Abstract
PURPOSE
To determine the role of epidermal growth factor (EGF) receptor (EGFR)–mediated signaling pathways in preventing infection-induced apoptosis in human corneal epithelial cells (HCECs).
METHODS
Epithelial monolayers of a telomerase-immortalized HCEC line, HUCL, and primary culture of HCECs were infected with Pseudomonas aeruginosa in the presence of the EGFR inhibitor tyrphostin AG1478, the extracellular signal-regulated kinase (ERK) inhibitor U0126, the phosphoinositide 3-kinase (PI3K) inhibitor LY294002, the heparin-binding EGF-like growth factor (HB-EGF) antagonist CRM197, the HB-EGF neutralizing antibody, or the matrix metalloproteinase inhibitor GM6001. The activation of EGFR was analyzed by immunoprecipitation using EGFR antibodies, followed by Western blot analysis with phosphotyrosine antibody. Phosphorylation of ERK and Akt, a major substrate of PI3K, and generation of cleaved caspase-3 and poly (ADP-ribose) polymerase (PARP) were determined by Western blot analysis. Apoptotic cells were characterized by positive staining of active caspase-3, loss of mitochondrial cytochrome c, and condensation of chromosomes. Apoptosis was also confirmed by measuring caspase-3 activity and assessing the generation of cleaved caspase-3 and PARP.
RESULTS
P. aeruginosa infection of HUCL cells resulted in EGFR activation and EGFR-dependent ERK1/2 and PI3K phosphorylation. Inhibition of EGFR, ERK1/2, and PI3K activities with kinase-specific inhibitors (AG1478, U0126, and LY294002, respectively) resulted in an increase in the number of apoptotic cells, in elevated cellular caspase-3 activity, and/or in increased cleaved PARP in P. aeruginosa–infected HUCL cells or primary culture of HCECs. Blocking HB-EGF ectodomain shedding by inhibition of matrix metalloproteinase–mediated proteolysis, downregulation of HB-EGF, or neutralization of its activity retarded infection-induced EGFR transactivation and, as a consequence, increased infection-induced HUCL apoptosis.
CONCLUSIONS
Bacterial infection of HCECs induces EGFR transactivation through HB-EGF ectodomain shedding. EGFR and its downstream ERK and PI3K signaling pathways play a role in preventing epithelial apoptosis in the early stage of bacterial infection.
The bacterium Pseudomonas aeruginosa is an opportunistic pathogen that can cause bacterial keratitis in patients who use extended-wear contact lenses.1 Corneal epithelial cells, like other mucosal epithelial linings in the body,2,3 constitute the first line of defense against microbial pathogens and have been shown to possess the ability to sense the presence of pathogenic bacteria such as P. aeruginosa.4–6 Recent studies have shown that the ability of epithelial cells to recognize pathogens is largely due to the expression of toll-like receptors (TLRs), an evolutionarily conserved family of receptors that function in innate immunity through recognition of pathogen-associated molecular patterns.7–9 Pattern recognition by TLRs then leads to activation of NF-κB and the mitogen-activated protein kinases (MAPKs) (e.g., p38 and c-Jun N-terminal kinase, JNK), through MyD88-dependent signaling pathways and production of proinflammatory cytokines.2,10–13 The release of these cytokines in resident corneal cells can augment or prolong the inflammatory response, a consequence necessary to contain the infection.4,14 The host inflammatory response, however, also contributes to corneal destruction.4,15,16
MAPKs link a variety of extracellular signals to a diverse range of cellular responses such as proliferation, differentiation, and apoptosis. There are three groups of mammalian MAPKs. The JNK17 and p3818 MAPKs are strongly responsive to stress and inflammatory signals, whereas the extracellular signal–regulated kinases, (ERK1/2)19 are generally activated by mitogens and differentiation-inducing stimuli.20,21 Infection of epithelial cells has been shown to activate ERK1/2 as well as phosphoinositide 3-kinase (PI3K), although the underlying mechanisms for their activation are not well defined.11,22–26 Both ERK1/2 and PI3K are generally elicited through the activation of cell surface receptors, such as epidermal growth factor (EGF) receptor (EGFR). The EGF family is composed of more than 10 members, including EGF,27 transforming growth factor-α,28 and heparin-binding EGF-like growth factor (HB-EGF).29 They are synthesized as membrane-anchored forms, which are then processed to give bioactive soluble factors. These factors act through the stimulation of specific cell-surface receptors, EGFR.30,31 Four related receptor tyrosine kinases have been identified (reviewed in Refs. 32–34). These are EGFR/erbB1/HER1, erbB2/HER2/neu, erbB3/HER3, and erbB4/HER4.30 Three of them, erbB1, -2, and -3, have been detected in corneal epithelium.35,36 We recently showed that HB-EGF is an endogenous EGFR ligand that is released on wounding of epithelial cells and acts in an autocrine fashion to activate EGFR in the cornea.37 The release of proHB-EGF, termed ectodomain shedding, is generally mediated by a matrix metalloproteinase(s).38,39 Numerous stress conditions are known to cause transactivation of the EGFRs through HB-EGF ectodomain shedding, including keratinocyte migration in cutaneous wound healing,40 cardiac hypertrophy,41,42 and bacterial challenge of lung and gastric epithelial cells.43–45 Whether P. aeruginosa is capable of inducing EGFR phosphorylation and subsequent ERK1/2 and PI3K activation in epithelial cells has not been explored.
The ERK1/2 and PI3K pathways are also associated with cellular apoptosis and mainly prevent apoptosis.46–48 Apoptosis, or programmed cell death, is a central mechanism for regulating the number of cells in adult tissues and is an important process in corneal development, homeostasis, and disease.49–54 There is increasing evidence that apoptosis plays a central role in modulating the pathogenesis of a variety of infectious diseases caused by bacteria, viruses, protozoa, and fungi.55 In this study, we investigated whether infection-induced EGFR transactivation and its subsequent activation of the ERK and PI3K pathways protect human corneal epithelial cells (HCECs) from apoptosis. We demonstrated that P. aeruginosa infection transactivates EGFR in HCECs through proHB-EGF ectodomain shedding and that subsequent activation of both MAPK and PI3K pathways plays an antiapoptotic role in P. aeruginosa–infected HCECs.
MATERIALS AND METHODS
HCEC Cultures and P. aeruginosa Infection
Human telomerase-immortalized corneal epithelial (HUCL) cells, kindly provided by James G. Rheinwald and Irene K. Gipson,56 were maintained in defined keratinocyte–serum-free medium (SFM; Invitrogen Life Technologies, Carlsbad, CA) in a humidified 5% CO2 incubator at 37°C. Before treatment, cells were split into culture dishes precoated with FNC (fibronectin-collagen, 1:3 mixture) coating mix (Athena Environmental Service, Inc., Baltimore, MD) and cultured in antibiotic-free defined keratinocyte-SFM. After cells were attached, the medium was replaced with keratinocyte basic medium (KBM; BioWhittaker, Walkersville, MD), and the cultures were incubated overnight (growth factor starvation).
To verify the results obtained from HUCL cells, HCECs were isolated from human donor corneas obtained from the Georgia Eye Bank. The epithelial sheet was separated from underlying stroma after overnight dispase treatment. The dissected epithelial sheet was trypsinized, and the epithelial cells were collected by centrifugation (500g, 5 minutes). HCECs were cultured in KGM (supplemented with growth factors; BioWhittaker) in T25 flasks coated with FNC and used at passage 3.
P. aeruginosa (PAO1 strain from a Pseudomonas genetic stock center at East Carolina University) was maintained on tryptic soy agar (Difco Laboratory, Detroit, MI). For infection experiments, bacteria were shaken in tryptic soy broth (Sigma-Aldrich, St. Louis, MO) at 37°C until absorbance at 600 nm reached optic density (OD) of 0.3 to 0.4. The bacterial culture was centrifuged at 6,000g for 10 minutes. Bacteria were resuspended in KBM and then used to challenge the growth factor-starved HUCL cells at a ratio of 25:1 (bacteria to cell) as follows. Resuspended bacteria were added to HUCL culture dishes, which were then centrifuged at 150g for 5 minutes to allow the bacteria to contact the cells readily. After 2 hours in culture, the cells were washed with PBS three times to remove unattached bacteria, and fresh KBM containing 100 μg/mL gentamicin to kill the extracellular bacteria was added. Cells were processed for immunostaining and Western blot analyses at the indicated times. The control cells for the infection experiments were treated the same including centrifugation and addition of gentamicin in KBM, but in the absence of bacteria.
To block EGFR-mediated signaling, cells were preincubated for 30 minutes at 37°C with tyrphostin AG1478 (Sigma-Aldrich), LY294002 (Cell Signaling Technology, Inc., Beverly, MA), or U0126 (Calbiochem, La Jolla, CA), followed by incubation with P. aeruginosa in the presence of the same inhibitors. For blocking HB-EGF shedding or function, cells were pretreated with CRM197 (Sigma-Aldrich), HB-EGF neutralizing antibody (R&D Systems, Inc., Minneapolis, MN), or GM6001 (Calbiochem) for 1 hour at 37°C before incubation with bacteria in the presence of the same inhibitors.
Invasion Assay
In accordance with a published method,57 HCECs were cultivated in 24-well plates and infected with P. aeruginosa at a ratio of 25:1 (bacteria to cell). After 2 hours in culture, the cells were washed with PBS three times to remove unattached bacteria, and fresh KBM containing 100 μg/mL gentamicin was added. After two additional hours in culture, monolayers were washed twice with PBS, lysed, and homogenized in 1 mL of lysis solution (1% Triton X-100). Lysates were serially diluted and plated on tryptic soy agar, and colonies were enumerated after overnight incubation. For control, an HCEC monolayer was fixed with fresh-made 4% formaldehyde in PBS for 20 minutes before addition of bacteria. No viable bacteria were detected from control cell lysates.
Immunoprecipitation of EGFR
After infection with bacteria for the indicated times, HUCL cells (1 × 107 cells in 100-mm dishes) were lysed with 500 μL radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 100 mM Tris-HCl [pH 7.5], 1% deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 50 mM NaF, 100 mM sodium pyrophosphate, 3.5 mM sodium orthovanadate, proteinase inhibitor cocktails, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) and centrifuged at 12,000 rpm at 4°C for 30 minutes to remove debris. The protein concentration was determined with kit (Micro BCA; Pierce Biotechnology, Rockford, IL). Equal amounts of protein (800 μg) were subjected to immunoprecipitation with 3 μg anti-human EGFR antibody and 20 μL protein G beads (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. Immunoprecipitates were washed with the washing buffer (150 mM NaCl, 100 mM Tris-HCl [pH 8.0], 0.1% Triton X-100, 50 mM NaF, 100 mM sodium pyrophosphate, 3.5 mM sodium orthovanadate, proteinase inhibitor cocktails, and 0.1 mM PMSF) three times, dissolved in 10 μL 4 × Laemmli’s buffer,58 and boiled for 5 minutes. The samples were then subjected to Western blot analysis.
Western Blot Analysis
Activation of ERK and PI3K and cleavage of caspase-3 and poly (ADP-ribose) polymerase (PARP; EC 2.4.2.30) were also determined by Western blot analysis of HUCL cell lysates. The same amount of cell lysates, collected at indicated times, was applied to a 5% to 15% gradient SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The membranes were blocked with 5% nonfat skim milk in Tris-buffered saline containing 0.05% Tween 20 (TBST) for 1 hour and then incubated with specific antibodies for 1 hour at room temperature or overnight at 4°C. The antibodies used were: anti-phosphotyrosine (PY99) anti-phospho-ERK1/2, anti-ERK2, and anti-caspase-3 (Santa Cruz Biotechnology); anti-phospho-Akt and anti-Akt (Cell Signaling Technology, Inc.); and anti- PARP (Biomol Research Laboratories, Inc., Plymouth Meeting, PA). After primary antibody incubation, nitrocellulose membranes were washed three times with TBST and then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour. An enhanced chemiluminescence detection system (Supersignal; Pierce Biotechnology) was used to visualize the labeled protein bands. Molecular mass was estimated by comparison of sample bands with prestained molecular mass markers (Bio-Rad, Hercules, CA).
Immunostaining and Analysis of Apoptosis
HUCL cells were grown on glass coverslips (Fisher Scientific, Pittsburgh, PA) in six-well dishes precoated with FNC, starved in KBM overnight, and pretreated with inhibitors and challenged with P. aeruginosa as described earlier. After incubation with bacteria for 4 hours, cells were fixed with 4% freshly made formaldehyde (Sigma-Aldrich), permeabilized with 0.1% Triton X-100, blocked with 5% normal goat serum, and stained with rabbit anti-cleaved caspase-3 antibody (Cell Signaling Technology) and mouse anti-cytochrome c antibody (BD-Pharmingen, San Diego, CA). Secondary antibodies were FITC-conjugated goat anti-rabbit IgG and Texas red–conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Nuclei were stained with 5 μg/mL Hoechst 33342 (Sigma-Aldrich) in PBS for 5 minutes at room temperature. The coverslips were then mounted (Vectashield; Vector Laboratories, Inc., Burlingame, CA). The apoptotic characteristics examined included active caspase-3 staining, loss of mitochondrial cytochrome c, and nuclear condensation and fragmentation. For each condition, apoptosis was measured in five fields with approximately 500 to 600 cells per field. The results were expressed as mean ± SE of five experiments.
Measurement of Caspase-3 Activity
Caspase-3 activity was determined by an enzymatic assay, using the fluorogenic peptide substrate DEVD-AFC (carbobenzoxy-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin). After incubation, plates with cells were placed on ice. The incubation medium was centrifuged to collect floating cells and cellular debris. The collected floaters were then combined with the cells remaining in the dishes for lysis in 1% Triton X-100 buffer (1% Triton X-100, 1 mM dithiothreitol [DTT], 25 mM HEPES, 115 mM NaCl, 1 mM KH2PO4, 1 mM KCl [pH 7.4], and 1 μL/mL protease inhibitor cocktail). The resultant lysate was centrifuged at 12,000g for 5 minutes at 4°C to obtain the supernatant. The centrifuged lysate (25 μg protein in 50 μL) was added to 200 μL enzymatic reaction buffer (0.1% CHAPS [3-([3-cholamidopropyl]di-methylammonio-2-hydroxy-1-propanesulfonate], 10% sucrose, 1 mM EDTA, 10 mM DTT, and 100 mM HEPES [pH 7.4]) containing 50 μM DEVD-AFC. After 60 minutes of reaction at 37°C, fluorescence was monitored at excitation 360 nm and emission 530 nm on a plate reader (SpectroFluor; Tecan US, Inc., Research Triangle Park, NC). Background fluorescence was determined with 50 μL lysis buffer and 200 μL reaction buffer with 50 μM DEVD-AFC and subtracted from the reaction values. For each measurement, a standard curve was constructed with free AFC. Based on the standard curve, the fluorescence reading from the enzymatic reaction was calculated into the molar amount of liberated AFC. For each condition, measurements of caspase-3 activity were performed in triplicate. The results were expressed as the mean ± SE of three experiments.
Statistical Analysis
Each figure shows the results of experiments repeated at least three times. All statistical analyses were performed using the unpaired Student’s t-test. P < 0.05 was considered statistically significant.
RESULTS
P. aeruginosa–Induced EGFR, ERK1/2, and PI3K Activation
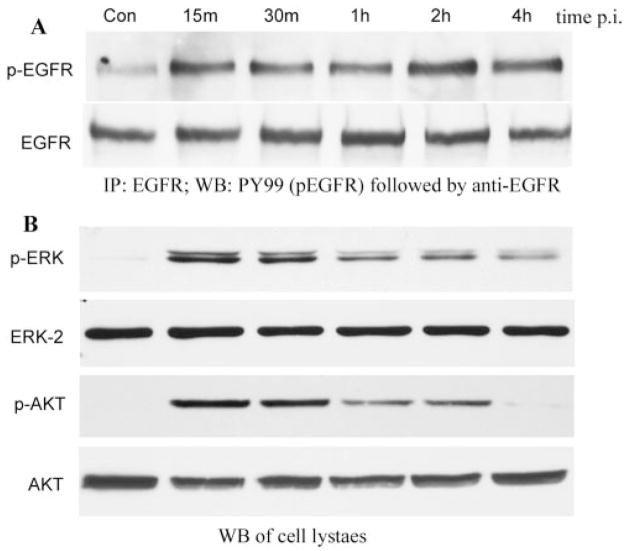
P. aeruginosa (PAO1 strain) induced EGFR phosphorylation in cultured HUCL cells (Fig. 1A). Uninfected control cells showed a low, but detectable, level of phosphorylated EGFR that increased 15 minutes after infection (PI) and remained at an elevated level for 4 hours PI whereas the levels of precipitated EGFR from control and P. aeruginosa–infected cells were similar. ERK1/2 and Akt (a major substrate of PI3K, also termed protein kinase B) showed increased levels of phosphorylation in response to P. aeruginosa infection (Fig. 1B). Control, noninfected cells exhibited undetectable levels of phosphorylated ERK1/2 and Akt. Phosphorylation increased to the highest levels within 15 minutes for both ERK1/2 and Akt and then slowly declined. Low, but detectable, levels of phosphorylated ERK1/2 (pERK, Fig. 1B) remained at 4 and 2 hours PI for Akt (pAKT, Fig. 1B). The protein levels of ERK2 and Akt remained constant throughout the infection period.
FIGURE 1.
P. aeruginosa–induced EGFR, ERK1/2, and Akt phosphorylation. HUCL cells were grown to ~90% confluence on 100-mm plates and serum-starved overnight. Cells were then infected with P. aeruginosa at a cell-to-bacterium ratio of 1:25 over a 4-hour time course. Cell lysates were prepared at the designated time points PI. (A) For immunoprecipitation, 800 μg protein for each sample was immunoprecipitated (IP) with 2.5 μg agarose-conjugated EGFR antibodies, subjected to SDS-PAGE, and probed (WB) with mouse anti-PY99 antibody (top); after stripping, reproved with mouse anti-EGFR (bottom). (B) To assess phosphorylation of ERK1/2 and Akt, 10 and 30 μg cell lysates of the same samples were subjected to Western blot analysis (WB) with either anti-phospho-ERK1/2 (pERK) or anti-phospho-Akt (pAKT). To normalize protein loading onto blots, anti-ERK2 (ERK2), and anti-Akt (AKT) antibodies were used as probes. The results are representative of three independent experiments.
EGFR-Dependent ERK1/2 and PI3K Activation in P. aeruginosa–Infected HUCL Cells
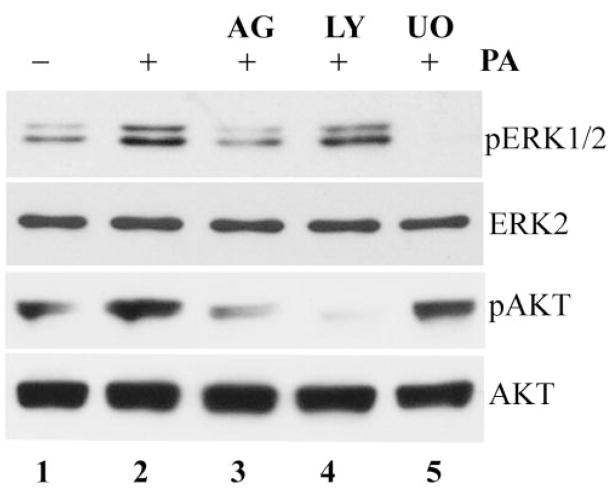
Figure 2 shows that infection-induced ERK and Akt phosphorylation was EGFR dependent. The presence of the EGFR inhibitor AG1478 in culture medium (lane 3) blocked infection-induced ERK1/2 and Akt phosphorylation (Fig. 2, compare lanes 3 and 2). As expected, the presence of the PI3K inhibitor LY294002 (lane 4) blocked infection-induced Akt phosphorylation and exhibited minimal effect on ERK1/2 phosphorylation. Similarly, U0126, an MEK inhibitor, inhibited P. aeruginosa–induced ERK1/2, but not Akt, phosphorylation (lane 5). The protein levels of ERK2 and Akt remained constant in inhibitor-treated and control cells.
FIGURE 2.
EGFR-dependent ERK1/2 and PI3K activation in P. aeruginosa–infected HUCL cells. HUCL cells were pretreated with 500 nM AG1478 (lane 3), 10 μM LY294002 (lane 4), or 10 μM U0126 (lane 5) for 30 minutes and stimulated with P. aeruginosa at a cell-to-bacterium ratio of 1:25 in the presence of the inhibitors for 1 hour. Uninfected cells (lane 1) and cells infected with P. aeruginosa alone (lane 2) were used as controls. Cell lysates (10 and 30 μg) were used for detection of phospho-ERK1/2 (pERK1/2) and phospho-Akt (pAKT), respectively. To normalize protein loading and determine the change of ERK2 and Akt in infected cells, anti-ERK2 (ERK2) and anti-Akt (AKT) were used to probe the samples.
Increased P. aeruginosa–Induced Apoptosis by Inhibition of EGFR-Mediated Signaling Pathways
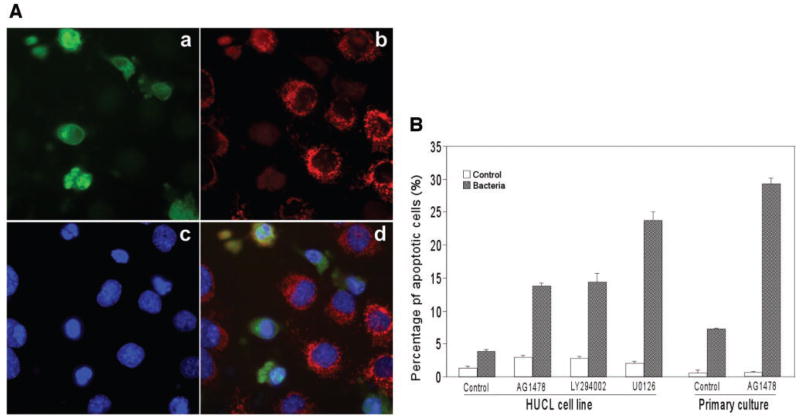
Monolayers of HUCL cells were infected with P. aeruginosa, and apoptosis was measured by indirect immunofluorescence for the presence of cleaved caspase-3 and released cytochrome c. Figure 3A shows the staining of P. aeruginosa–infected cells treated with EGFR inhibitor AG1478. Many cells stained positive for the active (cleaved) form of caspase-3 (Fig. 3Aa). These caspase-3 active cells lacked typical mitochondria-associated cytochrome c staining (Fig. 3Ab) but showed nuclear condensation and fragmentation (Fig. 3Ac). Taken together, the immunostaining and morphologic features (Fig. 3Ad) indicate that these HUCL cells are apoptotic.
FIGURE 3.
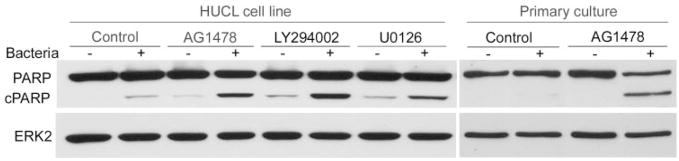
Augmentation of P. aeruginosa–induced apoptosis by inhibition of EGFR-mediated signaling pathways. HUCL cells were preincubated with 250 nM AG1478, 10 μM LY294002, and 5 μM U0126 for 30 minutes and challenged with P. aeruginosa at a cell-to-bacteria ratio of 1:25 in the presence of the inhibitors for 4 hours. Cells were fixed with formaldehyde for immunofluorescence of active caspase-3, and cytochrome c, and nuclear staining with Hoechst 33342. (A) Immunostaining of (Aa) cleaved caspase-3, (Ab) released cytochrome c from mitochondria, and (Ac) nuclear condensation with Hoechst 33342 staining; (Ad) merged image of the previous three panels. The cells with these three characteristics were identified as apoptotic cells. (B) Percentage of cellular apoptosis induced by inhibition of ERK1/2 and PI3K pathways in P. aeruginosa–infected HUCL and primary HCECs. Apoptotic cells and total cells were measured in five fields with approximately 500 to 600 cells per field. The numbers of total cells were calculated by Hoechst 33342 staining. Values are expressed as means ± SE; n = 5). **P < 0.01 (compared with results for inhibitors alone).
Figure 3B shows the percentage of cleaved caspase-3–positive cells in infected cultures of HUCL and primary HCECs in the presence or absence of signaling pathway inhibitors; the control group was not subjected to P. aeruginosa infection. There was a slight increase in apoptosis for infection alone (4 hours, 2.54% after subtraction of control, nontreated cells, 1.34%) or AG1478 alone (1.58%). At this time (4 and 2 hours after addition of gentamicin), there were 14 viable intracellular bacteria per cell as determined by invasion assay. Treatment of infected cells with AG1478 significantly increased the number of apoptotic cells to 12.4% (P < 0.01). We also treated infected cells with the PI3K and ERK inhibitors LY294002 and U0126. Although LY294002 had effects similar to those of AG1478 in inducing apoptosis in control (1.46%) and P. aeruginosa–infected HUCL cells (13.2%), more apoptotic cells (22.32%) were seen with U0126 treatment. In the absence of infection, U0126 alone showed almost no apoptosis (0.68%).
Primary HCECs were also subjected to apoptosis analysis in the presence of EGFR inhibitor. Almost no apoptosis was detected in control (0.6%) and AG1478-treated (0.54%) cells. P. aeruginosa infection resulted in 6.66% of cells becoming apoptotic, whereas treatment of infected cells with AG1478 greatly increased the number of apoptotic cells (to 28.64%, P < 0.01).
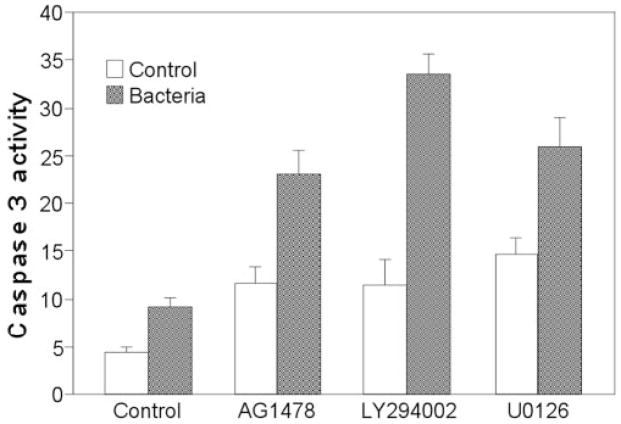
Caspase-3, an effector caspase, is known to be a major contributor to the apoptotic machinery in many cell types. Therefore, we assessed caspase-3 enzymatic activity and the effects of EGFR signaling inhibitors in P. aeruginosa – infected cells (Fig. 4). Infection alone and treatment with EGFR signaling inhibitors alone slightly increased caspase-3 activity, whereas each of the three inhibitors (AG1478, LY294002, and U0126) induced greatly increased caspase-3 activity in P. aeruginosa–infected HUCL cells.
FIGURE 4.
Effects of blocking EGFR-mediated pathways on caspase-3 activity in P. aeruginosa–infected HUCL cells. HUCL cells were preincubated with 250 nM AG1478, 10 μM LY294002, or 5 μM U0126 for 30 minutes and challenged with P. aeruginosa at a cell-to-bacterium ratio of 1:25 in the presence of the inhibitors for 4 hours. Cells were lysed and subjected to a DEVD-AFC cleavage assay. Enzymatic activity of caspase was indicated by the amount of DEVD-AFC cleavage (nano-moles per milligram protein per hour). Values are expressed as mean ± SE; n = 3). **P < 0.01 (compared with results for inhibitors without P. aeruginosa stimulation).
The cleavage of PARP by caspases is a characteristic feature of apoptosis, and the timing of apoptosis-associated events is often determined relative to the onset of PARP cleavage. Thus, the generation of cleaved PARP was assessed in P. aeruginosa–infected HCUL cells and primary HCECs (Fig. 5) with Western blot analysis. Whereas no detectable PARP cleavage was seen in control HUCL cultures, P. aeruginosa infection or treatment of cultured cells with EGFR and its downstream signaling pathway inhibitors resulted in detectable amounts of cleaved PARP. Treatment of P. aeruginosa–infected cells with these inhibitors generated a relatively large amount of cleaved PARP. In primary HCECs, no cleaved PARP was generated in control and AG1478-treated cells, consistent with the fact that no apoptosis was seen in those cells (Fig. 3B). Infection of primary HCECs resulted in an almost nondetectable level of cleaved PARP, whereas the presence of AG1478 induced a significant increase in cleaved PARP and a decrease in intact enzyme. The protein level of ERK2 (to normalize the loading) remained constant in all conditions.
FIGURE 5.
Effects of blocking EGFR-mediated pathways on the cleavage of PARP in P. aeruginosa–infected HUCL cells and primary HCECs. Cells were preincubated with 250 nM AG1478, 10 μM LY294002, or 5 μM U0126 for 30 minutes and challenged with P. aeruginosa at a cell-to-bacterium ratio of 1:25 in the presence of the inhibitors for 5 hours. Cells were lysed and 15 μg cell lysates were immunoblotted with anti-PARP antibody. cPARP represents the cleaved form. To normalize protein loading, anti-ERK2 was applied as probe to the same membrane.
Ectodomain Shedding of proHB-EGF in P. aeruginosa–Induced EGFR Transactivation
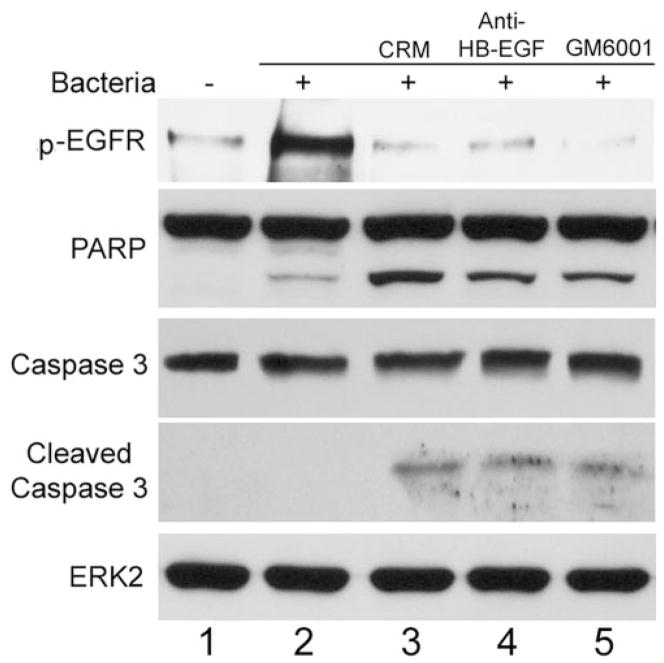
To understand the underlying mechanism for EGFR transactivation, the effects of HB-EGF neutralization and inhibition of ectodomain shedding on EGFR phosphorylation and epithelial apoptosis in response to P. aeruginosa infection were assessed (Fig. 6). Incubation of HUCL cells with P. aeruginosa for 30 minutes induced elevated EGFR phosphorylation; addition of CRM197, a highly specific antagonist of HB-EGF (lane 3)59,60; HB-EGF neutralizing antibody (lane 4); or GM6001 (a MMP inhibitor, lane 5) blocked infection-induced EGFR phosphorylation. Treatment of the cells with these HB-EGF targeting reagents also resulted in increased apoptosis in P. aeruginosa–infected cells as assessed by the appearance of cleaved PARP as well as cleaved caspase-3, whereas the protein level of ERK2 (to normalize the loading) remained constant.
FIGURE 6.
Effects of HB-EGF inhibitors and metalloprotease inhibitor on EGFR phosphorylation and augmented apoptosis in P. aeruginosa–infected HUCL cells. HUCL cells were preincubated with 20 μg/mL CRM197, 10 μg/mL HB-EGF neutralizing antibody, or 0.5 μg/mL GM6001 for 1 hour and challenged with P. aeruginosa at a cell-to-bacterium ratio of 1:25 in the presence of the inhibitors for 30 minutes. Cells were lysed and immunoprecipitated with anti-EGFR antibody, and subjected to Western blot analysis with anti-phosphotyrosine antibody (top). For apoptosis assay, cells were immunoblotted with anti-caspase-3 and anti-PARP antibodies; immunoblot analysis with anti-ERK antibody was used for normalization of protein loading.
DISCUSSION
In our study, P. aeruginosa infection of HUCL cells led to specific cellular responses involving the release of proHB-EGF through ectodomain shedding and subsequent transactivation of EGFR. The P. aeruginosa–induced EGFR activation elicited both the ERK1/2 and PI3K pathways. We demonstrated that inhibition of any step leading to ERK1/2 and PI3K activation augmented P. aeruginosa–induced apoptosis in HUCL cells, suggesting a protective role of EGFR transactivation in preventing P. aeruginosa–induced apoptosis.
P. aeruginosa infection of HCECs induced activation of ERK and PI3K. To date, the mechanisms by which infection induces epithelial ERK and PI3K activation are still unclear. Our results showed that the presence of the EGFR inhibitor AG1478 not only inhibited EGFR activation, as expected, but also blocked infection-induced ERK and Akt phosphorylation. Thus, we conclude that the ERK and PI3K signaling pathways are downstream signaling pathways of EGFR transactivation in response to P. aeruginosa infection in HCECs.
How might EGFR be transactivated in HUCL cells in response to P. aeruginosa-infection? Constitutive gene expression of EGFR ligands such as EGF, HB-EGF, TGF-α, and their receptors have also been demonstrated in HCECs.36,61–64 Recently, ectodomain shedding of HB-EGF, which is proteolytic release of the extracellular domain from the membrane-anchored form to give bioactive soluble factors, has been shown to be a major pathway leading to EGFR transactivation in a variety of cells.65,66 In the present study, transactivation of EGFR by P. aeruginosa infection was dependent on HB-EGF ectodomain shedding, since the induced EGFR phosphorylation and its downstream signaling were blocked by reagents that inhibit ectodomain shedding or impair HB-EGF function. These reagents also induced apoptosis in P. aeruginosa–infected HUCL cells. The mechanisms underlying infection-induced HB-EGF shedding remain to be determined.
What might be the role for EGFR-mediated antiapoptosis in epithelial cells during P. aeruginosa infection? There is increasing evidence that apoptosis plays an important role in modulating the pathogenesis of a variety of infectious diseases caused by bacteria, viruses, protozoa, and fungi.55,67 Intracellular bacterial pathogens, such as the PAO1 strain used in this study, can either induce apoptosis to destroy host cells or prevent epithelial apoptosis so they may maintain their intracellular niche.55,68,69 Recently, Huang and Hazlett,70 using microarray analysis, demonstrated the upregulation of several apoptosis-inhibiting related genes in the P. aeruginosa–infected cornea of susceptible mice and proapoptotic genes in resistant mice. Upregulation of these antiapoptotic genes may prevent epithelial apoptosis, allowing entry and multiplication of bacteria in the epithelial cells. The concept that pathogens affect host death mechanisms to enhance their survival has long been studied with viruses,71 but studies have just begun to provide support for bacterial pathogens in recent years.67,68,70 EGFR transactivation may serve as a link between P. aeruginosa infection and inhibition of host cell apoptosis.
Alternatively, EGFR-transactivation–based antiapoptosis may be essential for proper host response at an early stage of infection. There is increasing experimental evidence that the epithelium, the first line of defense against infection, is an essential component of innate immune responses after infection with bacterial pathogens.10 The role of the epithelium is twofold: First, in response to the presence of pathogens, the epithelial cells recognize the molecular patterns of pathogens such as flagellin5 and LPS6 and send initial signals, such as the release of proinflammatory cytokines, for recruiting the neutrophils and mononuclear lymphocytes into the cornea. Second, in response to infection, epithelial cells produce innate defense molecules such as β-defensin, either through direct interaction between bacteria and epithelium or by autocrine regulation of the produced cytokines in the infected corneas.72,73 Production of defensin by epithelial cells would provide an innate protection against infection. Thus, the proper function of epithelium is necessary for the early responses of the host to infection. Consequently, apoptosis of epithelial cells at an early stage of P. aeruginosa infection would result in a decrease in the effectiveness of host innate immune responses. As such, we suggest that transactivation of EGFR in HCECs in response to P. aeruginosa infection may contribute to the cornea’s innate defense and inflammatory response through activation of antiapoptotic mechanisms in epithelial cells. Further study is warranted to determine whether the antiapoptotic strategy is used by bacteria to their advantage or used by hosts as one of the defense mechanisms. However, as the infection progresses, it may be necessary for HCECs containing invasive bacteria to undergo apoptosis.70 Apoptotic cell death in the infected cornea would permit other cells to phagocytize the apoptotic bodies containing bacteria, allowing an epithelial cell to eliminate bacteria in the cornea effectively without significant inflammation, as in a P. aeruginosa–infected lung.74
In conclusion, the mechanism by which P. aeruginosa–epithelial cell interactions activate EGFR bears striking resemblance to a paradigm described recently in a variety of cells, in which EGFR is transactivated by the release of HB-EGF from the cell surface through metalloproteinase activation. Our data linked EGFR transactivation to the observed ERK signaling as well as PI3K activation in bacteria-infected epithelial cells. Furthermore, our study showed that EGFR-induced ERK1/2 and PI3K activation plays a role in preventing apoptosis of HCECs induced by P. aeruginosa infection. Further studies are needed to identify factor(s) triggering HB-EGF shedding and the mechanism by which P. aeruginosa infection induces epithelial apoptosis in the cornea.
Acknowledgments
Supported by National Eye Institute Grants EY14080 and EY10869.
The authors thank Rhea-Beth Markowitz (IMMAG, MCG) for critical reading of the manuscript and Yu Ding for technical assistance.
Footnotes
Disclosure: J. Zhang, None; H. Li, None; J. Wang, None; Z. Dong, None; S. Mian, None; F.-S.X. Yu, None
References
- 1.Cohen E, Laibson P, Arentsen J, Clemons C. Corneal ulcers associated with cosmetic extended wear soft contact lenses. Ophthalmology. 1987;94:109–114. doi: 10.1016/s0161-6420(87)33491-8. [DOI] [PubMed] [Google Scholar]
- 2.Gewirtz AT. Intestinal epithelial toll-like receptors: to protect and serve? Curr Pharm Des. 2003;9:1–5. doi: 10.2174/1381612033392422. [DOI] [PubMed] [Google Scholar]
- 3.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-κB activation in epithelial cells in a toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 4.Kurpakus-Wheater M, Kernacki KA, Hazlett LD. Maintaining corneal integrity how the “window” stays clear. Prog Histochem Cytochem. 2001;36:185–259. [PubMed] [Google Scholar]
- 5.Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–4254. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- 6.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877. [PubMed] [Google Scholar]
- 7.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila toll protein signals activation of adaptive immunity (see comments) Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 10.Philpott DJ, Girardin SE, Sansonetti PJ. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr Opin Immunol. 2001;13:410–416. doi: 10.1016/s0952-7915(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 11.Li JD. Exploitation of host epithelial signaling networks by respiratory bacterial pathogens. J Pharmacol Sci. 2003;91:1–7. doi: 10.1254/jphs.91.1. [DOI] [PubMed] [Google Scholar]
- 12.Keates S, Keates AC, Warny M, Peek RM, Jr, Murray PG, Kelly CP. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J Immunol. 1999;163:5552–5559. [PubMed] [Google Scholar]
- 13.Guillot L, Medjane S, Le-Barillec K, et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 14.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 15.Steadman R, Irwin MH, St John PL, Blackburn WD, Heck LW, Abrahamson DR. Laminin cleavage by activated human neutrophils yields proteolytic fragments with selective migratory properties. J Leukoc Biol. 1993;53:354–365. doi: 10.1002/jlb.53.4.354. [DOI] [PubMed] [Google Scholar]
- 16.Steuhl KP, Doring G, Henni A, Thiel HJ, Botzenhart K. Relevance of host-derived and bacterial factors in Pseudomonas aeruginosa corneal infections. Invest Ophthalmol Vis Sci. 1987;28:1559–1568. [PubMed] [Google Scholar]
- 17.Kyriakis JM, Banerjee P, Nikolakaki E, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 18.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 19.Owaki H, Makar R, Boulton TG, Cobb MH, Geppert TD. Extracellular signal-regulated kinases in T cells: characterization of human ERK1 and ERK2 cDNAs. Biochem Biophys Res Commun. 1992;182:1416–1422. doi: 10.1016/0006-291x(92)91891-s. [DOI] [PubMed] [Google Scholar]
- 20.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 21.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 22.Nozawa Y, Nishihara K, Peek J, et al. Identification of a signaling cascade for interleukin-8 production by Helicobacter pylori in human gastric epithelial cells. Biochem Pharmacol. 2002;64:21–30. doi: 10.1016/s0006-2952(02)01030-4. [DOI] [PubMed] [Google Scholar]
- 23.Mynott TL, Crossett B, Prathalingam SR. Proteolytic inhibition of Salmonella enterica Serovar Typhimurium-induced activation of the mitogen-activated protein kinases ERK and JNK in cultured human intestinal cells. Infect Immun. 2002;70:86–95. doi: 10.1128/IAI.70.1.86-95.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbie S, Chen L, Davis R, Galan J. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 25.Bierne H, Dramsi S, Gratacap MP, et al. The invasion protein InIB from Listeria monocytogenes activates PLC-gamma1 downstream from PI 3-kinase. Cell Microbiol. 2000;2:465–476. doi: 10.1046/j.1462-5822.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 26.Coombes BK, Mahony JB. Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of HEp2 cells. Cell Microbiol. 2002;4:447–460. doi: 10.1046/j.1462-5822.2002.00203.x. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S. Isolation and biological effects of an epidermal growth-stimulating protein. Nat Cancer Inst Monogr. 1964;13:13–27. [PubMed] [Google Scholar]
- 28.Derynck R, Roberts AB, Winkler ME, Chen EY, Goeddel DV. Human transforming growth factor: precursor structure and expression in E. coli. Cell. 1984;38:287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- 29.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 30.Hynes NE, Horsch K, Olayioye MA, Badache A. The ErbB receptor tyrosine family as signal integrators. Endocr Relat Cancer. 2001;8:151–159. doi: 10.1677/erc.0.0080151. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter G, Wahl M. The epidermal growth factor family. In: Sporn MB, Roberts AB, editors. Peptides, Growth Factors and their Receptors. Vol. 1. New York: Springer-Verlag; 1991. pp. 69–171. [Google Scholar]
- 32.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 33.Riese DJ, II, Komurasaki T, Plowman GD, Stern DF. Activation of ErbB4 by the bifunctional epidermal growth factor family hormone epiregulin is regulated by ErbB2. J Biol Chem. 1998;273:11288–11294. doi: 10.1074/jbc.273.18.11288. [DOI] [PubMed] [Google Scholar]
- 34.Olayioye M, Neve R, Lane Ha, Hynes N. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Carvajal M, Carraway CA, Carraway K, Pflugfelder SC. Expression of the receptor tyrosine kinases, epidermal growth factor receptor, ErbB2, and ErbB3, in human ocular surface epithelia. Cornea. 2001;20:81–85. doi: 10.1097/00003226-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]
- 37.Xu K, Ding Y, Lin J, Dong Z, Yu F. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:813–820. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112:3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto R, Mekada E. Heparin-binding EGF-like growth factor: a juxtacrine growth factor. Cytokine Growth Factor Rev. 2000;11:335–344. doi: 10.1016/s1359-6101(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 40.Tokumaru S, Higashiyama S, Endo T, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asakura M, Kitakaze M, Takashima S, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 42.Liao JK. Shedding growth factors in cardiac hypertrophy. Nat Med. 2002;8:20–21. doi: 10.1038/nm0102-20. [DOI] [PubMed] [Google Scholar]
- 43.Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat Med. 2002;8:41–46. doi: 10.1038/nm0102-41. [DOI] [PubMed] [Google Scholar]
- 44.Keates S, Sougioultzis S, Keates AC, et al. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Biol Chem. 2001;276:48127–48134. doi: 10.1074/jbc.M107630200. [DOI] [PubMed] [Google Scholar]
- 45.Wallasch C, Crabtree JE, Bevec D, Robinson PA, Wagner H, Ullrich A. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem Biophys Res Commun. 2002;295:695–701. doi: 10.1016/s0006-291x(02)00740-4. [DOI] [PubMed] [Google Scholar]
- 46.Choi IJ, Kim JS, Kim JM, Jung HC, Song IS. Effect of inhibition of extracellular signal-regulated kinase 1 and 2 pathway on apoptosis and bcl-2 expression in Helicobacter pylori-infected AGS cells. Infect Immun. 2003;71:830–837. doi: 10.1128/IAI.71.2.830-837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem. 2000;79:355–369. [PubMed] [Google Scholar]
- 48.Chang F, Lee JT, Navolanic PM, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 49.Hartel S, Zorn-Kruppa M, Tykhonova S, Alajuuma P, Engelke M, Diehl H. Staurosporine-induced apoptosis in human cornea epithelial cells in vitro. Cytometry. 2003;55:15–23. doi: 10.1002/cyto.a.10068. [DOI] [PubMed] [Google Scholar]
- 50.Stapleton F, Kim J, Kasses J, Willcox M. Mechanisms of apoptosis in human corneal epithelial cells. Adv Exp Med Biol. 2002;506:827–834. doi: 10.1007/978-1-4615-0717-8_117. [DOI] [PubMed] [Google Scholar]
- 51.Yeh S, Song X, Farley W, Li D, Stern M, Pflugfelder S. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44:124–129. doi: 10.1167/iovs.02-0581. [DOI] [PubMed] [Google Scholar]
- 52.Esco MA, Wang Z, McDermott ML, Kurpakus-Wheater M. Potential role for laminin 5 in hypoxia-mediated apoptosis of human corneal epithelial cells. J Cell Sci. 2001;114:4033–4040. doi: 10.1242/jcs.114.22.4033. [DOI] [PubMed] [Google Scholar]
- 53.Yew DT, Sha O, Li WW, Lam TT, Lorke DE. Proliferation and apoptosis in the epithelium of the developing human cornea and conjunctiva. Life Sci. 2001;68:2987–3003. doi: 10.1016/s0024-3205(01)01099-2. [DOI] [PubMed] [Google Scholar]
- 54.Miles D, Athmanathan S, Thakur A, Willcox M. A novel apoptotic interaction between HSV-1 and human corneal epithelial cells. Curr Eye Res. 2003;26:165–174. doi: 10.1076/ceyr.26.3.165.14899. [DOI] [PubMed] [Google Scholar]
- 55.Menaker RJ, Jones NL. Fascination with bacteria-triggered cell death: the significance of Fas-mediated apoptosis during bacterial infection in vivo. Microbes Infec. 2003;5:1149–1158. doi: 10.1016/j.micinf.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Rheinwald JG, Hahn WC, Ramsey MR, et al. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korth MJ, Lara JC, Moseley SL. Epithelial cell invasion by bovine septicemic Escherichia coli. Infect Immun. 1994;62:41–47. doi: 10.1128/iai.62.1.41-47.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 59.Uchida T, Pappenheimer A, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973;248:3838–3844. [PubMed] [Google Scholar]
- 60.Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- 61.Wilson S, Lloyd S, He Y. EGF, basic FGF, and TGF beta-1 messenger RNA production in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1987–1989. [PubMed] [Google Scholar]
- 62.Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999;68:377–397. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- 63.Li D, Tseng S. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 64.Zieske JD, Wasson M. Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J Cell Sci. 1993;106:145–152. doi: 10.1242/jcs.106.1.145. [DOI] [PubMed] [Google Scholar]
- 65.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 66.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 67.Zychlinksy A, Sansonetti P. Perspectives series: host/pathogen interactions—apoptosis in bacterial pathogenesis. J Clin Invest. 1997;100:493–495. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knodler LA, Finlay BB. Salmonella and apoptosis: to live or let die? Microbes Infect. 2001;3:1321–1326. doi: 10.1016/s1286-4579(01)01493-9. [DOI] [PubMed] [Google Scholar]
- 69.Steele-Mortimer O, Knodler LA, Marcus SL, et al. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J Biol Chem. 2000;275:37718–37724. doi: 10.1074/jbc.M008187200. [DOI] [PubMed] [Google Scholar]
- 70.Huang X, Hazlett LD. Analysis of Pseudomonas aeruginosa corneal infection using an oligonucleotide microarray. Invest Ophthalmol Vis Sci. 2003;44:3409–3416. doi: 10.1167/iovs.03-0162. [DOI] [PubMed] [Google Scholar]
- 71.Hasnain SE, Begum R, Ramaiah KV, et al. Host-pathogen interactions during apoptosis. J Biosci. 2003;28:349–358. doi: 10.1007/BF02970153. [DOI] [PubMed] [Google Scholar]
- 72.McNamara N, Van R, Tuchin OS, Fleiszig SM. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res. 1999;69:483–490. doi: 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- 73.McDermott AM, Redfern RL, Zhang B, Pei Y, Huang L, Proske RJ. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grassme H, Kirschnek S, Riethmueller J, et al. CD95/CD95 Ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science. 2000;290:527–530. doi: 10.1126/science.290.5491.527. [DOI] [PubMed] [Google Scholar]