Abstract
Objective
The purpose of this study was to investigate the reliable factors influencing the surgical outcome of the patients with traumatic acute subdural hematoma (ASDH) and to improve the functional outcome of these patients.
Methods
A total of 256 consecutive patients who underwent surgical intervention for traumatic ASDH between March 1998 and March 2008 were reviewed. We evaluated the influence of perioperative variables on functional recovery and mortality using multivariate logistic regression analysis.
Results
Functional recovery was achieved in 42.2% of patients and the overall mortality was 39.8%. Age (OR=4.91, p=0.002), mechanism of injury (OR=3.66, p=0.003), pupillary abnormality (OR=3.73, p=0.003), GCS score on admission (OR=5.64, p=0.000), and intraoperative acute brain swelling (ABS) (OR=3.71, p=0.009) were independent predictors for functional recovery. And preoperative pupillary abnormality (OR=2.60, p=0.023), GCS score (OR=4.66, p=0.000), and intraoperative ABS (OR=4.16, p=0.001) were independent predictors for mortality. Midline shift, thickness and volume of hematoma, type of surgery, and time to surgery showed no independent association with functional recovery, although these variables were correlated with functional recovery in univariate analyses.
Conclusion
Functional recovery was more likely to be achieved in patients who were under 40 years of age, victims of motor vehicle collision and having preoperative reactive pupils, higher GCS score and the absence of ABS during surgery. These results would be helpful for neurosurgeon to improve outcomes from traumatic acute subdural hematomas.
Keywords: Acute subdural hematoma, Functional recovery, Mortality
INTRODUCTION
Traumatic acute subdural hematoma (ASDH) is one of the most common traumatic neurosurgical emergencies and often requires surgical intervention. ASDH occurs in 12% to 30% of patients with severe head injury30) and reported mortality rates are various from 36% to 79% for patients who underwent surgery17,24). Although developments and improvements in emergency medical service systems, neuro-intensive monitoring and treatment, ASDH is a disorder with a still very high mortality rate and extremely poor prognosis among traumatic brain injuries11,23,27). Therefore, identifying reliable prognostic factors for ASDH to improve the surgical results in these patients is important. However relatively few studies have focused on the factors that affect the outcome of patients with surgically treated traumatic ASDH. In 1998, a Wide Regional Emergency Center was established in our hospital and has been practicing as a main referral emergency center of Kyungnam Province for about ten years. Through this center, we have experienced over 1,600 cases of surgically treated brain injury patients. Among these patients, we retrospectively reviewed patients who were surgically treated for traumatic ASDH and tried to find out which factors are related to functional recovery and mortality of this lethal disorder and to improve functional outcome of these patients hereafter. On the basis of our experiences and other literatures, we evaluated 256 patients with surgically treated ASDH with following questions in mind : 1) which factors best predict functional recovery and mortality for the patients with surgically treated traumatic ASDH?; 2) which would be better surgical option for traumatic ASDH between craniotomy (CO) and craniectomy (CE)?; 3) the sooner we do operate on, the better will it be?
MATERIALS AND METHODS
Study population
The records of 256 patients admitted to our neurosurgical department and underwent surgical intervention for traumatic ASDH between March 1998 and March 2008 were reviewed. Patients with sustained open or penetrating wounds, concomitant epidural hematoma, intracerebral hematoma or severe subarachnoid hemorrhage, serious extracranial injuries, and ASDH of the posterior fossa were excluded. We also excluded patients whose time from trauma to surgery exceeded 24 hours to clearly define the acutely injured patients who could potentially benefit from a surgical intervention. According to above exclusion criteria, we finally selected and analyzed 256 isolated traumatic ASDH patients who underwent surgery. We categorized all variables which might have been related to the functional recovery and mortality into three groups : 1) clinical variables; gender, age, mechanism of injury, preoperative GCS34) scores, eloquence of lesion, preoperative pupillary abnormalities, use of preoperative high dose mannitol for control of intracranial pressure (ICP); 2) computerized tomography (CT) variables; midline shift, widest thickness and volume of hematoma; 3) surgical variables; type of surgery, time elapsed from accident to surgery, and the presence of acute brain swelling (ABS) during operation.
General patient management
All patients were resuscitated and underwent craniocerebral CT scan shortly after arrival to the emergency center, and were operated on within 24 hours after injury. Surgical treatment was performed in all cases of rapid deterioration of level of consciousness and the presence of neurological deficits. In minimally symptomatic patients, surgery was indicated if the diameter of the hematoma was 1 cm or greater on preoperative CT scan. Hematoma evacuation via craniotomy or decompressive craniectomy with large bone flap was done and an enlarged duroplasty was performed using patient's fascia or artificial materials. The attending neurosurgeon decided whether to implant the bone flap (CO or CE) depending on the intra-operative presence of cerebral swelling after removal of subdural clot. All patients were evaluated and treated according to the "Guidelines for the Management of Severe Head Injury"3). ICP monitors were used at the discretion of the attending neurosurgeon and usually were placed in patients with GCS score less than 8 or in those with evidence of increased ICP. Monitoring of ICP was performed using an infrared parenchymal catheter (Camino, Integra Life Science Corporation, Plainsboro, NJ, USA). Increased ICP values were defined as 20 mmHg or greater.
Clinical variables
The GCS score was determined on admission and all patients were divided into those with GCS scores of 3 to 8 and those with GCS scores of 9 to 15 for statistical analysis. When comparing two discrete groups for pupillary abnormalities, patients with both pupils not reacting to light were considered to be not reactive. For logistic regression analysis, patients were classified as having 0, 1, or 2 reactive pupils. In order to evaluate the efficacy of secondary pre-operative intravenous administration of high dose mannitol, we divided all patients into two groups : the group 1 was conventional dose mannitol (CDM) group (0.6 to 0.7 g/kg) and the group 2 was high dose mannitol (HDM) group which were received an additional dose of mannitol (0.6 to 0.7 g/kg) immediately before surgical intervention at operating room, and compared the postoperative results each other.
CT variables
CT scans were obtained on admission and/or before surgery and the maximum thickness (<15 mm, ≥15 mm) and volume of subdural clot (<50 mL, ≥50 mL), midline shift at the septum pellucidum were analyzed on preoperative CT scan. The hematoma volume was calculated using computer assisted analysis with formula (0.5 height×width×length). The midline shift was divided into two categories according to the modified classification by Lobato et al.20) (<10 mm, ≥10 mm) for statistical analysis.
Surgical variables
The American College of Surgeons Committee on Trauma has in place an audit filter for patients in whom an traumatic ASDH is not evacuated within 4 hours8). Consulting this criteria, we divided all patients into early surgery group (<4 hours) and delayed surgery group (≥4 hours) concerning about the time elapsed from injury to surgery. With regard to type of operation, the neurosurgeon decided whether to implant the bone flap after hematoma removal depending on the degree of intra-operative cerebral swelling, preoperative neurological state and CT findings. We compared the mortality and functional recovery rates of the patients underwent craniectomy or craniotomy. Intraoperative ABS was visualized directly at surgery and recorded. Based on operation records, we divided all patients into the patients who showed intraoperative ABS (yes) and no ABS (no), and compared the outcomes.
Assessment of neurosurgical outcome
The neurosurgical outcome was determined according to Glasgow Outcome Scale (GOS)15) and final outcome was assessed and recorded 3 months after admission or at death. The five GOS categories are : good recovery (score 5), moderately disabled (score 4), severely disabled (score 3), persistent vegetative (score 2) and death (score 1). For statistical purpose, outcome categories were divided into functional recovery (GOS 5, 4) and nonfunctional recovery (GOS 3, 2, 1). And mortality was defined as postoperative death within 30 days after surgery.
Statistical analysis
Discrete variables were compared using the Chi-square test and continuous variables were compared using the Students' t-test. Logistic regression model were run to determine which variables were independently associated with functional recovery and mortality, and all variables with p<0.05 were considered statistically significant. All statistical analysis was performed using SPSS win. statistical package (ver. 12.0).
RESULTS
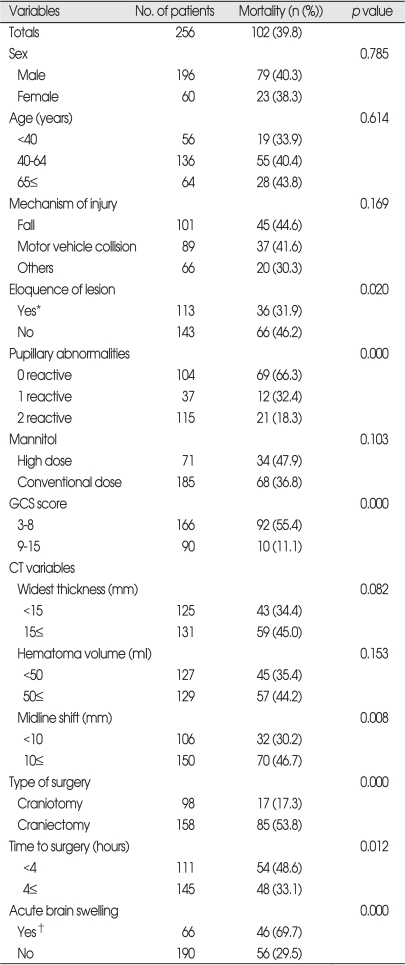
Demographic characteristics and mortality (Table 1)
Table 1.
Variables related to mortality in patients who underwent surgery for traumatic ASDH (n=256)
*when the hematoma is located on eloquent hemisphere, †when acute brain swelling is observed after clot removal. ASDH : acute subdural hematoma, GCS : Glasgow coma scale
The mean age was 51.8 years (range 2-83 years) and 76.6% of 256 patients were male. The most common cause of injury weres fall (39.5%) and traffic accident (34.8%). The overall mortality rate was 39.8% and the following seven factors were found to be significantly related to perioperative death; eloquence of lesion, pupillary abnormality, GCS score on surgical intervention, degree of midline shift on pre-operative CT scan, type of surgery, time from injury to surgical intervention and presence of intraoperative ABS. In the group with both pupils not reacting to light, the mortality rate was significantly higher (66.3%, p=0.000) than those with bilaterally reacting pupils (18.3%) and 32.4% who had unilaterally not reactive pupil died within 30 days after admission. The preoperative GCS score was highly correlated with mortality (p=0.000) and 55.4% of patients with preoperative GCS scores of 8 or less, comparing to 11.1% of GCS 9-15, died. The degree of midline shift measured on preoperative CT scans was closely related to the mortality (p=0.008). The patients with displacement over 10 mm had significantly higher mortality (46.7%) than those with less than 10 mm (30.2%). The presence of ABS was significantly correlated with the mortality. There were 66 (25.8%) cases where ABS occurred suddenly during surgery and it was usually impossible to control ICP. The mortality for the patients with intra-operative ABS was 69.7% compared to 29.5% for the patients with no ABS. Not only the length of time from trauma to surgery (0.002%) but the type of operation (0.000) significantly influenced the mortality. But, the patients who were operated on for less than 4 hours after the injury and undergone craniectomy had higher mortalities (48.6% and 53.8%). These results were somewhat contrary to our expectation.
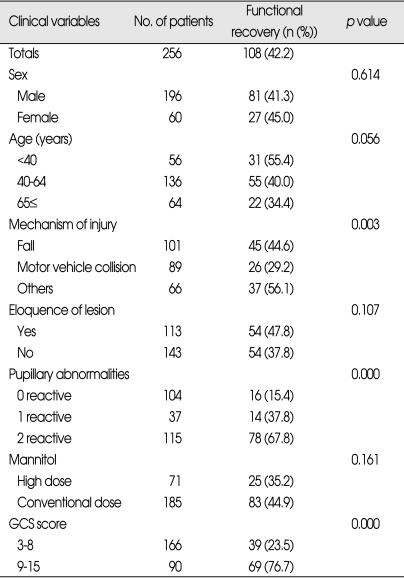
Clinical variables related to functional recovery (Table 2)
Table 2.
Clinical variables related to functional recovery in patients who underwent surgery for traumatic ASDH (n=256)
ASDH : acute subdural hematoma, GCS : Glasgow coma scale
Among various admission characteristics, mechanism of injury, pupillary abnormality, and preoperative GCS score were significantly associated with functional recovery. The patients showing high preoperative GCS (scores of 9 to 15) were more likely to have functional recovery (76.7%, p=0.000). The patients involved in motor vehicle collision were less likely to have functional recovery (29.2%, p=0.003) than those involved in other accidents. 67.8% of patients who had bilateral reactive pupils achieved a functional recovery compared with 21.3% of patients who had abnormal pupillary responses (p=0.000).
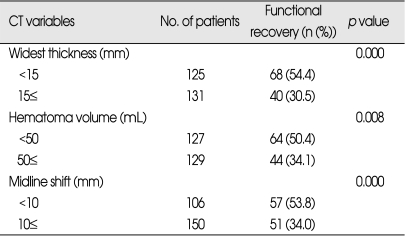
CT variables related to functional recovery (Table 3)
Table 3.
CT variables related to functional recovery in patients who underwent surgery for traumatic ASDH (n=256)
ASDH : acute subdural hematoma, CT : computed tomography
All of CT variables were found to be significantly related to functional recovery. With increasing midline shift the outcome became worse. The patient with less than 10 mm shift of midline on preoperative CT scan showed 53.8% of functional recovery compared to 34% of those with more than 10 mm shift (p=0.000). The smaller (<50 mL) the volume of hematoma, the better was the outcome (50.4% of functional recovery versus 34.1%, p=0.008). As to the thickness of hematoma, 54.4% of the patients with less than 15 mm of widest thickness of hematoma showed functional recovery and this compared with 30.5% for those with more than 15 mm (p=0.000).
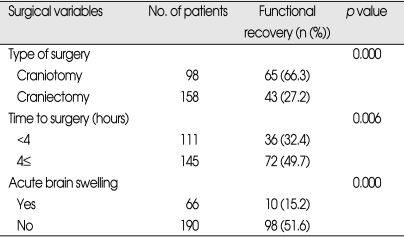
Surgical variables related to functional recovery (Table 4)
Table 4.
Surgical variables related to functional recovery in patients who underwent surgery for traumatic ASDH (n=256)
ASDH : acute subdural hematoma
All surgical variables were found to be significantly associated with functional recovery. The patients undergone craniectomy were found to have lower rate of functional recovery (27.2%) than those undergone craniotomy (66.3%) (p=0.000). And, the patients operated on within 4 hours from injury were less likely to show functional recovery (32.4%) while those operated later than 4 hours achieved 49.7% (p=0.006). The patients with ABS during surgery were less likely to have functional recovery (15.2%) compared to 51.6% for those without ABS (p=0.000).
Multivariate analysis
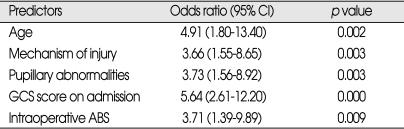
When the entire study population was subjected to logistic regression analysis, pupillary reactivity, admission GCS scores and occurrence of intraoperative ABS were found to be significantly independently predictive of postoperative death (Table 5). The age, mechanism of injury, pupillary reactivity, GCS scores on admission, and intraoperative ABS were significantly independent predictors for functional recovery (Table 6). Among these patients, type and timing of operative intervention did not significantly affect outcome.
Table 5.
Independent predictors for mortality by multivariate logistic regression analysis
ABS : acute brain swelling, CI : confidence interval
Table 6.
Independent predictors for functional recovery by multivariate logistic regression analysis
ABS : acute brain swelling, CI : confidence interval
DISCUSSION
In spite of more rapid diagnosis and aggressive neurosurgical intervention, the mortality rate of traumatic ASDH is still high in majority of series ranging between 39%9,14) and 75%26). Wilberger et al.37) recently reported that the overall mortality from traumatic ASDH is 66% and functional recovery 19%. In our series, the overall mortality was 39.8% and functional recovery 42.2%.
Increasing age is associated with a higher mortality from traumatic brain injury. Howard et al.13) compared 33 young patients (aged 18-40 years) with old patients (aged over 65) and they reported significantly higher mortality rate in the older group (74% versus 18%). Similar findings were reported by other authors18,21,23). In the study by Wilberger et al.37,38), the mean age of survivors was 41 years and of non survivors was 59 years. We observed a similar trend and found that age was an independent predictor of outcome in traumatic ASDH. In our study, those patients younger than 40 years showed significantly higher rate (OR=4.91, p=0.002) of functional recovery by multivariate logistic regression analysis. The mechanism by which age has such an effect on outcome is unknown12), but suggestions include a poor regenerative capacity of the older brain and predisposition to develop a more lethal injury13).
Interestingly, mechanism of injury (particularly injury resulting from motor vehicle collision) correlated with functional recovery. Stening et al.32) demonstrated that road traffic victims had a higher mortality rate (87%) when compared with other accidents (61%). In our study, mechanism of injury was closely related with outcome and the patients involved in motor vehicle collision showed lowest rate of functional recovery (29.2%). But, most common cause of injury was a fall (39.5%) and it represents that many industrial area were massed around our Emergency Center and there was high frequency of industrial disaster victims among our study population.
Cruz et al.5) reported that clinical outcome after ASDH treatment were significantly better for patients who received high secondary preoperative dose of mannitol. We compared the outcomes of this HDM group with CDM group but there were no statistically significant differences between two groups.
Pupillary abnormalities are associated with a significantly worse outcome. Many authors reported that patients with bilateral fixed pupils at surgery had favorable outcome from 0 to 13% and a mortality rate from 64 to 93%11,23,27,32,37). In case of one non-reacting pupil, favorable outcomes were 25 to 31% and mortality from 48 to 68%16,33). This finding was confirmed by another report10) where the presence of anisocoria did not adversely affect the outcome unless it was associated with decerebrated rigidity or respiratory depression. In our series, 67.8% of patients who had bilateral reactive pupils achieved a functional recovery (mortality 18.3%) and we speculated that preoperative pupillary abnormalities were strong predictor for functional recovery and mortality of traumatic ASDH after surgery.
The preoperative GCS score was another important predictor of outcome. Many authors reported that there is highly significant correlation between outcome and GCS score at admission10,16,18,23). Patients scoring GCS 3 at treatment have a mortality from 90 to 100% with favorable outcomes of 0 to 5%. Patients with a GCS 3 to 5 presented a mortality of 60 to 84% and favorable recoveries from 33 to 51%11,37). These findings confirm recent studies indicating that the severity of injury determines the outcome. We observed significantly lower incidence of functional recovery (23.5%) and higher mortality rate (55.4%) in the patients with GCS score of 3 to 8, similar to a previous report35).
Yanaka et al.40) showed that the mean hematoma volume was 31 cc for those patients with a favorable recovery and 104 cc for those cases with a poor (GOS 3, 2, 1) outcome, and the similar difference was shown for hematoma thickness (mean 7.8 mm versus 14.1 mm respectively). Marshall et al.22) reported that a greater midline shift was associated with a worse recovery, but some studies only showed differences comparing extreme degrees of shift. Becker et al.1) concluded that the mortality in patients with 10 mm or greater midline shift was 53% and this was compared with 25% for those patients with less than 10 mm of shift. Another author reported if the hematoma volume is less than 25 cc with a midline shift of less than 5 mm, even ASDHs in comatose patients may present a benign course with clinical improvement together with reabsorption of hematoma39). On the contrary, patients suffering from traumatic ASDHs with mass effect and midline shift sufficient to warrant emergency evacuation of hematoma demonstrated clear reduction of the cerebral blood flow. These reductions were significant in the first two post-traumatic days28) and contributed to the poor outcomes of these patients. D'Amato et al.7) reported that the presence and size of midline shift was a more important determinant of outcome than ASDH volume or its thickness. In our studies, all CT variables (including midline shift, thickness and volume of hematoma) were significantly correlated with outcomes in univariate analysis (p=0.000-0.008) but these were not independent predictors for outcomes in multi-variate logistic analysis.
Despite the increasing acceptance of CE in patients with traumatic brain injury, the value of early decompressive CE in patients with acute subdural hematoma is still under debate. Ransohoff et al.25) presented encouraging results with hemicraniectomy followed by hematoma evacuation for ASDH. They reported a recovery rate of 40%, but could not confirm these results in a later study2,4). Yanaka et al.40) also found no significant difference between CE group and CO group for ASDH but this finding may have been affected by differences in patient population. Shigemori et al.31) presented a significant reduction of ICP after decompressive CE. And Tokumi et al.36) compared different surgical treatment in a series of 120 traumatic ASDH patients and concluded that craniotomy was shown to yield a higher rate of good recovery than craniectomy. In our series, those who underwent CE had a significantly lower incidence of functional recovery than those who underwent CO (27.2 vs. 66.3, p=0.000) in univariate analysis. But, we think these results do not indicate CO is better surgical modality than CE in the patients with traumatic ASDH because there were significantly more patients with clinical signs of herniation in the CE group, which may have also affected outcome and those with lower preoperative GCS score and intraoperative ABS underwent CE rather than CO.
Seelig et al.29) in their study concluded that a delay from injury to operation was the factor of greatest therapeutic importance in traumatic ASDH. But the relationship between time to surgery and outcome is still controversial. Haselsberger et al.10) reported that 47% died and 32% had a favorable outcomes among the patients operated within two hours after the onset of coma. However, mortality in patients operated on later was 80% with only 4% favorable outcomes. In another series of 101 comatose patients, the mortality rate for those operated on within 4 hours was 59% versus 69% for those operated later and favorable recovery rates for these groups were 26% and 16% respectively with no statistical significance37). On the other hand, Stone et al.33) reported no difference in patients operated within 4 hours of injury compared with those operated later. In our series, patients who underwent subdural evacuation within 4 hours had worse outcome (functional recovery 32.4%, mortality 48.6%) than those operated later (functional recovery 49.7%, mortality 33.1%). This does not suggest that delaying operation leads to better outcomes, but patients with the most severe brain injuries, who require emergent evacuation of hematoma, are sent to neurosurgical unit more rapidly than those with less severe brain injury.
Intraoperative ABS seems to be the strong predictor of poor outcome for the patients with traumatic ASDH. Tian et al.35) reported the mortality for patients with intraoperative ABS was significantly high (62.16%), and avoiding the occurrence of acute encephalocele was crucial to improve the survival rate for traumatic ASDH patients. In our series, the mortality for patients with intraoperative ABS was 69.7% and functional recovery 15.2% (OR=3.71, p=0.009). Marmarou et al.20) found that brain swelling after severe head injury was caused by brain edema and not by increase in cerebral blood volume. They postulated that vascular engorgement might occur immediately following the removal of mass. We recommend that maximal removal of temporal bone flap, the administration of barbiturates and hyperventilation should be considered and a dural incision before controlling the increased ICP should be avoided.
Not only does raised ICP correlate with poor outcome, but its aggressive management in an intensive care unit setting has been associated with an improvement of outcome6). In our series, we surprisingly found that ICP monitoring was performed in only 29% (74/256) of patients, who showed a GCS score of 8 or lower. Therefore, inclusion of variables related to ICP in the statistical model would skew the analysis to the most severely injured. Also, the measurements of ICPs were not included in the analysis of the entire population. Consequently, all patients with surgically treated traumatic ASDH and presenting with a GCS score 8 or less should undergo ICP monitoring.
CONCLUSION
Among 256 consecutive patients with surgically treated traumatic ASDH, we found a functional recovery rate of 42.2% and overall mortality 39.8%. Intraoperative ABS, preoperative pupillary abnormalities and GCS score were predictors for mortality of surgically treated traumatic ASDH. The age, mechanism of injury, intraoperative ABS, preoperative pupillary abnormalities and GCS score were independently significant predictors for functional recovery of surgically treated traumatic ASDH. These results would be helpful for neurosurgeon to improve outcomes from traumatic ASDHs.
References
- 1.Becker DP, Miller JD, Ward JD, Greenberg RP, Young HF, Sakalas R. The outcome from severe head injury with early diagnosis and intensive management. J Neurosurg. 1977;47:491–502. doi: 10.3171/jns.1977.47.4.0491. [DOI] [PubMed] [Google Scholar]
- 2.Berger MS, Pitts LH, Lovely M, Edwards MS, Bartkowski HM. Outcome from severe head injury in children and adolescents. J Neurosurg. 1985;62:194–199. doi: 10.3171/jns.1985.62.2.0194. [DOI] [PubMed] [Google Scholar]
- 3.Brain Trauma Foundation; American Association of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care. Guidelines for the management of severe head injury. J Neurotrauma. 1996;13:641–734. doi: 10.1089/neu.1996.13.641. [DOI] [PubMed] [Google Scholar]
- 4.Cooper PR, Rovit RL, Ransohoff J. Hemicraniectomy in the treatment of acute subdural hematoma : a reappraisal. Surg Neurol. 1976;5:25–28. [PubMed] [Google Scholar]
- 5.Cruz J, Minoja G, Okuchi K. Improving clinical outcomes from acute subdural hematomas with the emergency preoperative administration of high doses mannitol : a randomized trial. Neurosurgery. 2001;49:864–871. doi: 10.1097/00006123-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–821. doi: 10.1136/jnnp.2003.033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Amato L, Piazza O, Alliata L, Sabia G, Zito G, Frassanito L, et al. Prognosis of isolated acute post-traumatic subdural hematoma. J Neurosurg Sci. 2007;51:107–111. [PubMed] [Google Scholar]
- 8.Dent DL, Croce MA, Menke PG, Young BH, Hinson MS, Kudsk KA, et al. Prognostic factors after acute subdural hematoma. J Trauma. 1995;39:36–42. doi: 10.1097/00005373-199507000-00005. discussion 42-43. [DOI] [PubMed] [Google Scholar]
- 9.Fell DA, Fitzgerald S, Moiel RH, Caram P. Acute subdural hematomas. Review of 144 cases. J Neurosurg. 1975;42:37–42. doi: 10.3171/jns.1975.42.1.0037. [DOI] [PubMed] [Google Scholar]
- 10.Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural hemorrhage. Acta Neurochir (Wien) 1988;90:111–116. doi: 10.1007/BF01560563. [DOI] [PubMed] [Google Scholar]
- 11.Hatashita S, Koga N, Hosaka Y, Takagi S. Acute subdural hematoma : severity of injury, surgical intervention, and mortality. Neurol Med Chir (Tokyo) 1993;33:13–18. doi: 10.2176/nmc.33.13. [DOI] [PubMed] [Google Scholar]
- 12.Hernesniemi J. Outcome following head injuries in the aged. Acta Neurochir (Wien) 1979;49:67–69. doi: 10.1007/BF01809175. [DOI] [PubMed] [Google Scholar]
- 13.Howard MA, 3rd, Gross AS, Dacey RG, Jr, Winn HR. Acute subdural hematomas : an age-dependant clinical entity. J Neurosurg. 1989;71:858–863. doi: 10.3171/jns.1989.71.6.0858. [DOI] [PubMed] [Google Scholar]
- 14.Jang HS, Lee YB, Chung C, Lee KC, Park YS, Mok JH. Acute subdural hematoma : an analysis of 244 operated cases. J Korean Neurosurg Soc. 1996;25:111–118. [Google Scholar]
- 15.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 16.Klun B, Fettich M. Factors influencing the outcome in acute subdural hematoma. A review of 330 cases. Acta Neurochir (Wien) 1984;71:171–178. doi: 10.1007/BF01401312. [DOI] [PubMed] [Google Scholar]
- 17.Koc RK, Akdemir H, Oktem IS, Meral M, Menkü A. Acute subdural hematoma : outcome and outcome prediction. Neurosurg Rev. 1997;20:239–244. doi: 10.1007/BF01105894. [DOI] [PubMed] [Google Scholar]
- 18.Kotwica Z, Brzezinski J. Acute subdural hematoma in adults : an analysis of outcome in comatose patients. Acta Neurochir (Wien) 1993;121:95–99. doi: 10.1007/BF01809257. [DOI] [PubMed] [Google Scholar]
- 19.Lobato RD, Cordobes F, Rivas JJ, de la Feunte M, Montero A, Barcena A, et al. Outcome from severe head injury related to the type of intracranial lesion. A computerized tomography study. J Neurosurg. 1983;59:762–774. doi: 10.3171/jns.1983.59.5.0762. [DOI] [PubMed] [Google Scholar]
- 20.Marmarou A, Fatouros PP, Barzo P, Portella G, Yoshihara M, Tsuji O, et al. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J Neurosurg. 2000;93:183–193. doi: 10.3171/jns.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 21.Marshall LF, Gautille T, Klauber MR. The outcome of severe closed head injury. J Neurosurg. 1991;75:528–536. [Google Scholar]
- 22.Marshall LF, Toole BM, Bowers SA. The National Traumatic Coma Data Bank, Part 2 : Patients who talk and deteriorate : implications for treatment. J Neurosurg. 1983;59:285–288. doi: 10.3171/jns.1983.59.2.0285. [DOI] [PubMed] [Google Scholar]
- 23.Phuenpathom N, Choomuang M, Ratanalert S. Outcome and outcome prediction in acute subdural hematoma. Surg Neurol. 1993;40:22–25. doi: 10.1016/0090-3019(93)90164-v. [DOI] [PubMed] [Google Scholar]
- 24.Piotrowski WP, Mühl BJ. [Results of surgery in acute subdural hematoma] Unfauchirurg. 1995;98:432–436. [PubMed] [Google Scholar]
- 25.Ransohoff J, Benjamin MV, Gage EL, Jr, Epstein F. Hemicraniectomy in the management of acute subdural hematoma. J Neurosurg. 1971;34:70–76. doi: 10.3171/jns.1971.34.1.0070. [DOI] [PubMed] [Google Scholar]
- 26.Richards T, Hoff J. Factors affecting survival from acute subdural hematoma. Surgery. 1974;75:253–258. [PubMed] [Google Scholar]
- 27.Sakas DE, Bullock MR, Teasdale GM. One-year outcome following craniotomy for traumatic hematoma in patients with fixed dilated pupils. J Neurosurg. 1995;82:961–965. doi: 10.3171/jns.1995.82.6.0961. [DOI] [PubMed] [Google Scholar]
- 28.Salvant JB, Jr, Muizelaar JP. Changes in cerebral blood flow and metabolism related to the presence of subdural hematoma. Neurosurgery. 1993;33:387–393. doi: 10.1227/00006123-199309000-00006. discussion 393. [DOI] [PubMed] [Google Scholar]
- 29.Seelig JM, Becker DP, Miller JD, Greenberg RP, Ward JD, Choi SC. Traumatic acute subdural hematoma : major mortality reduction in comatose patients treated within four hours. New Engl J Med. 1981;304:1511–1518. doi: 10.1056/NEJM198106183042503. [DOI] [PubMed] [Google Scholar]
- 30.Servadei F. Prognostic factors in severely head injured adult patients with acute subdural hematomas. Acta Neurochir (Wien) 1997;139:279–285. doi: 10.1007/BF01808822. [DOI] [PubMed] [Google Scholar]
- 31.Shigemori M, Syojima K, Nakayama K, Kojima T, Ogata T, Watanabe M, et al. The outcome from acute subdural hematoma following decompressive hemicraniectomy. Acta Neurochir (Wien) 1980;54:61–69. doi: 10.1007/BF01401944. [DOI] [PubMed] [Google Scholar]
- 32.Stening WA, Berry G, Dan NG, Kwok B, Mandryk JA, Ring I, et al. Experience with acute subdural hematomas in New South Wales. Aust N Z J Surg. 1986;56:549–556. doi: 10.1111/j.1445-2197.1986.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 33.Stone JL, Rifai MH, Sugar O, Lang RG, Oldershaw JB, Moody RA. Subdural hematomas. I. Acute subdural hematoma : progress in definition, clinical pathology, and therapy. Surg Neurol. 1983;19:216–231. doi: 10.1016/s0090-3019(83)80005-6. [DOI] [PubMed] [Google Scholar]
- 34.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 35.Tian HL, Chen SW, Xu T, Hu J, Rong BY, Wang G, et al. Risk factors related to hospital mortality in patients with isolated traumatic acute subdural hematoma: analysis of 308 patients undergone surgery. Chin Med J (Engl) 2008;121:1080–1084. [PubMed] [Google Scholar]
- 36.Tokumi T, Shigemori M , Kikuchi N. Treatment of acute subdural hematoma. In: Nakamura N, Hashimoto T, Yasue M, editors. Recent advance in neurotraumatology. Berlin/Heidelberg/New York/Tokyo: Springer Verlag; 1992. pp. 367–370. [Google Scholar]
- 37.Wilberger JE, Jr, Harris M, Diamond DL. Acute subdural hematoma : morbidity, mortality, and operative timing. J Neurosurg. 1991;74:212–218. doi: 10.3171/jns.1991.74.2.0212. [DOI] [PubMed] [Google Scholar]
- 38.Wilberger JE, Jr, Harris M, Diamond DL. Acute subdural hematoma : morbidity and mortality related to timing of operative intervention. J Trauma. 1990;30:733–736. [PubMed] [Google Scholar]
- 39.Wong CW. Criteria for conservative treatment of supratentorial acute subdural hematomas. Acta Neurochir (Wien) 1995;135:38–43. doi: 10.1007/BF02307412. [DOI] [PubMed] [Google Scholar]
- 40.Yanaka K, Kamezaki T, Yamada T, Takano S, Meguro K, Nose T. Acute subdural hematoma : prediction of outcome with a linear discriminant function. Neurol Med Chir (Tokyo) 1993;33:552–558. doi: 10.2176/nmc.33.552. [DOI] [PubMed] [Google Scholar]