Abstract
The advent of endovascular therapy for intracranial aneurysms and the rapid advances in that field have supplanted microsurgical treatment for many intracranial aneurysms. Applying current outcome data and other parameters, nuances of selecting the modality of treatment for intracranial aneurysms are reviewed. Patient factors, such a age, co-morbidities, vasospasm and other medical conditions, are addressed. A custom-tailored multimodality treatment paradigm for the management of ruptured and unruptured aneurysms will maximize the favorable results seen in this difficult patient population.
Keywords: Aneurysm, Subarachnoid hemorrhage, Aneurysm clip, Endovascular treatment
INTRODUCTION
The advent of endovascular therapy for intracranial aneurysms, and the rapid advances in that filed have supplanted microsurgical treatment for many intracranial aneurysms. The less invasive nature of endovascular therapy is quite appealing to patients as well as practitioners.
These endovascular advances have led to a sharp decline in the use of microsurgical techniques for many aneurysms without clear scientific evidence of superiority of endovascular techniques.
Microsurgical clipping has the advantage of being a time-honored, durable and versatile method for treating most intracranial aneurysms. It is rare for an intracranial aneurysm to recur once it has been properly clipped, and there are very few aneurysms that are not amenable to some microsurgical repair technique9,50). The primary disadvantage of surgery is the fact that it requires an open operation and some manipulation of the brain.
Endovascular therapy has the advantage of being less invasive and multiple aneurysms at distant sites can be treated. There are, however, some important disadvantages of endovascular therapy. Like surgery, endovascular therapy has risks. As with surgery, if endovascular therapy in provided by individuals with significant experience, those risks are relatively small, but not eliminated9,37). The primary disadvantage of endovascular therapy is its inferior durability9,39).
Endovascular treatment requires routine maintenance in the form of follow-up imaging because of the higher likelihood of recurrence of the aneurysm and the potential need for additional treatment8). Another disadvantage of endovascular therapy is the fact that it simply is not suitable for all aneurysms. There remain a large number of aneurysms whose angioarchitectural features make them unsuitable for current endovascular techniques, as suggested in the International Subarachnoid Aneurysm Trial (ISAT), where only 22% of the 9,559 patients were randomized into the trial for either endovascular or open surgical treatment37). The majority of those not randomized underwent surgical management of their aneurysms. Finally, endovascular coiling does not have the ability to remove intracerebral hemorrhages or necessarily eliminate mass effect from large or giant aneurysms that present as an intracranial mass. In some rare instances, endovascular treatment can occasionally exacerbate the condition34,41,47).
With a rapid shift in the philosophy concerning the treatment of intracranial aneurysms, it is important to periodically review the role of therapeutic options, as well as the scientific evidence supporting the shift. In this review article, we will assess the current role of microsurgical clip ligation of selected intracranial aneurysms based upon available evidence supporting its use. It is our opinion that the best results in the management of intracranial aneurysms will occur in high-volume institutions that utilize a truly multidisciplinary approach to the management of patients harboring these lesions7,33,48). Many aneurysms are amenable to both microsurgical or endovascular therapies. There remain, however, some aneurysms that are not ideal for microsurgical clip ligation and a number of aneurysms which are currently not suitable for available endovascular techniques.
ANEURYSMS NOT IDEAL FOR MICROSURGICAL CLIP LIGATION
A number of characteristics of either the patient and/or the aneurysm can make them undesirable for surgical management. In these situations, an available endovascular option may be a much more appropriate choice because of the known increased risks of surgical management in such situations, including aneurysms in elderly patients, patients in very poor medical condition or who present with cerebral vasospasm, or aneurysms that have calcified necks or unfavorable surgical anatomy.
Elderly patients
It is well-recognized that surgical treatment of aneurysms, whether ruptured or unruptured, carries increased morbidity in the elderly population16,40,49). These patients simply do not tolerate intracranial surgery as well as their younger counterparts12,27). As older patients do not have as long a life expectancy as younger individuals, the marginally inferior durability of endovascular techniques is less of a concern in this population9). Most institutions utilize general anesthesia for endovascular procedures, so this aspect of treatment and its attendant risks not eliminated by endovascular therapy. However, though it has not been clearly demonstrated that endovascular therapy in the elderly population is associated with less risk than an open procedure, intuition would certainly suggest that this is the case.
Patients in poor neurological or medical condition
The advantages of early elimination of a ruptured aneurysm from the intracranial circulation have been addressed in the literature17). Early removal eliminates the risk or rehemorrhage, which is most likely to occur during the first six to twenty four hours after the initial rupture26,31). Furthermore, if the patient develops vasospasm in the days to come, it can be treated more aggressively without fear of causing a re-hemorrhage form the aneurysm. These observations of the advantages of early surgical management of the aneurysms led to a shift in the mid-1980s and early 1990s to earlier surgery for ruptured intracranial aneurysms17,27,37). In many institutions, early surgery was withheld from patients in poor neurological or medical condition for fear of unacceptable morbidity in operating on patients with attendant medical or neurological comorbidities. Although several authors have demonstrated that acceptable outcomes can occur with early surgery in patients who are initially in poor neurological condition, there certainly is additional morbidity of early manipulation of a severely injured and edematous brain. The advent of endovascular techniques has provided an alternative to early surgery and remains a very appealing option for patients in poor medical or neurological condition.
Patients presenting with cerebral vasospasm
With increasing regionalization of aneurysm care, many patients presenting with aneurysmal subarachnoid hemorrhage are transferred to regional facilities; it has been shown that patients that receive care at high-volume centers have statistically better outcomes11). However, the delays inherent in such transfers, and the attendant delays in resuscitation and work-up may result in a patient presenting to the treating institution in angiographic and/or clinical vasospasm. In such a situation, neurovascular surgeons may be concerned about operating in the face of significant vasospasm for fear of exacerbating the underlying condition or increasing the risks of intraoperative ischemic injuries because of poor perfusion54). If the ruptured intracranial aneurysm is appropriate for endovascular therapy, an endovascular approach to eliminate the aneurysm from the circulation and treat the vasospasm during the same procedure is a very appealing and likely superior option for these patients.
Difficult location
Although virtually any aneurysm can be addressed by some microsurgical treatment, it is clear that some aneurysms carry a greater risk of exposure and treatment than others. Even within the same anatomic location, local factors of the aneurysm can influence the risks of microsurgical clip ligation. While most neurovascular surgeons agree that basilar apex aneurysms are particularly treacherous due to the surrounding perforating vessels, those lesions with a very high or low bifurcation can make microsurgical treatment even more difficult than if the aneurysm arises at the level of the posterior clinoid processes4). The orientation of a basilar apex aneurysm should also be considered, as anteriorly-pointing domes allow easier visualization and dissection of the thalamoperforating vessels. Anterior communicating artery aneurysms pointing anteriorly are very straightforward to treat surgically, while those that point superiorly between the A2 segments present a greater challenge to the neurovascular surgeon, and often require some degree of gyrus rectus resection, which may have subtle influences on future neuropsychiatric performance. Those aneurysms that arise in difficult locations or have anatomic variations that increase the degree of surgical difficulty are often more ideal for endovascular therapy if their angioarchitecture is suitable for an endovascular option. In general, basilar trunk or apex aneurysms, high-riding and posterior-pointing anterior communicating aneurysms, or aneurysms of the very proximal internal carotid artery with a partially extradural neck are often at least considered for endovascular therapy.
Calcified neck
Intracranial aneurysms associated with any degree of calcification, particularly if the calcification involves the neck, present significant difficulties for the neurovascular surgeon. Thin-slice CT through the aneurysm and its neck can identify area of calcification, allowing the neurovascular team to anticipate significant difficulty in securing the aneurysm by clip ligation (Fig. 1). If these lesions are suitable for endovascular therapy, this will often be a far superior option than attempts at reconstructing the calcified neck by clipping.
Fig. 1.

Axial computed tomography scan demonstrating significant calcification of a distal internal carotid aneurysm.
Multiple aneurysms requiring multiple operations
Approximately 20% to 30% of patients who harbor an intracranial aneurysm will have multiple aneurysms43). This can vary from two to several aneurysms. Ideally, one would wish to choose a therapeutic option that allows the largest number of aneurysms to be eliminated from the circulation. Although a number of aneurysm locations can be exposed through the standard surgical approaches, with some aneurysms, there are limitations to those surgical exposures. For example, a right-sided pterional approach will allow one to clip aneurysms throughout most of the anterior circulation, as well as proximal aneurysms on the left carotid circulation and aneurysms of rostral basilar artery. This approach, however, may not allow adequate exposure of a left middle cerebral artery aneurysm or aneurysms of the vertebral artery. Endovascular therapy, however, is not limited by these anatomic constraints. Multiple aneurysms at multiple sites can potentially be treated at the same endovascular setting. However, in the setting of subarachnoid hemorrhage, whichever modality is chosen should, of course, focus foremost on the ruptured aneurysm.
ANEURYSMS NOT IDEAL FOR ENDOVASCULAR COILING
Despite the many advances in endovascular therapy and the appeal of this less invasive technique, there remain a significant number of aneurysms which are simply unsuitable or not ideal for endovascular therapy. Advances in endovascular technology may address many of these shortcomings in the foreseeable future but, for the present time, microsurgical clip ligation remains a better option in many of these situations. The ISAT trial has shown that complete aneurysm obliteration was only seen in 66% of those coiled at one year, as compared with more than 80% of the surgically ligated aneurysms, suggesting that many aneurysms that were treated by endovascular means are not ideally suited to be treated as such37). In the literature, angiographically demonstrated recurrences of aneurysms during follow-up ranged from 17-50%, depending on the aneurysm neck size, and most studies include relatively short-term follow-up11,44,52).
Fusiform aneurysms
With some rare exceptions, fusiform aneurysms, particularly those on the distal aspects of intracranial arteries, are not amenable to endovascular therapy without sacrificing the parent artery (Fig. 2). There are reports of an endovascular 'bypass' with a combination of stent and possible coiling, however the long-term stent patency and recurrence rates are unclear10,22). If it is important to maintain patency of the distal vessel, as is usually the case for many intracranial aneurysms may be challenging and may require use of extracranial to intracranial bypass, intracranial to intracranial bypass, flow reversal, clip wrapping techniques or other innovative means to eliminate the aneurysm (Fig. 3).
Fig. 2.

Three dimensional cerebral arteriogram demonstrating a small fusiform aneurysm of the posterior inferior cerebellar artery that is not amenable to endovascular therapy. The vessel was reconstructed and the aneurysm ligated using a transcondylar approach.
Fig. 3.
Antero-posterior right internal carotid angiogram (A) showing a large aneurysm of the MCA. 3-D angiogram (B) demonstrates the complex nature of the aneurysm. This aneurysm was treated by placement of a radial artery bypass graft (C) from the common carotid to the middle cerebral artery (D) with proximal occlusion of the M1 segment (arrow) to reverse flow.
Blister-like aneurysms
"Blister-like" aneurysms at non-branching points, most commonly occur on the dorsal proximal internal carotid artery, although these may also occur in other locations on the intracranial circulation45,46) and are often misclassified initially with a negative angiogram. These aneurysms are relatively rare, comprising less than 1% of cerebral aneurysms1,45). They are characterized as very small, broad-based aneurysms without a neck that are extremely thin-walled and may be seen in association with other aneurysms. Pathologically, they lack an internal elastic lamina and media, and are covered only by adventitia and fibrinous tissue25). Currently, there is no appropriate endovascular option for managing these nefarious lesions. Perhaps, in the future, covered stents may provide an alternative to microvascular repair when needed. Even surgical repair of these lesions is challenging, with a high intraoperative rupture rate. Often times, management requires considerable creativity, utilizing such techniques as clip wrapping or even a sacrifice with extracranial to intracranial bypass6,45).
Wide-necked aneurysms
With the advent of endovascular adjuncts, such as balloon-remodeling and stent-coiling, many wide-necked aneurysms previously deemed unsuitable for endovascular coiling are now able to be treated by endovascular techniques. For many wide-necked aneurysms, however, microsurgical clip ligation remains a better option, as it allows for complete, immediate and permanent clip ligation of the aneurysm with a very low chance of recurrence (Fig. 4). Although the current literature regarding wide-necked aneurysm treatment and recurrence varies tremendously, there is little doubt that wide-necked aneurysm, often defined as 4 mm or greater, have a higher recurrence rate23,39). We are very hesitant to use stents in treating recently ruptured aneurysms because the need for use of powerful antiplatelet agents that may increase the risks of patient management, particularly in the setting of hydrocephalus requiring ventricular drainage or additional surgical procedures51). Additionally, the long term efficacy of intracranial stents is unknown but significant morbidity in this population.
Fig. 4.

3-D angiogram in the anterior-posterior projection demonstrating the wide-necked nature, involving both proximal posterior cerebral arteries. This aneurysm was successfully treated by clip ligation.
Complex aneurysm configuration
Aneurysms with highly complex anatomy are oftentimes unsuitable for endovascular treatment. If endovascular therapy is used, a variety of adjuncts, including balloon-remodeling and the use of stents, may be necessary, and may increase the risk of the endovascular procedure. Oftentimes, these complex aneurysms can be best treated by microsurgical clip ligation with reconstruction of the parent artery, utilizing a variety of clipping strategies (Fig. 5).
Fig. 5.
A : The vascular anatomy of aneurysms such as this basilar tip requires a complex clip configuration to reconstitute the native vessels and obliterate the aneurysm. B : Post-operative 3-D angiogram demonstrating successful clipping of the aneurysm and preservation of the native vasculature.
Thrombotic aneurysms
It is well-recognized that aneurysms with a significant amount of intraluminal thrombus are poor candidates for endovascular therapy, although they are poorly described in the literature because they are often included into larger endovascular therapy databases. Most of the thrombotic aneurysms reported in the literature are large or giant aneurysms32). There are rare case reports suggesting that endovascularly-treated thrombotic aneurysms behave differently, with less thrombus organization and deposition of fibrous connective tissue38). In addition to the delayed or altered healing, endovascular coils rapidly migrate into the adjacent thrombus, leading to early recurrence of these aneurysms. On the other hand, microsurgical treatment, including aneurysmorraphy, the evacuation of the intraluminal thrombus, and clip reconstruction are more durable options for managing these complicated aneurysms (Fig. 6)32).
Fig. 6.
Sagittal magnetic resonance image (A) reveals a partially thrombosed distal MCA aneurysm. Antero-posterior (B), lateral (C) and 3D (D) right ICA angiograms demonstrate the aneurysm. Postoperative ICA (E) and ECA (F) angiograms document successful trapping of the aneurysm and patency of the superficial temporal artery bypass to the distal MCA.
Giant aneurysms
Many large and giant aneurysms have been demonstrated to be associated with significantly lower rates of obliteration and significantly higher recurrence rates following endovascular therapy, with recurrence rates higher than 50% in some larger studies20,39).
Furthermore, the mass effect of these aneurysms often present with is not reliably eliminated by filling the aneurysm with endovascular devices, with some studies suggesting up to 25% of patients experiencing no improvement or worsening of their neurologic symptoms (Fig. 6B, C)24,34). There is also a suggestion that local tissue reaction secondary to bioactive ('second generation') endovascular coils may cause unwanted sequelae, such as chemical meningitis24,36).
Very small aneurysms
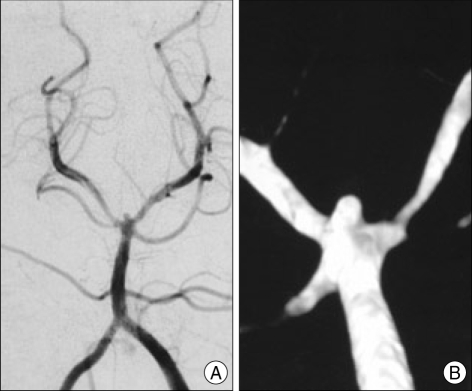
Small aneurysms, such as those that are less than 3 mm in size, are likely treated by routine surveillance in most patients. However, in those patients presenting with subarachnoid hemorrhage, or unruptured aneurysms in patients that are young, have other intracranial aneurysms or a personal of family history of subarachnoid hemorrhage, or other medical or social reasons that necessitate treatment of these very small aneurysms, open surgical management remains the standard. Aneurysms of this size are very difficult or impossible to treat by endovascular means (Fig. 7)53). Currently, there are few endovascular coils small enough to treat these very small aneurysms adequately21). Endovascular treatment of such small lesions also presents significant technical challenges, including more difficult and unstable catheter positioning and a higher risk of coil migration and aneurysm perforation. In contrast, however, small aneurysms are much more readily amenable to microsurgical clip ligation and do not typically lesions more suitable for microsurgical clip ligation at the present time.
Fig. 7.
Anterior-posterior angiogram (A) and 3-D angiogram (B) of a patient with subarachnoid hemorrhage demonstrating a tiny basilar tip aneurysm. This was treated with microsurgical clip ligation.
Failed endovascular treatment
Aneurysms that have undergone endovascular therapy and present with recurrence should be strongly considered for microsurgical treatment. Although the data available varies considerably, the ISAT treatment rate was 6.9 times more likely in the endovascular therapy group than in the open surgical group (Fig. 8)9). Most experienced centers now report a 3-6% endovascular re-treatment rate, compared to a historical surgical recurrence rate of approximately 1% (though the true rate is unknown, as most surgeons do not routinely perform surveillance of clipped aneurysms). If an initial endovascular procedure has failed, questions regarding the cause of failure and what new endovascular options can be brought to bear must be answered. If the initial endovascular treatment did not utilize all current technology and a suitable endovascular option exists that will likely eliminate the aneurysm on a permanent basis, endovascular therapy is still the primary option. However, if the new endovascular option carries a significant risk of another recurrence, future microsurgical treatment may be rendered much more complicated and dangerous; clearly, in such a situation, a definitive surgical treatment strategy is preferred over a second failed endovascular attempt. With every retreatment, one also has additive medical and procedural-related risks associated with additional procedures. In a recent report of 350 endovascular-retreated aneurysms, there was a 2.2% morbidity-mortality rate associated with retreatment, although with subsequent retreatment sessions, the authors did note a decreased complication rate as compared with the initial treatment session19). It must also be remembered that surgical treatment of previously coiled aneurysms is also associated with challenges55).
Fig. 8.
Lateral left carotid angiogram (A) demonstrates a large proximal ICA aneurysm that was treated by endovascular coiling. Follow-up angiogram (B) reveals significant recurrence of the aneurysm. Intraoperative angiogram (C) documents complete obliteration of the aneurysm and normal filling of the ICA and ophthalmic artery.
Intracerebral hemorrhage
Those aneurysms presenting with an intracerebral hemorrhage with mass effect should be strongly considered for microsurgical repair at the time of clot evacuation (Fig. 9) 1. Currently, there is no endovascular means for the removal of an intracerebral hemorrhage and, if an operation is to be performed to evacuate the hematoma, one should treat the aneurysm by a more durable microsurgical procedure during the same operation18,42).
Fig. 9.
Brain CT (A) and 3-D angiogram (B) showing A large middle cerebral aneurysm with associated intaparenchymal clot with mass effect. The hemorrhage was surgically evacuated and the aneurysm clipped in the same procedure.
Multiple aneurysms not amenable to endovascular therapy
If a patient presents with multiple aneurysms that are not all amenable to endovascular therapy, one should strongly consider microsurgical clip ligation if this is a viable option for treating all of the aneurysms. To treat one or more aneurysms by endovascular therapy when the patient will have to be subjected to an intracranial operation at a later time is an illogical choice, since surgery is not avoided. The obvious exception is if endovascular therapy can be provided for an aneurysm that would not be able to be addressed during a single microsurgical procedure.
Subarachnoid hemorrhage with high-risk of vasospasm
Vasospasm is a common complication, identified angiographically in 40-70% of patients and causing delayed ischemic neurological deficits (DIND) in 17-40% of patients with subarachnoid hemorrhage2,13-15,29). Of this population with DIND, approximately 50% will develop an ischemic infarction35). Although a recent review suggested there was no statistical difference between coiling vs. clipping of aneurysms with regard to vasospasm, there have been several smaller studies examining pharmacological or technical nuances suggesting that surgical management may provide decreased vasospasm risk that were not addressed in the review which bear discussion and consideration13). Andaluz et al.3) prospectively examined the effect of microsurgical opening of the lamina terminals in ruptured anterior communicating artery patients and found that there was as statistically significant decrease in vasospasm and hydrocephalus requiring a shunt. Another group has shown that placement of nicardipine prolonged-release pellets into the surgical bed and cisterns significantly reduced the incidence of vasospasm in their patient population30). Although reports suggest that microsurgical management of ruptured intracranial aneurysms is far from a mature art, it is also clear that we as physicians are far from solving many of the vexing problems that our patients experience.
CONCLUSION
Endovascular therapy for the treatment of intracranial aneurysms represents an innovative and welcome addition to the armamentarium for management of these challenging lesions.
Currently, there is a paucity of definitive scientific evidence to support the superiority of endovascular or microsurgical treatment in the management of the vast majority of aneurysms. Undeniably, the International Subarachnoid Aneurysm Trial (ISAT) published in 2002 has changed the practice patterns of many referring physicians and neurosurgeons. However, many experts in the field have pointed out significant design and implementation flaws within the study5). In such an environment, individualized clinical decision-making for each aneurysm and each patient is undoubtedly best accomplished by a truly multidisciplinary group with both endovascular and microsurgical skills. Despite the substantial advances in endovascular therapy, microsurgery also is not a static field and a number of advances have been made in the past several years. These include advances in skull-base surgery to minimize brain retraction and injury, three-dimensional rotational, intraoperative and indocyanine green videoangiography, advanced pharmacologic mechanisms for brain protection, improved temporary and permanent aneurysm clips, and the increasing use and refinement of cerebral bypass techniques.
As endovascular therapy has become more popular and been utilized for an increasing number of intracranial aneurysms, those aneurysms requiring surgical treatment have become increasingly complex and this trend will continue for the foreseeable future. These more challenging aneurysms will tax the skills of the neurovascular surgeons and require increasingly innovative and challenging microsurgical solutions.
References
- 1.Abe M, Tabuchi K, Yokoyama H, Uchino A. Blood blisterlike aneurysms of the internal carotid artery. J Neurosurg. 1998;89:419–424. doi: 10.3171/jns.1998.89.3.0419. [DOI] [PubMed] [Google Scholar]
- 2.Adams HP, Jr, Kassell NF, Torner JC, Haley EC., Jr Predicting cerebral ischemia after aneurysmal subarachnoid hemorrhage : influences of clinical condition, CT results, and antifibrinolytic therapy. A report of the Cooperative Aneurysm Study. Neurology. 1987;37:1586–1591. doi: 10.1212/wnl.37.10.1586. [DOI] [PubMed] [Google Scholar]
- 3.Andaluz N, Zuccarello M. Fenestration of the lamina terminalis as a valuable adjunct in aneurysm surgery. Neurosurgery. 2004;55:1050–1059. doi: 10.1227/01.neu.0000140837.63105.78. [DOI] [PubMed] [Google Scholar]
- 4.Aziz A, Khaled M, Andaluz N. Basilar bifurcation aneurysms : strategies for surgical approach selection. Neurosurgery Quarterly. 2007;17:101–112. [Google Scholar]
- 5.Barrow DL. Bad science ISAT : the impact on neurosurgical practice. Clin Neurosurg. 2004;51:126–131. [PubMed] [Google Scholar]
- 6.Bederson JB, Zabramski JM, Spetzler RF. Treatment of fusiform intracranial aneurysms by circumferential wrapping with clip reinforcement. J Neurosurg. 1992;77:478–480. doi: 10.3171/jns.1992.77.3.0478. [DOI] [PubMed] [Google Scholar]
- 7.Berman MF, Solomon RA, Mayer SA, Johnston SC, Yung PP. Impact of hospital-related factors on outcome after treatment of cerebral aneurysms. Stroke. 2003;34:2200–2207. doi: 10.1161/01.STR.0000086528.32334.06. [DOI] [PubMed] [Google Scholar]
- 8.Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms : outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656–663. doi: 10.3171/jns.1999.90.4.0656. [DOI] [PubMed] [Google Scholar]
- 9.Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT) Stroke. 2007;38:1538–1544. doi: 10.1161/STROKEAHA.106.466987. [DOI] [PubMed] [Google Scholar]
- 10.Chiaradio JC, Guzman L, Padilla L, Chiaradio MP. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery : technical case report. Neurosurgery. 2002;50:213–216. doi: 10.1097/00006123-200201000-00034. discussion 216-217. [DOI] [PubMed] [Google Scholar]
- 11.Cowan JA, Jr, Dimick JB, Wainess RM, Upchurch GR, Jr, Thompson BG. Outcomes after cerebral aneurysm clip occlusion in the United States : the need for evidence-based hospital referral. J Neurosurg. 2003;99:947–952. doi: 10.3171/jns.2003.99.6.0947. [DOI] [PubMed] [Google Scholar]
- 12.Cowan JA, Jr, Ziewacz J, Dimick JB, Upchurch GR, Jr, Thompson BG. Use of endovascular coil embolization and surgical clip occlusion for cerebral artery aneurysms. J Neurosurg. 2007;107:530–535. doi: 10.3171/JNS-07/09/0530. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira JG, Beck J, Ulrich C, Rathert J, Raabe A, Seifert V. Comparison between clipping and coiling on the incidence of cerebral vasospasm after aneurysmal subarachnoid hemorrhage : a systematic review and meta-analysis. Neurosurg Rev. 2007;30:22–30. doi: 10.1007/s10143-006-0045-5. discussion 30-31. [DOI] [PubMed] [Google Scholar]
- 14.Dehdashti AR, Mermillod B, Rufenacht DA, Reverdin A, de Tribolet N. Does treatment modality of intracranial ruptured aneurysms influence the incidence of cerebral vasospasm and clinical outcome? Cerebrovasc Dis. 2004;17:53–60. doi: 10.1159/000073898. [DOI] [PubMed] [Google Scholar]
- 15.Dorsch NW. Therapeutic approaches to vasospasm in subarachnoid hemorrhage. Curr Opin Crit Care. 2002;8:128–133. doi: 10.1097/00075198-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Forrest JB, Rehder K, Cahalan MK, Goldsmith CH. Multicenter study of general anesthesia. III. Predictors of severe perioperative adverse outcomes. Anesthesiology. 1992;76:3–15. doi: 10.1097/00000542-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Haley EC, Jr, Kassell NF, Torner JC. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992;23:205–214. doi: 10.1161/01.str.23.2.205. [DOI] [PubMed] [Google Scholar]
- 18.Heiskanen O, Poranen A, Kuurne T, Valtonen S, Kaste M. Acute surgery for intracerebral haematomas caused by rupture of an intracranial arterial aneurysm. A prospective randomized study. Acta Neurochir (Wien) 1988;90:81–83. doi: 10.1007/BF01560559. [DOI] [PubMed] [Google Scholar]
- 19.Henkes H, Fischer S, Liebig T, Weber W, Reinartz J, Miloslavski E, et al. Repeated endovascular coil occlusion in 350 of 2759 intracranial aneurysms : safety and effectiveness aspects. Neurosurgery. 2006;58:224–232. doi: 10.1227/01.NEU.0000194831.54183.3F. discussion 224-232. [DOI] [PubMed] [Google Scholar]
- 20.Henkes H, Fischer S, Weber W, Miloslavski E, Felber S, Brew S, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54:268–280. doi: 10.1227/01.neu.0000103221.16671.f0. discussion 280-285. [DOI] [PubMed] [Google Scholar]
- 21.Henkes H, Reinartz J, Preiss H, Miloslavski E, Kirsch M, Kühne D. Endovascular treatment of small intracranial aneurysms : three alternatives to coil occlusion. Minim Invasive Neurosurg. 2006;49:65–69. doi: 10.1055/s-2005-919150. [DOI] [PubMed] [Google Scholar]
- 22.Higashida RT, Smith W, Gress D, Urwin R, Dowd CF, Balousek PA, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944–949. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 23.Hope JK, Byrne JV, Molyneux AJ. Factors influencing successful angiographic occlusion of aneurysms treated by coil embolization. AJNR Am J Neuroradiol. 1999;20:391–399. [PMC free article] [PubMed] [Google Scholar]
- 24.Im SH, Han MH, Kwon BJ, Jung C, Kim JE, Han DH. Aseptic meningitis after embolization of cerebral aneurysms using hydrogel-coated coils : report of three cases. AJNR Am J Neuroradiol. 2007;28:511–512. [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa T, Nakamura N, Houkin K, Nomura M. Pathological consideration of a "blister-like" aneurysm at the superior wall of the internal carotid artery : case report. Neurosurgery. 1997;40:403–405. doi: 10.1097/0006123-199702000-00038. discussion 405-406. [DOI] [PubMed] [Google Scholar]
- 26.Kassell NF, Torner JC. Aneurysmal rebleeding : a preliminary report from the Cooperative Aneurysm Study. Neurosurgery. 1983;13:479–481. doi: 10.1227/00006123-198311000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Kassell NF, Torner JC, Jane JA, Haley EC, Jr, Adams HP. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2 : Surgical results. J Neurosurg. 1990;73:37–47. doi: 10.3171/jns.1990.73.1.0037. [DOI] [PubMed] [Google Scholar]
- 28.Kazekawa K, Tsutsumi M, Aikawa H, Iko M, Kodama T, Go Y, et al. Internal carotid aneurysms presenting with mass effect symptoms of cranial nerve dysfunction : efficacy and imitations of endosaccular embolization with GDC. Radiat Med. 2003;21:80–85. [PubMed] [Google Scholar]
- 29.Keller E, Krayenbühl N, Bjeljac M, Yonekawa Y. Cerebral vasospasm : results of a structured multimodal treatment. Acta Neurochir Suppl. 2005;94:65–73. doi: 10.1007/3-211-27911-3_11. [DOI] [PubMed] [Google Scholar]
- 30.Krischek B, Kasuya H, Onda H, Hori T. Nicardipine prolonged-release implants for preventing cerebral vasospasm after subarachnoid hemorrhage : effect and outcome in the first 100 patients. Neurol Med Chir (Tokyo) 2007;47:389–394. doi: 10.2176/nmc.47.389. discussion 394-396. [DOI] [PubMed] [Google Scholar]
- 31.Laidlaw JD, Siu KH. Ultra-early surgery for aneurysmal subarachnoid hemorrhage : outcomes for a consecutive series of 391 patients not selected by grade or age. J Neurosurg. 2002;97:250–258. doi: 10.3171/jns.2002.97.2.0250. discussion 247-249. [DOI] [PubMed] [Google Scholar]
- 32.Lawton MT, Quinõnes-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms : classification scheme and management strategies in 68 patients. Neurosurgery. 2005;56:441–454. doi: 10.1227/01.neu.0000153927.70897.a2. discussion 441-454. [DOI] [PubMed] [Google Scholar]
- 33.Lawton MT, Quinones-Hinojosa A, Sanai N, Malek JY, Dowd CF. Combined microsurgical and endovascular management of complex intracranial aneurysms. Neurosurgery. 2003;52:263–274. doi: 10.1227/01.neu.0000043642.46308.d1. discussion 274-275. [DOI] [PubMed] [Google Scholar]
- 34.Malisch TW, Guglielmi G, Viñuela F, Duckwiler G, Gobin YP, Martin NA, et al. Unruptured aneurysms presenting with mass effect symptoms : response to endosaccular treatment with Guglielmi detachable coils. Part I. Symptoms of cranial nerve dysfunction. J Neurosurg. 1998;89:956–961. doi: 10.3171/jns.1998.89.6.0956. [DOI] [PubMed] [Google Scholar]
- 35.Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation. 1994;90:2592–2605. doi: 10.1161/01.cir.90.5.2592. [DOI] [PubMed] [Google Scholar]
- 36.Meyers PM, Lavine SD, Fitzsimmons BF, Commichau C, Parra A, Mayer SA, et al. Chemical meningitis after cerebral aneurysm treatment using two second-generation aneurysm coils : report of two cases. Neurosurgery. 2004;55:1222. doi: 10.1227/01.neu.0000140987.71791.df. [DOI] [PubMed] [Google Scholar]
- 37.Miyaoka M, Sato K, Ishii S. A clinical study of the relationship of timing to outcome of surgery for ruptured cerebral aneurysms. A retrospective analysis of 1622 cases. J Neurosurg. 1993;79:373–378. doi: 10.3171/jns.1993.79.3.0373. [DOI] [PubMed] [Google Scholar]
- 38.Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with Guglielmi detachable coils. Report of two cases with autopsy correlation. J Neurosurg. 1995;83:129–132. doi: 10.3171/jns.1995.83.1.0129. [DOI] [PubMed] [Google Scholar]
- 39.Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen T, Eliasen K, Henriksen E. A prospective study of mortality associated with anaesthesia and surgery : risk indicators of mortality in hospital. Acta Anaesthesiol Scand. 1990;34:176–182. doi: 10.1111/j.1399-6576.1990.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 41.Pickett GE, Laitt RD, Herwadkar A, Hughes DG. Visual pathway compromise after hydrocoil treatment of large ophthalmic aneurysms. Neurosurgery. 2007;61:E873–E874. doi: 10.1227/01.NEU.0000298918.55119.7C. discussion E874. [DOI] [PubMed] [Google Scholar]
- 42.Prat R, Galeano I. Early surgical treatment of middle cerebral artery aneurysms associated with intracerebral haematoma. Clin Neurol Neurosurg. 2007;109:431–435. doi: 10.1016/j.clineuro.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi AI, Suarez JI, Parekh PD, Sung G, Geocadin R, Bhardwaj A, et al. Risk factors for multiple intracranial aneurysms. Neurosurgery. 1998;43:22–26. doi: 10.1097/00006123-199807000-00013. discussion 26-27. [DOI] [PubMed] [Google Scholar]
- 44.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 45.Sekula RF, Jr, Cohen DB, Quigley MR, Jannetta PJ. Primary treatment of a blister-like aneurysm with an encircling clip graft : technical case report. Neurosurgery. 2006;59(1 Suppl 1):E168. doi: 10.1227/01.neu.0000220058.17532.b5. discussion E168. [DOI] [PubMed] [Google Scholar]
- 46.Shigeta H, Kyoshima K, Nakagawa F, Kobayashi S. Dorsal internal carotid artery aneurysms with special reference to angiographic presentation and surgical management. Acta Neurochir (Wien) 1992;119:42–48. doi: 10.1007/BF01541780. [DOI] [PubMed] [Google Scholar]
- 47.Sluzewski M, Menovsky T, van Rooij WJ, Wijnalda D. Coiling of very large or giant cerebral aneurysms : long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003;24:257–262. [PMC free article] [PubMed] [Google Scholar]
- 48.Solomon RA, Mayer SA, Tarmey JJ. Relationship between the volume of craniotomies for cerebral aneurysm performed at New York state hospitals and in-hospital mortality. Stroke. 1996;27:13–17. doi: 10.1161/01.str.27.1.13. [DOI] [PubMed] [Google Scholar]
- 49.Taylor CL, Yuan Z, Selman WR, Ratcheson RA, Rimm AA. Mortality rates, hospital length of stay, and the cost of treating subarachnoid hemorrhage in older patients : institutional and geographical differences. J Neurosurg. 1997;86:583–588. doi: 10.3171/jns.1997.86.4.0583. [DOI] [PubMed] [Google Scholar]
- 50.Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms : results of long-term follow-up angiography. Stroke. 2001;32:1191–1194. doi: 10.1161/01.str.32.5.1191. [DOI] [PubMed] [Google Scholar]
- 51.Tumialán LM, Zhang YJ, Cawley CM, Dion JE, Tong FC, Barrow DL. Intracranial hemorrhage associated with stent-assisted coil embolization of cerebral aneurysms : a cautionary report. J Neurosurg. 2008;108:1122–1129. doi: 10.3171/JNS/2008/108/6/1122. [DOI] [PubMed] [Google Scholar]
- 52.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm : perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 53.Wiebers DO. Patients with small, asymptomatic, unruptured intra-cranial aneurysms and no history of subarachnoid hemorrhage should generally be treated conservatively : for. Stroke. 2005;36:408–409. doi: 10.1161/01.STR.0000152270.22970.48. [DOI] [PubMed] [Google Scholar]
- 54.Yundt KD, Grubb RL, Jr, Diringer MN, Powers WJ. Cerebral hemodynamic and metabolic changes caused by brain retraction after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1997;40:442–450. doi: 10.1097/00006123-199703000-00003. discussion 450-451. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YJ, Barrow DL, Cawley CM, Dion JE. Neurosurgical management of intracranial aneurysms previously treated with endovascular therapy. Neurosurgery. 2003;52:283–293. doi: 10.1227/01.neu.0000043643.93767.86. discussion 293-295. [DOI] [PubMed] [Google Scholar]