanding of the treatment situation was also reliable, r = .89. Assessment of the appreciation of consequences showed acceptable but lower reliability, with r = .60. We could not establish interrater reliability for assessment of the provision of rational reasons for the treatment choice, r = .39. Thus, we deemed further analysis regarding capacity to provide rational reasons for one's treatment choice inappropriate, and we dropped the relation of this measure to other study variables from further analysis.
Descriptive Statistics
Figures 1 through 3 illustrate residents' distribution of scores for three aspects of capacity. As shown in Figure 1, 82% of residents retained some capacity to state a simple treatment preference (scores of 1 or 2). Notably, a sizable number of residents did not retain measurable capacity to understand treatment alternatives (27.0% with a score of 0; Figure 2) or to appreciate the consequences of their choice (35.1% with a score of 0; Figure 3).
Figure 1.
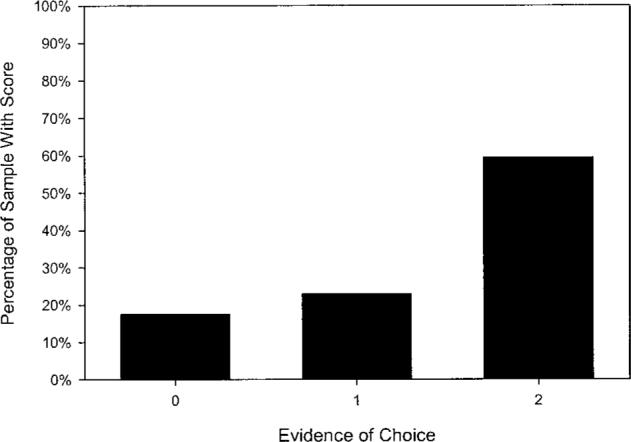
Frequency distribution (N = 78) for residents' capacity to state a treatment choice (possible score range 0, 1, 2).
Figure 3.
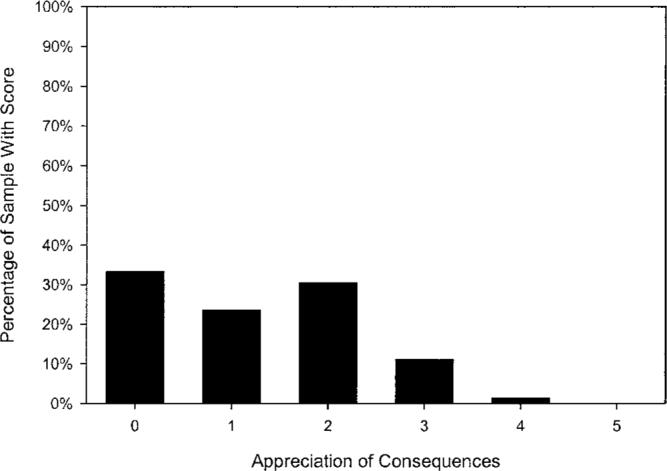
Frequency distribution (N = 78) for residents' capacity to appreciate the consequences of the treatment choice made (possible score range 0 to 5; M = 1.20, SD = 1.09).
Figure 2.
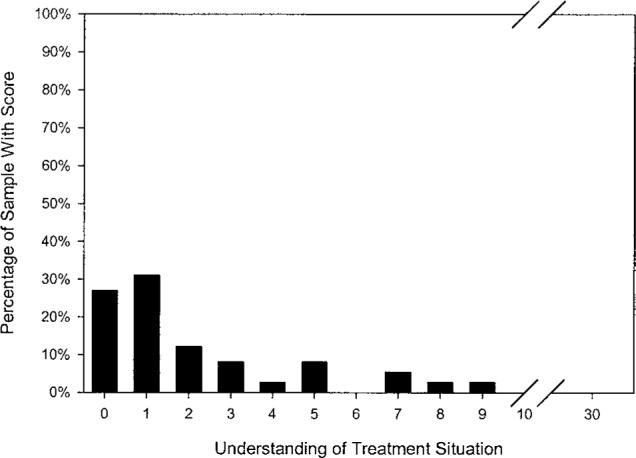
Frequency distribution (N = 78) for residents' capacity to understand the treatment situation (possible score range 0 to 30; M = 2.15, SD = 2.51).
Associations With MMSE
The simple capacity to state a treatment preference was not significantly associated with any other variable. Better global cognitive ability was related to residents' capacity to understand the treatment situation (r = .61, p = .001 and, to a lesser extent, residents capacity to appreciate the consequences of their treatment choice (r = .24, p = .042). Residents' age, degree of comorbid illness, and self-reported pain were not associated with any aspect of capacity.
Hypothesis Two: Relation of Understanding and Appreciation With Resident Behavior
After we removed associations with global cognitive ability (i.e., MMSE), residents' capacity to participate in decisions about future feeding tube placement was related significantly to observable verbal interactions. Specifically, residents with higher scores on understanding spent more time talking with others (partial r = .28, p = .015), and more time being spoken to by others (partial r = .25, p = .036). Residents' capacity to appreciate the consequences of their treatment choice also was related to more time spent talking with others (partial r = .34, p = .003).There were no associations between capacity and residents' engagement with their physical environment after we removed statistically the effects of global cognitive ability.
Hypothesis Three: Relation of Study Variables With Advance Directive Possession
Descriptive Statistics
The resident's possession of advance directives was marginally associated with documentation of a DNR order in the residents' medical chart, χ2 (1, N = 73) = 2.72, p = .10. Fifty-four percent of residents had a documented DNR order. Seventy-six percent of residents with a DNR order also had advance directives. Overall, 69% of residents and 41% of proxies had advance directives. Among individuals who did not possess an advance directive, 11% of residents had discussed their treatment preferences with their proxies, whereas 37% of proxies had discussed their treatment preferences with others in a position to make medical decisions for them in the future. Twenty percent of residents and 22% of proxies had neither formal nor informal advance care plans. Forty-one of the 54 residents with advance directives (76%) had also discussed their end-of-life treatment wishes with their proxy decision maker; 28 of the 33 proxies with advance directives (85%) had discussed their treatment wishes with someone who might be in the position of making treatment decisions for them in the future.
Because of the relatively few proxies who reported making informal plans for their residents or for themselves, the two categories of informal and no plans were collapsed and contrasted with possession of advance directives. As expected, the chi-square revealed a significant relation between formal planning for the resident and for the proxy, χ2 (1, N = 78) = 8.50, p = .004. Twenty-eight resident-proxy dyads possessed advance directives for each member of the dyad. Another 20 dyads did not possess formal plans for either residents or proxies. Twenty-six proxies had advance directives for their resident but not for themselves. Only four proxies had formal plans for themselves but not their resident.
Associations With Other Study Variables
Residents' possession of advance directives was significantly related to the proxy's religiosity (r = -.24, p = .04). Proxies with a greater degree of organizational, nonorganizational, and subjective religiosity were less likely to have advance directives for their resident. Residents' possession of advance directives also was significantly related to the amount of time others spent talking with the resident (r = .24, p = .04). Notably, none of the following variables were associated with residents' advance directive possession: capacity, global cognitive ability, age, gender, severity of illness, self-report of pain, or staff report of resident functional ability.
Discussion
This study extends prior research by examining psychosocial and behavioral correlates of capacity and possession of advance directives among nursing home residents. We found evidence that although most residents retain the ability to state a treatment preference, many lack the capacity to understand the treatment situation or appreciate the consequences of the treatment choice made. We also found that resident capacity and global cognitive ability are not related to possession of advance directives. Instead, proxy possession of advance directives, proxy religiosity, and resident social engagement are related to residents' possession of formal advance care plans. These results have implications for the identification of residents that may participate in familial advance planning for the end of life (Allen & Shuster, 2002) and highlight the need for further development of objective measures of resident capacity that enhance compromised verbal ability. The results also suggest proxies' need for structured educational interventions to facilitate familial advance planning for the end of life.
Understanding is the most frequently used legal standard for the assessment of consent capacity (Appelbaum & Grisso, 1995, 1998; Marson et al., 1996; Stanley, 1987). Our measure of understanding is moderately related to global cognitive ability as measured by the MMSE (37% shared variance). Although the MMSE is a reasonable tool for initial cognitive screening, its usefulness as a measure of consent capacity for understanding the treatment situation is limited. Use of the MMSE as a screening measure for a resident's ability to appreciate the consequences of the treatment choice made is even more problematic, given that MMSE score accounts for only 6% of variance in appreciation in this study. We recommend that measures developed to assess capacity to consent to treatment (Appelbaum & Grisso, 1998; Marson et al., 1996; Marson et al., 1995) be used rather than the MMSE to assess directly the capacity of nursing home residents to participate in end-of-life treatment planning. Such objective measurement tools should be used as part of a clinical assessment of a resident's decision-making capacity, along with an understanding of the resident's physical functioning, values and preferences, resources available to assist in the decision-making process, and the complexity of the decisions involved.
It is noteworthy that we are unable to establish reliability for the capacity to state rational reasons for the treatment choice made in this study (i.e., long term placement of a feeding tube). The average cognitive ability of our residents is much lower than that of the participants in previous research with normal older adults (M MMSE = 29.1, SD = 0.9; Marson et al., 1995) and individuals with mild to moderate probable Alzheimer's disease (M MMSE = 19.4, SD = 4.6; Marson et al., 1995). It appears that discrimination of capacity for rational reasoning is hampered in this study by the restricted range in the cognitive ability of our sample. It could be that rational reasoning is the most cognitively challenging aspect of treatment consent capacity to express as an individual and to assess as a clinician. Indirect support for this notion comes from the differential findings regarding the relations between global cognitive ability, understanding, and appreciation. Future research should address the hierarchical nature of treatment consent capacity as a theoretical construct. Clinicians interested in testing the limits of a resident's capacity to participate in treatment planning discussions may consider using cognitive supports such as prompts (Allen & Shuster, 2002) and different response options (i.e., forced choice versus free recall; Hebert, 2002).
We found support for our notion that residents who retain some decisional capacity will be more actively engaged. Specifically, our measures of understanding and appreciation are associated with the amount of time residents spend verbally engaged with others during direct computer-assisted behavioral observations. These relations hold true even when we remove statistically the association with global cognitive ability. It should be noted that capacity assessments heavily rely on verbal expression, so these relations are not surprising. However, a resident's engagement in verbal interaction with others is an easily observable behavior that can be used by nursing home staff to identify those residents who may still retain capacity to participate in advance planning. CNAs could be taught to rate residents' verbal ability on a simple ordinal scale (Bourgeois, 1993), and care plan coordinators and social service staff could then attempt familial advance planning for end-of-life discussions with these residents and their proxy decision makers.
Our findings regarding correlates of residents' possession of advance directives are somewhat surprising. Although we expected that residents with advance directives would have proxies who also had advance directives, we expected residents' cognitive ability and capacity to be significantly associated with residents' advance directive possession. These relations are not found. We also expected that proxies would have advance directives for residents who were more ill, and thus less engaged with their environment. Instead, residents who talk more with others are more likely to possess advance directives.
We offered no hypothesis about the relation between proxy religiosity and advance directive possession. The finding that residents with advance directives have proxies who express less religiosity contrasts with previous research showing that individuals who have stronger spiritual beliefs are more likely to possess advance directives (DeLuca Havens, 2000; Kaldjian, Jekel, & Friedland, 1998). However, it supports previous research that shows adult children who express a higher degree of religiosity and greater closeness in their relationship with a terminally ill parent typically request continuation or initiation of life-sustaining treatments, even when this request does not comply with parental wishes (Sonnenblick, Friedlander, & Steinberg, 1993). Thus, religiosity and spirituality may play different roles in the end-of-life medical treatment decisions of older adults and their adult children, individuals who are likely to become proxy decision makers. Further research addressing religiosity and spirituality in the context of familial advance planning is needed (Allen & Shuster, 2002).
Several limitations of this study are acknowledged. The CABOS data were collected as part of a larger intervention study designed to improve effective communication between staff and residents in nursing homes (Allen-Burge et al., 2001; Burgio et al., 2001) and were not intended to directly measure residents' ability to engage in advance care planning. Second, our inclusion criteria retain residents with compromised cognitive status but screen out those who can not use multiword phrases in spontaneous speech. Thus, our residents may not reflect the nursing home population in general. Perhaps the major limitation is that this study is cross-sectional and correlational. Therefore, no conclusions about causality or time-dependent relationships such as the temporal sequence of actions preceding the execution of advance directives can be made. These data require longitudinal replication to adequately explore the intriguing relations between resident behavior, capacity, and possession of advance directives. Greater attention to establishing clinically meaningful cut-off scores for aspects of capacity, particularly in less verbal individuals, is needed. Exploration of the potential differential impact of individual and proxy religiosity and/or spirituality on end-of-life medical decision making is also needed.
Acknowledgments
The research reported in this article was supported by funding from the National Institute on Aging (RO1AG13008) to M. Bourgeois and L. Burgio. Funding from the National Institute on Aging (K01AG00943) to R. S. Allen supported preparation of the manuscript. Portions of this article were presented at the 53rd Annual Scientific Meeting of The Gerontological Society of America, Washington, DC, November, 2000.
We thank the nurses, nursing assistants, and administrative staff of the many Birmingham area nursing homes for their support and assistance. Special thanks are extended to John Gerstle for data management and to the staff of the Applied Gerontology Program for assistance in manuscript preparation.
References
- Allen RS, Shuster JL. The role of proxies in treatment decisions: Evaluating functional capacity to consent to end-of-life treatments within a family context. Behavioral Science and the Law. 2002;20:235–252. doi: 10.1002/bs1.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RS, Thorn BE, Fisher SE, Gerstle J, Quarles K, Bourgeois MS, et al. Prescription and dosage of analgesic medication in relation to resident behaviors in the nursing home. Journal of the American Geriatrics Society. 2003;51:534–538. doi: 10.1046/j.1532-5415.2003.51164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Burge R, Burgio LD, Bourgeois MS, Sims R, Nunnikhoven J. Increasing communication among nursing home residents. Journal of Clinical Geropsychology. 2001;7:213–230. [Google Scholar]
- Allen-Burge R, Haley WE. Individual differences and surrogate medical decisions: Differing preferences for life-sustaining treatments. Aging and Mental Health. 1997;1:121–131. [Google Scholar]
- American Health Care Association . Facts and trends 1999: The nursing facility sourcebook. Author; Washington, DC: 1999. [Google Scholar]
- Appelbaum PS, Grisso T. Comparison of standards for assessing patients' capacities to make treatment decisions. American Journal of Psychiatry. 1995;152:1033–1038. doi: 10.1176/ajp.152.7.1033. [DOI] [PubMed] [Google Scholar]
- Appelbaum PS, Grisso T. Assessing patients' capacities to consent to treatment. New England Journal of Medicine. 1998;319:1635–1638. doi: 10.1056/NEJM198812223192504. [DOI] [PubMed] [Google Scholar]
- Berger JT, Majerovitz D. Stability of preferences for treatment among nursing home residents. The Gerontologist. 1998;38:217–223. doi: 10.1093/geront/38.2.217. [DOI] [PubMed] [Google Scholar]
- Blevins D, Deason-Howell LM. End-of-life care in nursing homes: The interface of policy, research, and practice. Behavioral Sciences and the Law. 2002;20:271–286. doi: 10.1002/bsl.486. [DOI] [PubMed] [Google Scholar]
- Bourgeois MS. Effects of memory aids on the didactic conversations of individuals with dementia. Journal of Applied Behavior Analysis. 1993;26:77–87. doi: 10.1901/jaba.1993.26-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E, Walker L, Blechner B, Wetle T. Assessing capacity to participate in discussions of advance directives in nursing homes: Findings from a study of the Patient Self-Determination Act. Journal of the American Geriatrics Society. 1997;45:79–83. doi: 10.1111/j.1532-5415.1997.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Burgio LD, Allen-Burge R, Roth DL, Bourgeois MS, Dijkstra K, Gerstle J, et al. "Come talk with me": Improving communication between nursing assistants and nursing home residents during care routines. The Gerontologist. 2001;41:449–460. doi: 10.1093/geront/41.4.449. [DOI] [PubMed] [Google Scholar]
- Burgio L, Scilley K, Hardin JM, Janosky J, Bonino P, Slater SC, et al. Studying disruptive vocalization and contextual factors in the nursing home using computer-assisted real-time observation. Journal of Gerontology: Psychological Sciences. 1994;49:P230–P239. doi: 10.1093/geronj/49.5.p230. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cohen J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychology Bulletin. 1968;70:213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- Coon DW, Schultz R, Ory MG. Innovative intervention approaches for Alzheimer's disease caregivers. In: Beigel D, Blum A, editors. Innovations in practice and service delivery across the lifespan. Oxford University Press; New York: 1999. pp. 295–325. [Google Scholar]
- DeLuca Havens GA. Differences in the execution/nonexecution of advance directives by community dwelling adults. Research in Nursing Health. 2000;23:319–333. doi: 10.1002/1098-240x(200008)23:4<319::aid-nur8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Diamond EL, Jernigan JA, Moseley RA, Messina V, McKeown RA. Decision-making ability and advance directive preferences in nursing home patients and proxies. The Gerontologist. 1989;29:622–626. doi: 10.1093/geront/29.5.622. [DOI] [PubMed] [Google Scholar]
- Ditto PH, Danks JH, Smucker WD, Bookwala J, Coppola KM, Dresser R, et al. Advance directives as acts of communication: A randomized controlled trial. Archives of Internal Medicine. 2001;161:421–430. doi: 10.1001/archinte.161.3.421. [DOI] [PubMed] [Google Scholar]
- Ferrell BA. Pain evaluation and management in the nursing home. Annals of Internal Medicine. 1995;123:681–687. doi: 10.7326/0003-4819-123-9-199511010-00007. [DOI] [PubMed] [Google Scholar]
- Field M, Cassel C. Approaching death: Improving care at the end of life. Institutes of Medicine, National Academy Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gabrel CS. Characteristics of elderly nursing home residents and discharges: Current data from the 1997 National Nursing Home Survey. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Washington, DC: 2000. [Google Scholar]
- Gage B, Dao T. Medicare's hospice benefit: Use and expenditures, 1996 cohort. U.S. Department of Health and Human Services; Washington, DC: 2000. Retrieved September 16, 2002, from http://www.aspe.hhs.gov/daltcp/reports/96useexp.htm. [Google Scholar]
- Hamilton BB, Laughlin JA, Fielder RC, Granger CV. Inter-rater reliability of the 7-level Functional Independence Measure (FIM) Scandinavian Journal of Rehabilitation Medicine. 1994;26:115–119. [PubMed] [Google Scholar]
- Hanson LC, Danis M, Garrett J. What is wrong with end-of-life care? Opinions of bereaved family members. Journal of the American Geriatrics Society. 1997;45:1339–1344. doi: 10.1111/j.1532-5415.1997.tb02933.x. [DOI] [PubMed] [Google Scholar]
- Hanson LC, Henderson M, Rodgman E. Where will we die? A national study of nursing home death [Letter to the editor] Journal of General Internal Medicine. 1999;14:101. [Google Scholar]
- Hebert KR. Decision making capacity regarding research participation in persons with Alzheimer's disease. University of Texas-Southwestern; Dallas: 2002. Unpublished master's thesis. [Google Scholar]
- Jacobson JA, Kasworm E, Battin MP, Francis LP, Green D, Botkin J, et al. Advance directives in Utah. Information from death certificates and informants. Archives of Internal Medicine. 1996;156:1862–1868. [PubMed] [Google Scholar]
- Kafonek S, Ettinger WH, Roca R, Kittner S, Taylor N, German PS. Instruments for screening depression and dementia in a long-term care facility. Journal of the American Geriatrics Society. 1989;37:29–34. doi: 10.1111/j.1532-5415.1989.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Kaldjian LC, Jekel JF, Friedland G. End-of-life decisions in HIV-positive patients: The role of spiritual beliefs. AIDS. 1998;12:103–107. doi: 10.1097/00002030-199801000-00012. [DOI] [PubMed] [Google Scholar]
- Kidd D, Stewart G, Baldry J, Johnson J, Rossiter D, Petruckevitch A, et al. The Functional Independence Measure: A comparative validity and reliability study. Disability and Rehabilitation. 1995;17:10–14. doi: 10.3109/09638289509166622. [DOI] [PubMed] [Google Scholar]
- King DA, Kim SYH, Conwell Y. Family matters: A social systems perspective on physician-assisted suicide and the older adult. Psychology, Public Policy, and the Law. 2000;6:434–451. [PubMed] [Google Scholar]
- Lynn J, Teno J, Dresser R, Brock D, Nelson HL, Nelson JL, et al. Dementia and advance-care planning: Perspectives from three countries on ethics and epidemiology. Journal of Clinical Ethics. 1999;10:271–285. [PubMed] [Google Scholar]
- Marson DC, Chatterjee A, Ingram KK, Harell LE. Toward a neurologic model of competency: Cognitive predictors of capacity to consent in Alzheimer's disease using three different legal standards. American Academy of Neurology. 1996;46:666–672. doi: 10.1212/wnl.46.3.666. [DOI] [PubMed] [Google Scholar]
- Marson DC, Ingram KK, Cody HA, Harrell LE. Assessing the competency of patients with Alzheimer's disease under different legal standards: A prototype instrument. Archives of Neurology. 1995;52:949–954. doi: 10.1001/archneur.1995.00540340029010. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Mezey MD, Mitty EL, Bottrell MM, Ramsey GC, Fisher T. Advance directives: Older adults with dementia. Clinics in Geriatric Medicine. 2000;16:255–268. doi: 10.1016/s0749-0690(05)70056-2. [DOI] [PubMed] [Google Scholar]
- Mezey MD, Mitty E, Rappaport M, Ramsey G. Implementation of the Patient Self-Determination Act (PSDA) in nursing homes in New York City. Journal of the American Geriatrics Society. 1997;45:43–49. doi: 10.1111/j.1532-5415.1997.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Miller SC, Gozalo P, Mor V. Outcomes and utilization for hospice and non-hospice nursing facility decedents. 2001 Retrieved October 14, 2002, from http://www.aspe.hhs.gov/daltcp/reports/oututil. htm.
- Mitchell SL, Berkowitz RE, Lawson FM, Lipsitz LA. A cross-national survey of tube-feeding decisions in cognitively impaired older persons. Journal of the American Geriatrics Society. 2000;48:391–397. doi: 10.1111/j.1532-5415.2000.tb04696.x. [DOI] [PubMed] [Google Scholar]
- Omnibus Budget Reconciliation Act of 1990 P. L. 101-508, §4206, and 4715, codified at 42 U.S.C. §§ 1395cc (a) (1) (q), 1395 mm (c) (8), 1395cc (f), 1396a (57), (58), 1396a (w)
- Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. Journal of Gerontology. Psychological Sciences. 1991;46:P15–P21. doi: 10.1093/geronj/46.1.p15. [DOI] [PubMed] [Google Scholar]
- Repp AC, Karsh KG, van Acker R, Felce D, Harmann M. A computer-based system for collecting and analyzing observational data. Journal of Special Education Technology. 1989;9:207–216. [Google Scholar]
- Rovner BW, Kafonek S, Filipp L, Lucas MJ, Folstein MF. Prevalence of mental illness in a community nursing home. American Journal of Psychiatry. 1986;143:1446–1449. doi: 10.1176/ajp.143.11.1446. [DOI] [PubMed] [Google Scholar]
- Silveira MJ, DiPiero A, Gerrity MS, Feudtner C. Patients' knowledge of options at the end of life: Ignorance in the face of death. Journal of the American Medical Association. 2000;284:2483–2488. doi: 10.1001/jama.284.19.2483. [DOI] [PubMed] [Google Scholar]
- Smyer MA, Allen-Burge R. Older adults' decision-making capacity: Institutional settings and individual choices. In: Cavanaugh JC, Whitbourne SK, editors. Gerontology: An interdisciplinary perspective. Oxford University Press; New York: 1999. pp. 391–413. [Google Scholar]
- Sonnenblick M, Friedlander Y, Steinberg A. Dissociation between the wishes of terminally ill parents and decisions by their offspring. Journal of the American Geriatrics Society. 1993;41:599–604. doi: 10.1111/j.1532-5415.1993.tb06729.x. [DOI] [PubMed] [Google Scholar]
- Stanley JM. More fiddling with the definition of death? Journal of Medical Ethics. 1987;13:21–25. doi: 10.1136/jme.13.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilden VP, Nelson CA, Dunn PM, Donius M, Tolle SW. Nursing's perspective on improving communication about nursing home residents' preferences for medical treatments at end of life. Nursing Outlook. 2000;48(3):109–115. doi: 10.1067/mno.2000.100434. [DOI] [PubMed] [Google Scholar]
- Zerzan J, Stearns S, Hanson L. Access to palliative care and hospice in nursing homes. Journal of the American Medical Association. 2000;284:2489–2494. doi: 10.1001/jama.284.19.2489. [DOI] [PubMed] [Google Scholar]


