Abstract
The innate immune system has been implicated in the pathogenesis of alcoholic liver disease. While innate immunity is usually considered an early response to injury, previous work implicating innate immunity in ethanol-induced liver injury focuses primarily on long-term ethanol exposure. Here we investigated the early period of ethanol exposure to determine whether there were temporal associations between activation of innate immune responses and known correlates of liver injury. Female C57BL/6 mice were allowed free access to an ethanol-containing Lieber-DeCarli diet or pair-fed a control diet. Within four days of ethanol exposure, we observed a striking spike in expression of hepatic pro-inflammatory cytokines, including TNF-α, IL-6 and IFNγ, prior to hepatic triglyceride accumulation or increased plasma ALT activities, as well as before the induction of CYP2E1 or oxidative stress. This early spike in inflammatory cytokines coincided with deposition of C3b-iC3b/C3c (C3b) in the liver. This deposition, resulting from the cleavage of complement factor 3 (C3), is evidence for activation of complement in response to ethanol. C3−/− mice were protected from the early, ethanol-induced increase in hepatic TNF-α expression. Ethanol increased C3b deposition in mice deficient in C3a receptor or C5a receptor, as well as in wild type mice depleted of hepatic macrophages; however, there was no increase in hepatic TNF-α in the absence of C3a receptor, C5a receptor, or hepatic macrophages. In contrast, the absence of TLR-4 had no effect on the early, ethanol-induced increase in either C3b or TNF-α.
In conclusion
For the first time, we have identified a complement- and macrophage-dependent, but TLR-4 independent, phase in the pathogenesis of ethanol-induced liver injury.
Introduction
The development of alcoholic liver disease (ALD) is a complex process involving both parenchymal and non-parenchymal cells in the liver, as well as the recruitment of other cell types to the liver in response to damage and inflammation (1). Accumulating evidence indicates that different components of the innate immune response are activated during chronic ethanol exposure. For example, Kupffer cells, the resident macrophages in the liver, play an essential role in chronic ethanol-induced liver injury (2). Endotoxin/lipopolysaccharide (LPS), a component of gram-negative bacterial cell walls, is an important activator of Kupffer cells during long-term ethanol exposure, resulting in increased production of pro-inflammatory cytokines and chemokines (3;4), as well as reactive oxygen species (ROS)(5). Mice lacking components of the toll-like receptor 4 (TLR-4) complex (TLR4−/− or CD14−/−), as well as the TNF receptor I (TNFRI−/−), are protected from chronic ethanol-induced steatosis, as well as inflammation and early stages of fibrosis (3;4).
Complement is also implicated in chronic ethanol-induced liver injury (6–8). Chronic ethanol feeding to mice for 6–8 weeks increases the activation of C3, the 3rd component of the complement pathway, increasing the concentration of C3a in the plasma (6), as well as deposition of C3 in the liver (9). Mice lacking C3 (C3−/−) do not develop steatosis in response to chronic ethanol feeding (6;8). In contrast, while mice lacking C5 (C5−/−) still exhibit increased hepatic triglycerides in response to long-term ethanol exposure, they are completely protected from ethanol-induced increases in ALT and inflammatory cytokines (6). Mice lacking CD55/DAF, a complement regulatory protein, exhibit exacerbated steatosis, ALT and inflammatory cytokines in response to chronic ethanol feeding, suggesting that CD55/DAF acts as a brake against chronic ethanol-induced liver injury (6).
Studies of the role of the innate immune response in ethanol-induced liver injury have usually focused on relatively long periods, from 4–8 weeks, of ethanol exposure. However, activation of innate immune responses can be an early response to a variety of insults. In particular, activation of complement is a rapid response to infection, tissue injury or stress; rapid activation of complement serves to orchestrate different aspects of innate immunity, as well as to provide links to the adaptive immune response (10). Therefore, we hypothesized that activation of innate immune responses, including increased expression of pro-inflammatory cytokines and activation of complement, would likely be an early response to ethanol exposure. Here we report that ethanol exposure results in an early, transient increase in both complement activation products and TNF-α expression, prior to the accumulation of triglycerides in the liver or increases in ALT in the plasma. Importantly, Kupffer cells, as well as both C3a receptor or C5a receptor, were required for the early, transient increase in TNF-α expression during ethanol feeding. However, in contrast to the contributions of TLR-4 to the long-term, chronic effects of ethanol in liver (3), the early ethanol-induced activation of complement and TNF-α expression was independent of TLR-4. These data illustrate the specific, dynamic interactions between multiple components of the innate immune response at early times in the hepatic response to ethanol.
Methods
Materials
Female mice (8–10 weeks old) of each genotype were used in the studies. C57BL/6J, TNRI−/− (B6.129-Tnfrsf1atm1Mak/J) and C3−/− (B6.129S4-C3129S4-C3tm1Crr/J) mice were purchased from Jackson Labs (Bar Harbor, Maine), C3aR −/− and C5aR−/− mice were on a mixed wild type background (WTaR), as previously described (11;12). TLR4−/− were on a C57BL/6 background (13). See Supplmental Information for further details on Materials.
Ethanol feeding
All procedures using animals were approved by the Case Western Reserve University or Cleveland Clinic Institutional Animal Care and Use Committees. 8–10 week old female mice were housed in shoe-box cages (2 animals/cage) with microisolator lids. Details of animal husbandry are in Supplemental Information. The ethanol-fed group was allowed free access to ethanol containing diet with increasing concentrations of ethanol: 1% (vol/vol) (1% d2) and 2% each for 2 days (2% d2), then 4% ethanol for 7 d (4% d3 and 4% d7), and finally 5% ethanol for up to 4 weeks (5% wk1, wk2, wk3 or wk4) (6;14). The 5% ethanol diet provides 26.9% of calories as ethanol. Control mice were pair-fed diets which iso-calorically substituted maltose dextrins for ethanol over the entire feeding period. Plasma ethanol concentrations were measured in samples collected three hours into the feeding/dark cycle. In one experiment, mice were intravenously injected with 0.2 ml of a 1 mg/ml suspension of clodronate encapsulated in liposomes, as previously described (15), prior to the start of ethanol feeding, to selectively deplete Kupffer cells (16).
Plasma measurements and liver triglycerides
Plasma samples were assayed for alanine aminotransferase (ALT) and ethanol using commercially available enzymatic assay kits (Diagnostic Chemicals, LTD, Oxford, CT) following the manufacturer’s instructions. Total liver triglycerides were measured using the Triglyceride Reagent Kit from Pointe Scientific Inc. (Lincoln Park, Michigan).
Immunohistochemistry
Immunohistochemical analysis of C3b, TNF-α, 4-HNE and F4/80 were carried out in liver sections using standard techniques (see Supplemental Information).
Preparation of liver lysates
Frozen liver tissue (0.5–1.0 g) was homogenized in 10 ml/g tissue in lysis buffer exactly as described (6). Protein concentrations were measured using the BCA assay. Lysates were then used to measure CYP2E1 expression by Western blotting and cytokine concentrations by ELISA, as previously described (6).
Statistical analysis
Values reported are means ± SEM. Because of the logistics of animal care, several feeding trials were carried out and combined for the final data analysis. Data were analyzed by general linear models procedure (SAS, Carey, IN), blocking for trial effects when data from more than one feeding trial was used in a data set. Data were log transformed if needed to obtain a normal distribution. Follow-up comparisons were made by least square means testing.
Results
To begin to address the interactions between innate immune responses and ethanol-induced liver injury, we allowed female C57BL/6 mice ad libitum access to the Lieber-DeCarli ethanol diet with increasing concentrations of ethanol (Figure 1). The ethanol-fed mice continued to grow at the same rate as their pair-fed controls (Figure 1A). In the ethanol-fed mice, hepatic triglycerides (Figure 1B) and plasma ALT (Figure 1C), a marker of hepatocyte injury, were increased as early as 7 days (4% d3). Liver triglycerides continued to accumulate at a steady rate over the entire ethanol feeding period (Figure 1B). Although plasma ALT was highest during the first week of exposure to 5% ethanol (Figure 1C), plasma ALT remained elevated over the ethanol feeding period.
Figure 1. Time course of hepatic triglyceride accumulation, plasma ALT and hepatic cytokine expression in C57BL/6 mice during ad libitum ethanol feeding.
Mice were allowed free access to a liquid diet containing increasing concentrations of ethanol (1% for 2 d, 2% for 2 d, 4% for 7 d and then 5% for an additional 4 weeks). A) Body weights of ethanol- and pair-fed mice. B) Total triglycerides and C) plasma ALT activity were measured. For panels A,B and C, values represent means ± SEM, n=at least 5 for pair-fed and 10 for ethanol-fed at each time point, except day 32, where n=7 for ethanol-fed. Values with different superscripts are significantly different from each other, p<0.05. For panels D, E, and F, lysates were prepared from frozen livers and TNFα (D), IL-6 (E) and IFN-γ (F) proteins measured by ELISA. Hepatic cytokines were normalized to percent of pair-fed. Hepatic cytokines concentrations did not change over time in pair-fed (data not shown). Concentrations of TNF-α were 42 ± 6 pg/mg protein, IL-6 45 ± 3 pg/mg protein and IFN-γ 133 ± 10 pg/mg protein in pair-fed at the 2% d2 time point. Values represent means ± SEM, n=at least 3 for pair-fed and 7 for ethanol-fed at each time point. *p<0.05 compared to pair-fed mice at each time point.
It is well known that expression of a number of inflammatory cytokines is increased in ethanol-induced liver injury; TNF-α plays an essential role in the development of ethanol-induced liver injury (4;17). Analysis of our ethanol-fed mice showed that TNF-α protein in the liver was transiently increased after only 4 days of ethanol feeding (2% d2) and dropped below baseline after 7 days (4% d3) (Figure 1D). IFN-γ and IL-6 expression also exhibited a similar rapid and transient induction early in the response to ethanol (Figure 1E and 1F). After this initial response, hepatic TNF-α and IFN-γ increased again after 5% wk4 of ethanol feeding (Figure 1D–E), typical of previous reports of increases in expression of hepatic TNF-α after chronic ethanol feeding (6). Immunohistochemical staining revealed that TNF-α protein was expressed throughout the liver (Figure 2A); the staining intensity paralled TNF-α concentrations determined by ELISA (Figure 1D). Co-localization studies with F4/80, a macrophage-specific marker, and TNF-α demonstrate that F4/80 positive cells had higher TNF-α immunostaining after ethanol feeding (2% d2) (Figure 2B)
Figure 2. Immunohistochemical analysis of TNF-α protein in livers from wild type mice during ethanol feeding and the effect of ethanol in TNFRI−/− mice.
A) Immunoreactive TNF-α was visualized in frozen liver sections from C57BL/6 mice. B) Co-localization of F4/80 (green) and TNF-α (red) was visualized in paraffin-embedded sections. Images are representative of at least 3 mice per time point. C/D/E) C57BL/6 and TNFRI−/− mice were allowed free access to ethanol containing diets 11 days (4% day 7) or pair-fed control diets. B) Total hepatic triglycerides and C) plasma ALT activity were measured. Values represent means ± SEM, n=6 for pair-fed and n=10 for ethanol-fed C57BL/6 and n=3 (pair- and ethanol-fed) for TNFRI−/−. Values with different superscripts are significantly different from each other, p<0.05.D) Immunoreactive 4-hydroxynonenal (4-HNE) adducts were visualized by immunohistochemistry in formalin-fixed liver sections. Images are representative of at least 3 mice per time point.
In order to evaluate the pathophysiological significance of this early peak in hepatic TNF-α, we assessed markers of ethanol-induced liver injury in TNFRI−/− mice after ethanol feeding for 11 days (4% d7), just after the transient elevation in hepatic TNF-α. In wild type mice, hepatic triglycerides (Figure 2C), plasma ALT (Figure 2D), as well as the accumulation of 4-hydroxynonenal adducts (Figure 2E), a measure of lipid peroxidation, were increased at day 11 (4% d7) of ethanol exposure. In contrast, while hepatic triglycerides still accumulated in TNFRI−/− mice (Figure 2C), ALT and 4-hydroxynonenal adducts were not increased by ethanol feeding in the absence of TNF receptor I (Figure 2D/E).
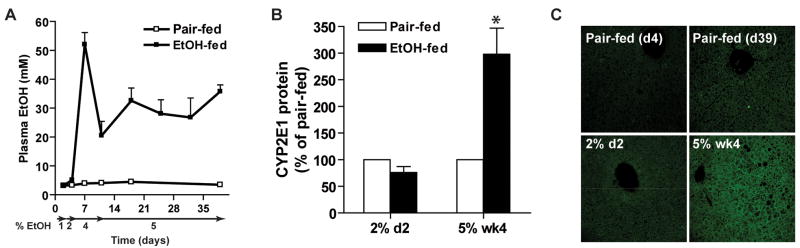
We next addressed the molecular mechanism for the early ethanol-induced increase in hepatic TNF-α. When mice consumed 1 or 2% ethanol, plasma ethanol concentrations, measured 3 h into the dark/feeding period, were undetectable (Figure 3A). In contrast, increased ethanol concentrations in the plasma were observed when the ethanol concentrations in the diet were 4% or higher (Figure 3A). CYP2E1 expression was induced after long-term ethanol feeding (5% wk4), but not yet increased at day 4 (2% d2) (Figure 3B). Similarly, while 4-hydroxynonenal adducts, a measure of lipid peroxidation, were increased at 5% wk4, no adducts were detected at day 4 (2% d2) (Figure 3C). Neither plasma adiponectin concentrations nor the concentration of endotoxin in platelet-rich plasma were affected by ethanol feeding at day 4 (2% d2) (data not shown).
Figure 3. Plasma ethanol, hepatic CYP2E1 expression and 4-hydroxynonenal adduct formation.
A) Plasma ethanol concentrations were measured 3h into the feeding/dark cycle. Values represent means ± SEM, n=3 for pair-fed and 6–10 for ethanol-fed. B) Immunoreactive CYP2E1 protein in liver lysates after day 4 (2% d2) and day 39 (5% wk 4) was measured by Western blot. Values are expressed as percent increase over pair-fed and represent means ± SEM, n=4, *p<0.05 compared to pair-fed. C) Immunoreactive 4-hydroxynonenal (4-HNE) adducts were visualized by immunohistochemistry in formalin-fixed liver sections from mice at day 4 (2% d2) and day 39 (5% wk 4). Images are representative of at least 3 mice per time point.
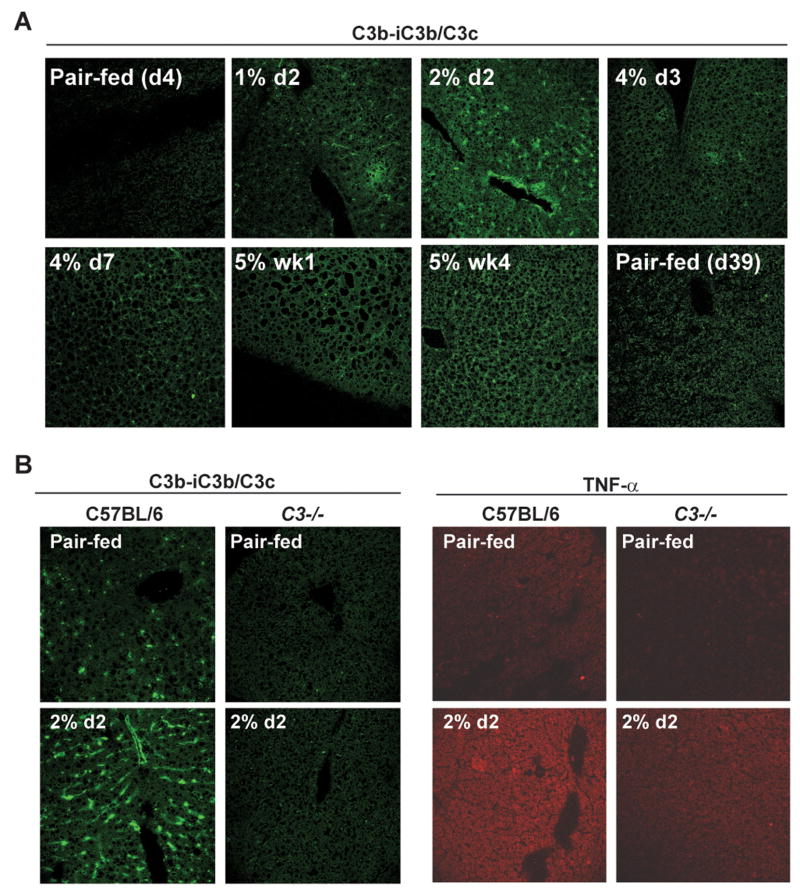
Recent studies by ourselves (6) and others (8) have causally implicated complement activation in chronic ethanol-induced liver injury. Since complement activation generates C3a and C5a, anaphylatoxins that increase inflammatory cytokine production, we next investigated the time course of complement activation during ethanol feeding. We have previously shown that 6 weeks of ethanol feeding increases serum C3a concentration (6). Here we have taken the alternative approach and used antibodies generated to detect neo-epitopes revealed on the C3b-iC3b/C3c (abbreviated here as C3b) portion of C3 after cleavage (18). Immunohistochemistry showed a rapid and transient increase in C3b deposition at day 4 (2% d2) (Figure 4A), paralleling the kinetics of TNF-α expression (Figure 2). This was followed by a later accumulation of C3b at 5% wk4 of ethanol feeding (Figure 4A). At day 4, in contrast to the localization of TNF-α, C3b deposition was concentrated in sinusoidal areas of the liver.
Figure 4. Ethanol rapidly and transiently increases C3b deposition in livers from wild type, but not C3−/−, mice during ethanol feeding.
A) Wild type mice were allowed free access to an ethanol-containing diet or pair-fed control diets. Immunoreactive C3b-iC3b/C3c was visualized in frozen liver sections. Images are representative of at least 3 mice per time point. B) Wild type and C3−/− were allowed free access to an ethanol-containing diet for 4 days (2% d2). C3b and TNF-α were detected by immunohistochemistry in frozen liver sections. Images are representative of at least 3 mice per experimental group.
If complement activation is required for ethanol-induced increases in TNF-α at these early time points, then TNF-α protein should not be detected in C3−/− mice during ethanol feeding. As expected in the absence of C3 protein, C3b deposition was not detectable in the livers of C3−/− mice at day 4 (2% d2) of ethanol feeding (Figure 4B). Moreover, ethanol-induced increases in TNF-α protein were suppressed in C3−/− compared to wild type mice at day 4 (2% d2)(Figure 4B), arguing that complement activation is required for the early ethanol-induced increase in TNF-α expression.
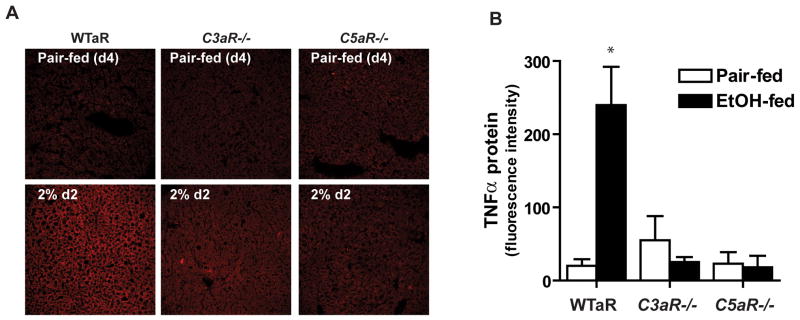
Complement activation generates C3a and C5a, which upon interaction with their receptors, C3aR or C5aR, induces expression of inflammatory cytokines. Therefore, we next made use of mice lacking either C3aR (C3aR−/−) or C5aR (C5aR−/−) to determine if one or both of these receptors is required for the early increase in hepatic TNF-α expression that accompanies ethanol feeding. Whereas wild type mice exhibited increased hepatic TNF-α expression after 4 days (2% d2) of ethanol feeding (Figure 5), no increase occurred in either C3aR−/− or C5aR−/− mice (Figure 5). C3b accumulation was detected in livers from C3aR−/− and C5aR−/− after ethanol feeding at day 4 (data not shown), indicating that the abrogation of ethanol-induced TNF-α expression was due to the absence of C3aR or C5aR in these knock-out strains, rather than the absence of complement activation.
Figure 5. Ethanol does not increase TNF-α expression in livers from C5aR−/− or C3aR−/− mice.
A) C3aR and C5aR−/− mice and wild type mice (WTaR), were allowed free access to an ethanol-containing liquid diet for 4 days (2% day 2) and TNF-α detected by immunohistochemistry in frozen liver sections. B) Quantification of immunoreactive TNF-α in liver sections. Values represent means ± SEM, n=4–6, *p<0.05 compared to pair-fed.
Previous work has shown that ethanol-induced increases in LPS and its interaction with TLR-4 on Kupffer cells are important contributors to chronic ethanol-induced hepatic TNF-α production and subsequent liver injury (2;4;17). Moreover, TLR-4 and C3a receptor/C5a receptor signaling synergistically increases the expression of cytokines (19;20). Therefore, we next investigated whether interactions among complement, Kupffer cells and TLR-4 were required for the early ethanol-induced increase in TNF-α expression. We treated mice with clodronate to deplete Kupffer cells prior to ethanol exposure (16). Localization of F4/80 demonstrated a profound decrease in Kupffer cells 4 days after clodronate treatment in both ethanol- and pair-fed mice (Figure 6A). While Kupffer cell depletion did not affect ethanol-induced C3b deposition (Figure 6B), the absence of Kupffer cells abolished the ethanol induction of hepatic TNF-α expression (Figure 6C). In contrast, the absence of TLR-4 had no effect on the early ethanol-induced increase in C3b deposition or TNF-α expression (Figure 7).
Figure 6. Depletion of hepatic macrophages prevents the early, ethanol-induced increase in hepatic TNF-a expression, but not C3b deposition.
Mice were pretreated with liposomes containing PBS or clodronate prior to ethanol exposure for 4 days (2% day 2) to deplete Kupffer cells. Immunohistochemical analysis of A) F4/80 was measured in formalin-fixed liver sections and B) C3b and C) TNF-α were assessed in frozen liver sections. Images are representative of at least 3 mice per experimental group.
Figure 7. Ethanol increases both hepatic C3b deposition and TNF-α expression in TLR4−/− mice.
Wild type and TLR4−/− mice were allowed free access to an ethanol-containing diet for 4 days (2% day 2). Immunohistochemical analysis of C3b and TNF-α was carried out in frozen liver sections. Images are representative of at least 3 mice.
Discussion
The pathophysiological development of ethanol-induced liver injury follows a characteristic pattern of steatosis, hepatocyte injury and inflammation, which in some cases progresses to fibrosis and cirrhosis. While in humans, this process can take years, in animal models the early stages of steatosis and hepatocyte injury can be reproduced over a period of weeks. Activation of hepatic macrophages by LPS, increased expression of inflammatory cytokines, particularly TNF-α, activation of complement and the generation of ROS are all important contributors to chronic ethanol-induced liver injury (3–5). However, the dynamics of these different contributors are not understood. Here we have identified an early increase in hepatic TNF-α expression that precedes the ethanol-induced accumulation of hepatic triglycerides and increased plasma ALT, as well as the induction of CYP2E1 and the accumulation of 4-HNE adducts, which result from oxidative stress. This early, transient increase in TNF-α was not observed in mice lacking C3, C3aR or C5aR, indicating that ethanol-mediated activation of complement and interaction of C3a and C5a with their receptors, contributed to the early induction of TNF-a expression (Figure 8). Activation of complement by ethanol did not involve hepatic macrophages; however, macrophages were required for the early, ethanol-induced increase in hepatic TNF-α. Interestingly, TLR-4 was not required for either the early, ethanol-induced increase in C3b deposition or TNF-α accumulation. Taken together, our data suggest that ethanol exposure rapidly activates multiple components of innate immunity, including complement, Kupffer cells, and inflammatory cytokine production; the synergistic interaction among these pathways is a critical mechanism contributing to ethanol-induced liver injury.
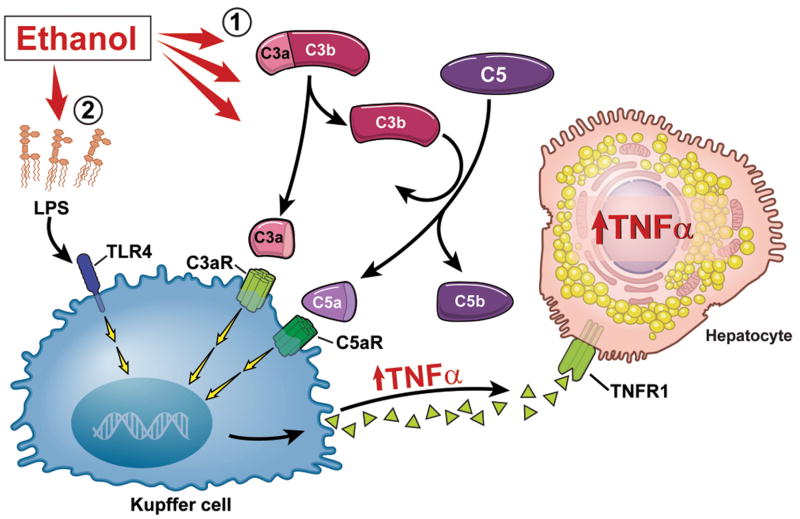
Figure 8. Diagram illustrating an early, complement-dependent phase in the pathogenesis of ethanol-induced liver injury.
1) Ethanol feeding to mice results in an early activation of complement, evidenced by the deposition of C3b in liver. C3a and C5a interact with their receptors, most likely on the surface of Kupffer cells, to stimulate TNF-α expression. TNF-α expression in the liver is then amplified as hepatocytes also produce TNF-α. This early phase of TNF-α expression is independent of TLR-4. 2) After longer periods of ethanol exposure, both TLR-4-dependent and complement-dependent pathways contribute to the further progression of ethanol-induced liver injury.
Recent studies have identified the complement pathway as an important regulator of chronic ethanol-induced liver injury, with both C3−/− and C5−/− mice protected from specific consequences of long-term ethanol feeding (6;8). CD55/DAF−/− mice, lacking an important complement regulatory protein, exhibit exacerbated injury after long-term ethanol feeding (6). Interestingly, the absence of C3 or C5 differentially protects from chronic ethanol-induced liver damage. After 6 weeks of ethanol feeding, C3 −/− mice, while protected from ethanol-induced steatosis, still exhibit increased expression of inflammatory cytokines, including TNF-α, IL-6 and IFN-γ, as well as moderately increased ALT. In contrast, chronic ethanol feeding does not increase liver cytokines or ALT in C5−/− mice, even though they develop steatosis (6). These data suggest that C3 and C5 differentially contribute to steatosis, hepatocyte injury and increased production of inflammatory cytokines during long periods of ethanol exposure.
Here we further characterized the role of complement activation in the hepatic response to ethanol, identifying an essential role for both C3aR and C5aR in an early, complement-dependent increase in hepatic TNF-α expression. C3a and C5a are potent anaphylatoxins, capable of increasing inflammatory cytokine responses (21), which contribute to a number of chronic inflammatory diseases, including myocardial ischemia reperfusion, respiratory distress syndrome, arthritis and antibody-dependent type II autoimmunity (21;22). In the healthy liver, Kupffer cells and stellate cells express both C3a and C5a receptors. C5a receptor expression can be induced in hepatocytes in response to inflammatory cytokines (23) or in regenerating hepatocytes (24). While chronic ethanol feeding (5% wk4) did not increase total hepatic C3aR or C5aR mRNA (data not shown), absence of either C3aR or C5aR prevented the early, ethanol-induced increase in hepatic TNF-α (Figure 5). While the present studies demonstrate that both C3a and C5a contribute to the early, ethanol-induced increase in TNF-α, our earlier studies suggest that C5a, rather than C3a, is more important for sustained ethanol-induced increases in inflammatory cytokine expression after chronic ethanol feeding (6). The greater effect of C5a in hepatic injury over longer periods of ethanol exposure could relate to its ability to recruit neutrophils to sites of infection/injury (25;26). Alternatively, while C3a has numerous pro-inflammatory properties, under some conditions, it also has anti-inflammatory activity. For example, C3a receptor knock-out mice show increased sensitivity in a model of endotoxemia, suggesting important anti-inflammatory functions for the C3a receptor (27).
While both C3aR and C5aR were required for the early, ethanol-induced increase in hepatic TNF-α, TLR-4 was not required for this response. This is in contrast to the critical role of TLR-4 in mediating increased TNF-α expression and liver injury in response to chronic ethanol exposure (3,4). The difference in the role of TLR-4 in early vs chronic ethanol-induced TNF-α expression may be due to the relatively low doses of ethanol and/or the short period of ethanol exposure used in this study. Thus, while our data demonstrate that the early increase in hepatic cytokine expression is primarily dependent on complement activation, additional factors, including activation of TLR-4 and elevated production of ROS (3,4), have been shown to contribute to sustained inflammatory cytokine production during the later phases of ethanol-induced liver injury.
Activation of systemic complement can be initiated via the classical, lectin and alternative pathways, although additional extrinsic pathways for activation of C5 by proteases can play a role in some pathophysiological conditions (28;29). Local production and activation of C5 by Kupffer cells also contributes to type II autoimmune injury in mice (21). More studies will be needed to determine if localized activation of C5 within the complex cellular environment of the liver contributes to ethanol-induced liver injury.
Previous reports demonstrated that TNFRI−/− mice are protected from chronic ethanol-induced liver injury, using intragastric infusion of ethanol over 4 weeks (3;4). Here we exposed TNFRI−/− to ethanol for a shorter time (4%d7), when increased markers of injury were initially observed in wild type mice, in order to more directly assess the role of the early increase in hepatic TNF-α in mediating subsequent ethanol-induced liver injury. The TNFRI−/− mice were completely protected from ethanol-induced increases in ALT and 4-hydroxynonenal adduct accumulation at day 11, but still exhibited hepatic steatosis. These data support the hypothesis that the early increase in TNF-α is required for increased ROS production and hepatocyte injury. This dichotomy between the role of TNF-α in ethanol-induced increases in ALT and steatosis is consistent with a growing body of evidence suggesting that distinct mechanisms contribute to ethanol-induced increases in ALT compared to triglyceride accumulation. For example, C5−/− are protected from increased cytokine and ALT, but not triglyceride accumulation, during chronic ethanol exposure (6). Alternatively, at these early times of ethanol exposure, hepatic steatosis may be more dependent on shifts in NADH/NAD ratios, resulting from ethanol metabolism, leading to decrease β-oxidation, rather than mechanisms for triglyceride accumulation that are dependent on complement and inflammatory cytokines that involve the up-regulation of genes regulating fatty acid synthesis and down-regulation of genes involved in fatty acid oxidation, as well as an impairment of VLDL secretion (30).
Out studies demonstrate that complement is necessary for early, ethanol-induced TNF-α expression and that Kupffer cells are also involved in mediating the early effects of ethanol on inflammatory cytokine expression in the liver. The contribution of Kupffer cells is likely due to C3aR and C5aR signal transduction during the early period of ethanol exposure and not related to activation of TLR-4. The present study thus illustrates the complex integration of multiple elements of the innate immune system during the response of the liver to ethanol. Remarkably, the absence of a single component, such as a deficiency in C3aR or C5aR, prevents the early, ethanol-induced increase in TNF-α expression. These data thus add to our understanding of the highly orchestrated response of the liver to insult, such as the injury that occurs in response to ethanol.
Acknowledgments
This work was supported by NIH grants AA016399 to LEN and AI23598 MEM. The authors are grateful to Dr. Shizuo Akira (Osaka University, Osaka, Japan) for the generous gift of TLR4 deficient mice.
Abbreviations
- ALT
alanine aminotransferase activity
- C3
third component of the complement system
- C3b
C3b-iC3b/C3c
- C5
fifth component of the complement system
- C3aR
C3a receptor
- C5aR
C5a receptor
- CYP2E1
cytochrome P450 2E1
- DAF
decay accelerating factor
- FBS
fetal bovine serum
- IL-6
interleukin 6
- IFNγ
interferon γ
- LPS
lipopolysaccharide
- OCT
optimal cutting temperature
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- SEM
standard error of the mean
- TLR-4
toll-like receptor 4
- TNF-α
tumor necrosis factor α
Reference List
- 1.Gressner AM, Bachem MG. Molecular mechanisms of liver fibrogenesis-a homage to the role of activated fat-storing cells. Digestion. 1995;56:335–346. doi: 10.1159/000201257. [DOI] [PubMed] [Google Scholar]
- 2.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- 3.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. New Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 4.Thurman RG. Mechanisms of Hepatic Toxicity II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 5.Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- 6.Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, et al. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117–26. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvelainen HA, Vakeva A, Lindros KO, Meri S. Activation of complement components and reduced regulator expression in alcohol-induced liver injury in the rat. Clin Immunol. 2002;105:57–63. doi: 10.1006/clim.2002.5267. [DOI] [PubMed] [Google Scholar]
- 8.Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Complement C3 contributes to ethanol-induced liver steatosis in mice. Ann Med. 2006;38:280–6. doi: 10.1080/07853890600664608. [DOI] [PubMed] [Google Scholar]
- 9.Bykov I, Jauhiainen M, Olkkonen VM, Saarikoski ST, Ehnholm C, Junnikkala S, et al. Hepatic gene expression and lipid parameters in complement C3(−/−) mice that do not develop ethanol-induced steatosis. J Hepatol. 2007;46:907–14. doi: 10.1016/j.jhep.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–27. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, et al. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 12.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–9. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 14.McMullen MR, Cocuzzi E, Hatzoglou M, Nagy LE. Chronic ethanol exposure increases the binding of HuR to the TNFalpha 3′-untranslated region in macrophages. J Biol Chem. 2003;278:38333–41. doi: 10.1074/jbc.M304566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 16.Van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology. 1996;23:1239–43. doi: 10.1053/jhep.1996.v23.pm0008621159. [DOI] [PubMed] [Google Scholar]
- 17.Nagy LE. New insights into the role of the innate immune response in the development of alcoholic liver disease. Expt Biol Med. 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 18.Mastellos D, Prechl J, Laszlo G, Papp K, Olah E, Argyropoulos E, et al. Novel monoclonal antibodies against mouse C3 interfering with complement activation: description of fine specificity and applications to various immunoassays. Mol Immunol. 2004;40:1213–21. doi: 10.1016/j.molimm.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Hawlisch H, Kohl J. Complement and Toll-like receptors: key regulators of adaptive immune responses. Mol Immunol. 2006;43:13–21. doi: 10.1016/j.molimm.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–36. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, et al. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512–20. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 23.Schieferdecker HL, Schlaf G, Jungermann K, Gotze O. Functions of anaphylatoxin C5a in rat liver: direct and indirect actions on nonparenchymal and parenchymal cells. Int Immunopharmacol. 2001;1:469–81. doi: 10.1016/s1567-5769(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 24.Daveau M, Benard M, Scotte M, Schouft MT, Hiron M, Francois A, et al. Expression of a functional C5a receptor in regenerating hepatocytes and its involvement in a proliferative signaling pathway in rat. J Immunol. 2004;173:3418–24. doi: 10.4049/jimmunol.173.5.3418. [DOI] [PubMed] [Google Scholar]
- 25.Jauneau AC, Ischenko A, Chan P, Fontaine M. Complement component anaphylatoxins upregulate chemokine expression by human astrocytes. FEBS Lett. 2003;537:17–22. doi: 10.1016/s0014-5793(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 26.DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao P. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J Immunol. 1999;162:1127–36. [PubMed] [Google Scholar]
- 27.Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol. 2000;165:5406–9. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- 28.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–7. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 29.Wetsel RA, Kolb WP. Expression of C5a-like biological activities by the fifth component of human complement (C5) upon limited digestion with noncomplement enzymes without release of polypeptide fragments. J Exp Med. 1983;157:2029–48. doi: 10.1084/jem.157.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy LE. Molecular mechanisms of alcohol metabolism. Ann Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]