Abstract
Objective
It is well known that changes in cerebral hemodynamics occur after traumatic brain injury (TBI). Osmo-regulation in the brain is important for maintaining a constant milieu in the central nervous system. Nevertheless, to our knowledge, early osmolarity changes after minor head injury have not been studied until now.
Methods
In this study, serum osmolarity was measured in 99 patients with minor head trauma. As a control group, blood samples were drawn from 99 patients who had a minor trauma in an extremity. Serum osmolarity was estimated using a fully automatic biochemical autoanalyzer within the first 3 hours after the trauma.
Results
The mean serum osmolarity levels were 286.08±10.17 mOsm/L in the study group and 290.94±5.65 mOsm/L in the control group (p<0.001). However, after age adjustment between the study and control groups, this statistical significance was found to be valid only for patients over 30 years of age.
Conclusion
It was noted that serum osmolarity levels decrease in the first 3 hours following minor head trauma in patients over 30 years of age. Further studies into this area could provide guidance for the management/treatment of elderly patients.
Keywords: Biomarkers, Brain injury, Head trauma, Neurophysiology, Osmolarity
INTRODUCTION
Unlike the heart, lung or liver, the brain is not a homogeneous organ. Moreover, it is not simply an electrical conduction system. The central nervous system is a unique morphofunctional unit consisting of an integrated, dynamic network of several subsystems37). Each part of the central nervous system has its own particular physical, chemical and immunological features. Hence, any neurological deficit after head trauma may be related not only to parenchymal and vascular damages revealed using radiological studies, but also to chemical, metabolic and immunological cerebral disturbances which cannot easily be shown using current diagnostic modalities.
It is well known that changes in cerebral hemodynamics occur after traumatic brain injury (TBI)32). Data using diffusion weighted imaging techniques on rats has shown that closed head injury is associated with a biphasic pathophysiological response and at least three forms of edema-vasogenic, ischemic, and neurotoxic edema- may contribute to increased tissue water following trauma3). The origin of cytotoxic brain edema is primarily related to disturbance of cellular osmo-regulation resulting in an intracellular increase in sodium and water3). Osmo-regulation in the brain is important for maintaining a constant milieu in the central nervous system29). Osmolality of the contused brain tissue increases rapidly, and in turn, strongly attracts water20). It has been reported that, in experimental rat models of moderate TBI, serum magnesium level decreases and calcium/magnesium level increases22). The use of hypertonic saline in the treatment of post-traumatic cerebral edema has been reported8).
As is evident from the importance of maintaining of optimum serum osmolarity during brain surgery, reciprocal changes between cerebrospinal fluid (CSF), blood and brain parenchyma occur in relation to a given time period38). However, the exact relationships are complex and very little information is available concerning how and the degree to which each compartment contributes to the volume-pressure response38). Are there similar detectable changes after minor head trauma? Can cerebral edema be involved in minor TBI? Minor head trauma is usually defined as occurring in a patient who is oriented and alert, corresponding to a Glasgow Coma Scale (GCS) of 1515,27). Minor TBI may be a structural lesion and result in life-threatening dangers, especially in patients with specific risk factors such as coagulapathies2,6). Early recognition of the changes related to minor TBI may reduce the unfavorable outcomes. However, in contrast to the cases of moderate or severe head injury, radiological studies of such patients including computed tomography (CT) and magnetic resonance imaging (MRI), do not usually show any abnormality13,19). Although guidelines for the management of head injury have been published, there is no consensus about the management of mild head injury1,23,25).
Unfortunately, biochemical markers do not serve as a substitute for neuroimaging at present time6). To our knowledge, early osmolarity changes after minor head injury have not previously been studied.
MATERIALS AND METHODS
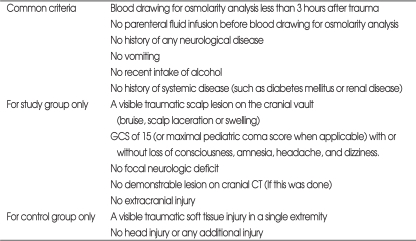
In this prospective study, 99 patients, who were admitted to the emergency department at our institution with an isolated minor head trauma and who met our selection criteria, were studied (Table 1). They were evaluated as a GCS score of 15 with or without loss of consciousness (LOC), detected amnesia, headache, or dizziness. All the patients had a visible sign of soft tissue injury over the surface of the head on the frontal, parietal, temporal or occipital regions, and this was considered objective evidence for head trauma. Trauma to the face was not accepted as head trauma. Patients with a demonstrable lesion on CT scan, or focal neurologic findings were excluded, since we aimed to find an early chemical change before a radiologically visible parenchymal or vascular brain injury occurred.
Table 1.
Inclusion criteria for the study and control groups
GCS: Glasgow coma scale, CT: computed tomography
Every patient who participated in the study underwent neurological examination including GCS. Each patient of 6 year of age or above was given a mini mental state examination (MMSE) that included a diagnostically valuable verbal retention test and tasks assessing basic orientation, short term memory, the ability to calculate and visual-motor ability.
The patients were examined by skull X-ray studies and/or CT of the brain without contrast media. A CT scan was preferred to skull radiography when there was no overloading of the CT facility.
As a control group, blood sample data from 99 subjects seen at the hospital's outpatient or emergency clinics with a minor soft tissue trauma in an extremity were used. The inclusion criteria for the control group were the same as for the study group with the exception of the site of visible traumatic soft tissue lesion (head versus extremity) (Table 1). The reason for selecting patients with a minor extremity injury as a control group instead of selecting patients without any injuries was to avoid the difference in osmolarity created by local inflammation of the soft tissue injury on the head. However, patients with severe extremity injuries such as bone fractures were not included either.
The following exclusion criteria were used when selecting patients for both study and control groups : 1-Patients who had received any kind of parenteral infusion before the blood for osmolarity determination was drawn. 2-Patients who had drunk any fluids in the period between the trauma and the blood withdrawal for analysis of osmolarity. 3-Patients who had recently consumed alcohol. 4-Patients who stated, or whose parents stated, that they had vomited or who were observed to vomit after admission. No patient with a known history of diabetes mellitus or any other systemic chronic diseases was allocated to either the study group or control group.
This study was approved by the local ethical committee, and written consent was obtained from all patients or from their parents.
Laboratory methods
In our study, serum determinations of osmolarity were done in our hospital laboratory within the first 3 hours after the trauma. Bloods samples of 5 cc were obtained from the cubital veins of the patients. These samples were centrifuged under 3,000 rpm and the sera were separated. Osmolarity measurements of the patients were conducted using a fully automatic biochemical autoanalyzer (Abbott Diagnostics-Aeroset, Abbott Laboratories, Abbott Park, IL, USA). Osmolarity values were obtained automatically after programming the autoanalyzer according to the formula below:16)
Serum osmolarity=(2×serum sodium [mEq/L])+(BUN [mg/dL]/2.8)+(glucose [mg/dL]/18)
The laboratory facility was available for osmolarity analysis 24 hours a day.
Statistical analysis
All results were expressed in mean¡¾standard deviation (SD). An SPSS (Statistical Package for the Social Sciences) for Windows 15.0 software package (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. During evaluation of the study data, along with descriptive statistical methods, parameters with normal distribution were evaluated using Student's t test. Results were given in 95% confidence interval and significance was accepted at p<0.05 level.
RESULTS
The ages of the 25 female and 74 male patients in the study group ranged from 1 to 66 yrs (mean=17.07 yrs±13.72 SD). The mean age of the control group was 48.36±19.96 (range 2-79). Neurological examination was normal in all patients. Mini mental state test scores were found to be normal or slightly decreased.
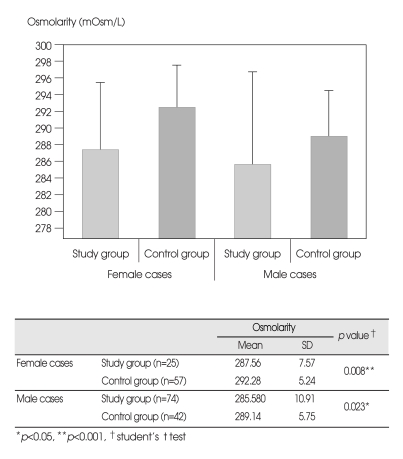
Osmolarity levels were lower in the study group than in the control group (p<0.001) (Fig. 1). The mean serum osmolarity levels were 286.08±10.17 mOsm/L in the study group and 290.94±5.65 mOsm/L in the control group. This difference was more marked in female patients than in male patients (p=0.008 and p=0.023 respectively).
Fig. 1.
Osmolarity levels are lower in the study group, in both females and males.
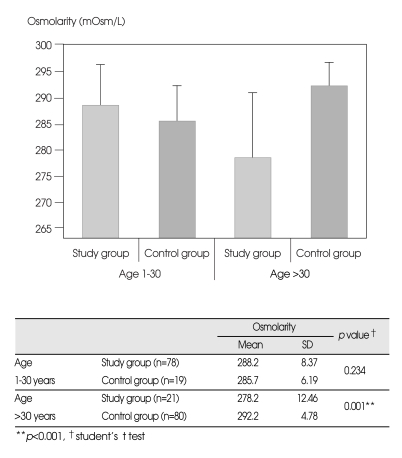
Serum osmolarity levels of patients aged ≤30 years were higher in the study group. However, this difference did not reach statistical significance (p>0.05). Contrary to this, in patients over 30 years of age, blood osmolarity levels were significantly lower in the study group as can be seen in Fig. 2 (p<0.001).
Fig. 2.
A statistical significance is found when comparing patients over 30 years of age.
DISCUSSION
It is generally believed that osmolarity has insufficient tissue specificity to be used as a clinical laboratory measurement. In fact, osmo-regulation for maintaining a constant milieu in the brain is a very complex and sensitive process. The hypothalamus integrates hormonal and osmotic cues sensing cell volume and the state of the extracellular space18). Osmotic stimuli can act directly on osmoreceptor cells, probably neurons in the hypothalamus. The feedback signals derive from many sources including arterial baroreceptors in the aortic arch and carotid sinus, the kidney that secretes renin, and the subfornical organ that is sensitive to low concentrations of angiotensin II. There is a neural pathway between the hypothalamus, the subfornical organ and the preoptic area. This pathway utilizes an angiotensin-like molecule as a transmitter. The preoptic area receives information from baroreceptors, which also sends information to the paraventricular nucleus and mediate the release of vasopressin.
A more accurate estimation of a correlation between trauma to the brain and changes in serum osmolarity as a reaction can be made only in patients with isolated minor head trauma. This study could not be carried out in cases with moderate and severe head injuries, because these patients are usually managed early and administered parenteral fluids which iatrogenically affect the serum osmolarity. Additionally, there are local and systemic effects of intracranial hemorrhages in these severely injured patients. Vomiting also affects osmolarity. Hence, it is clear that serum osmolarity may be found to be decreased or increased in patients with moderate and severe head trauma.
The importance of serum osmolarity changes after minor TBI
Age has previously been reported as a risk factor in delayed deterioration following minor head trauma12,30). Our studies indicate that older patients with minor head trauma may be more prone to osmolarity changes, manifesting as a decrease in serum osmolarity, and thus be more prone to injury. Further studies could provide guidance for more accurate observation of such patients and for their oral or parenteral treatment with hyperosmolar fluids in cases where the patient also complains of vague symptoms, such as dizziness, difficulty in concentration or prickling sensations. The questions of how long this observation and treatment should last and what kind of hyperosmolar fluids should be used are subjects for additional studies. As an example, mannitol has been shown to pass into the CSF and sudden discontinuation of its intravenous administration may create an osmolar gap because of increased CSF osmolarity31). This may cause increase in intracranial pressure. Hence, mannitol should be avoided in patients with minor head injury. The next step after our findings could be to investigate whether there is any association between decrease in serum osmolarity in elderly patients and clinical course after minor head trauma. In our study, the serum osmolarity values were found to be lower in the study group even after analyzing the findings according to the sexes. It is well known that direct brain trauma causes complex hormonal responses of the pituitary end-organ axis9,21). Hence, at least a slight difference in findings between the sexes is to be expected, as seen in our study.
Proposal for a panel of biomarkers including serum osmolarity monitoring after minor TBI
Even though many markers associated with neuronal and glial injury, hypoxia, and inflammation have been studied, currently there is no reliable and practical biochemical marker of head injury5,11,26,28). Only two markers seem to have a diagnostic adjunct in adults : neuron-specific enolase (NSE) and S100B protein5,7,10,13,14,17,28,34-36). An S100B has been found in astrocytes, bone marrow, fat and skeletal muscle, while NSE has been detected in neurons, platelets and red blood cells5). However, these markers are not specific to head injury and may be increased in meningitis, encephalitis, and neurodegenerative conditions4,28). Additionally, specificity of S100B and NSE is uncertain since it may increase in patients with body trauma without signs of head trauma13,24,34). In one study, it has been shown that serum cortisol concentrations markedly increased in a majority of patients with mild TBI shortly (about 3 hours) after the trauma33). This finding is also nonspecific and can be seen in other stress related conditions. It may be useful if data on the evolution of osmolarity over time could be studied after the initial measurement at the early post-traumatic period.
Study limitations
Osmotic concentration are typically expressed as either miliosmoles/kilogram (mOsm/kg) of solvent referred to as osmolality, or miliosmoles/liter (mOsm/L) of solution referred to as osmolarity16). Osmolality can be measured by an osmometer which usually uses a method such as freezing point depression of water.
Our method for determining osmolarity may be of concern. This is a calculated value rather than an actual measurement of serum osmolarity using an osmometer. Unfortunately, we did not regard the age related values of blood urea nitrogen (BUN) and glucose which could affect this calculation. The control and patient populations were not age balanced. A difference of about thirty years in the mean age of the two populations might have caused the higher calculated osmolarity in the control population. We were not able to simultaneously measure the accepted markers of brain injury such as S100B or NSE. Therefore, we could not compare the osmolarity change with values of other biochemical markers mentioned. Also, it is important to discover whether a change in serum osmolarity after head trauma could be detected earlier than other potential biomarkers in the serum. To indicate that osmolarity disturbances in minor or mild TBI are similar to more severe TBI, measurements of urinary osmolarity and anti-diuretic hormone (ADH) might be necessary. Although, we were aware of this fact during the study, clinical and technical difficulties prevented us acting upon it. The results might have been better if, in addition to serum osmolarity, urine osmolarity, ADH and sodium in the patients, had been measured.
CONCLUSION
It was found that serum osmolarity levels decrease in the first 3 hours following minor head trauma in patients over 30 years of age. Although a single blood analysis of serum osmolarity may not show any significance in an individual patient, serum osmolarity monitoring of patients with minor head trauma may be a cost-effective way to help identify those at greater risk. A panel of biomarkers including serum osmolarity monitoring could be beneficial in clinical diagnosis of otherwise silent brain injury. In patients where a decrease in serum osmolarity was associated with vague symptoms such as dizziness, hyperosmolar treatment would be advisable. Further studies in larger patient populations are needed for conclusive findings.
Acknowledgements
We would like to thank Antoin Rodgers, Corina Nino and Ann Hazinedar for their editorial assistance.
References
- 1.Balak N. A prospective and comparative study of referrals to neurosurgeons in an emergency department : does use of guidelines for head trauma affect the assessment made by non-neurosurgeons? Ulus Travma Acil Cerrahi Derg. 2008;14:292–298. [PubMed] [Google Scholar]
- 2.Balak N, Silav G, Kilic Y, Timur C, Elmaci I. Successful surgical treatment of a hemophiliac infant with nontraumatic acute subdural hematoma. Surg Neurol. 2007;68:537–540. doi: 10.1016/j.surneu.2006.11.053. discussion 540. [DOI] [PubMed] [Google Scholar]
- 3.Barzó P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg. 1997;87:900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- 4.Bazarian JJ, Zemlan FP, Mookerjee S, Stigbrand T. Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 2006;20:759–765. doi: 10.1080/02699050500488207. [DOI] [PubMed] [Google Scholar]
- 5.Berger RP, Kochanek PM, Pierce MC. Biochemical markers of brain injury : could they be used as diagnostic adjuncts in cases of inflicted traumatic brain injury? Child Abuse Negl. 2004;28:739–754. doi: 10.1016/j.chiabu.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Biasca N, Lovell MR, Collins MW, Jordan BD, Matser E, Weber J, et al. [The undetected brain lesion in sports. Minor traumatic brain injury and its sequelae] Unfallchirurg. 2006;109:101–111. doi: 10.1007/s00113-005-1046-5. [DOI] [PubMed] [Google Scholar]
- 7.Biberthaler P, Mussack T, Kanz KG, Linsenmaier U, Pfeifer KJ, Mutschler W, et al. [Identification of high-risk patients after minor craniocerebral trauma. Measurement of nerve tissue protein S 100] Unfallchirurg. 2004;107:197–202. doi: 10.1007/s00113-004-0730-1. [DOI] [PubMed] [Google Scholar]
- 8.Catrambone JE, He W, Prestigiacomo CJ, McIntosh TK, Carmel PW, Maniker A. The use of hypertonic saline in the treatment of post-traumatic cerebral edema : a review. Eur J Trauma Emerg Surg. 2008;34:397–409. doi: 10.1007/s00068-007-7068-7. [DOI] [PubMed] [Google Scholar]
- 9.Cernak I, Savic VJ, Lazarov A, Joksimovic M, Markovic S. Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Inj. 1999;13:1005–1015. doi: 10.1080/026990599121016. [DOI] [PubMed] [Google Scholar]
- 10.Chatfield DA, Zemlan FP, Day DJ, Menon DK. Discordant temporal patterns of S100beta and cleaved tau protein elevation after head injury : a pilot study. Br J Neurosurg. 2002;16:471–476. doi: 10.1080/0268869021000030285. [DOI] [PubMed] [Google Scholar]
- 11.Chiaretti A, De Benedictis R, Langer A, Di Rocco C, Bizzarri C, Iannelli A, et al. Prognostic implications of hyperglycaemia in paediatric head injury. Childs Nerv Syst. 1998;14:455–459. doi: 10.1007/s003810050259. [DOI] [PubMed] [Google Scholar]
- 12.Choi SW, Koh HS, Yeom JY, Kim SH, Song SH, Kim Y. Clinical analysis of the risk factors and prognostic factors of delayed deterioration following mild head injury. J Korean Neurosurg Soc. 1999;28:1216–1323. [Google Scholar]
- 13.de Boussard CN, Lundin A, Karlstedt D, Edman G, Bartfai A, Borg J. S100 and cognitive impairment after mild traumatic brain injury. J Rehabil Med. 2005;37:53–57. doi: 10.1080/16501970410015587. [DOI] [PubMed] [Google Scholar]
- 14.De Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Rutten J, Twijnstra A. Prediction of post-traumatic complaints after mild traumatic brain injury : early symptoms and biochemical markers. J Neurol Neurosurg Psychiatry. 2002;73:727–732. doi: 10.1136/jnnp.73.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Kruijk JR, Twijnstra A, Meerhoff S, Leffers P. Management of mild traumatic brain injury : lack of consensus in Europe. Brain Inj. 2001;15:117–123. doi: 10.1080/026990501458353. [DOI] [PubMed] [Google Scholar]
- 16.Erstad BL. Osmolality and osmolarity : narrowing the terminology gap. Pharmacotherapy. 2003;23:1085–1086. doi: 10.1592/phco.23.10.1085.32751. [DOI] [PubMed] [Google Scholar]
- 17.Jang WY, Kim JH, Joo HP, Lee JK, Kim TS, Kim SH. Serum S-100B Protein as a Prognostic Factor in Patients with Severe Head Injury. J Korean Neurosurg Soc. 2006;39:271–276. [Google Scholar]
- 18.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. ed 4. New York: The McGraw-Hill Companies; 2000. pp. 1006–1007. [Google Scholar]
- 19.Kara A, Celik SE, Dalbayrak S, Yilmaz M, Akansel G, Tireli G. Magnetic resonance imaging finding in severe head injury patients with normal computerized tomography. Turk Neurosurg. 2008;18:1–9. [PubMed] [Google Scholar]
- 20.Kawamata T, Mori T, Sato S, Katayama Y. Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg Focus. 2007;22:E5. doi: 10.3171/foc.2007.22.5.6. [DOI] [PubMed] [Google Scholar]
- 21.Keskil Z, Evrenkaya T, Gozil R, Calguner E, Keskil S. Effects of vasoconstriction on the acute anterior pituitary hormonal response to head injury. Neuropeptides. 2002;36:287–290. doi: 10.1016/s0143-4179(02)00047-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim JG, Park CO, Hyun DK, Youn SH, Kim EY, Park HC. Change in serum concentration of magnesium and calcium ions following moderate diffuse axonal injury in rats-preliminary study- J Korean Neurosurg Soc. 2004;36:229–234. [Google Scholar]
- 23.Kolodziejczyk D. [Mild head injury : diagnostic pitfalls and complications] Unfallchirurg. 2008;111:486–492. doi: 10.1007/s00113-008-1452-6. [DOI] [PubMed] [Google Scholar]
- 24.Korfias S, Stranjalis G, Psachoulia C, Vasiliadis C, Pitaridis M, Boviatsis E, et al. Slight and short-lasting increase of serum S-100B protein in extra-cranial trauma. Brain Inj. 2006;20:867–872. doi: 10.1080/02699050600832395. [DOI] [PubMed] [Google Scholar]
- 25.Lapierre F Neurosurgical Society of France. [Guide-lines for head injured patients management in adult age] Neurochirurgie. 1998;44:55–56. [PubMed] [Google Scholar]
- 26.Lazar L, Erez I, Gutermacher M, Katz S. Brain concussion produces transient hypokalemia in children. J Pediatr Surg. 1997;32:88–90. doi: 10.1016/s0022-3468(97)90102-0. [DOI] [PubMed] [Google Scholar]
- 27.Miller JD, Murray LS, Teasdale GM. Development of a traumatic intracranial hematoma after a "minor" head injury. Neurosurgery. 1990;27:669–673. doi: 10.1097/00006123-199011000-00001. discussion 673. [DOI] [PubMed] [Google Scholar]
- 28.Naeimi ZS, Weinhofer A, Sarahrudi K, Heinz T, Vecsei V. Predictive value of S-100B protein and neuron specific-enolase as markers of traumatic brain damage in clinical use. Brain Inj. 2006;20:463–468. doi: 10.1080/02699050600664418. [DOI] [PubMed] [Google Scholar]
- 29.Ohtsuki S. New aspects of the blood-brain barrier transporters; its physiological roles in the central nervous system. Biol Pharm Bull. 2004;27:1489–1496. doi: 10.1248/bpb.27.1489. [DOI] [PubMed] [Google Scholar]
- 30.Park YS, Kim HJ, Whang K, Pyen JS, Hu C, Hong SK, et al. Clinical characteristics and prognosis of mild head injury in the elderly. J Korean Neurosurg Soc. 2002;31:564–568. [Google Scholar]
- 31.Polderman KH, van de Kraats G, Dixon JM, Vandertop WP, Girbes AR. Increases in spinal fluid osmolarity induced by mannitol. Crit Care Med. 2003;31:584–590. doi: 10.1097/01.CCM.0000050287.68977.84. [DOI] [PubMed] [Google Scholar]
- 32.Sinson G, Reilly PM, Grady MS. Initial resuscitation and patient evaluation. In: Winn HR, editor. Youmans Neurological Surgery. Philadelphia: Saunders; 2004. pp. 5083–5101. [Google Scholar]
- 33.Sojka P, Stalnacke BM, Bjornstig U, Karlsson K. One-year follow-up of patients with mild traumatic brain injury : occurrence of post-traumatic stress-related symptoms at follow-up and serum levels of cortisol, S-100B and neuron-specific enolase in acute phase. Brain Inj. 2006;20:613–620. doi: 10.1080/02699050600676982. [DOI] [PubMed] [Google Scholar]
- 34.Stalnacke BM, Tegner Y, Sojka P. Playing soccer increases serum concentrations of the biochemical markers of brain damage S-100B and neuron-specific enolase in elite players : a pilot study. Brain Inj. 2004;18:899–909. doi: 10.1080/02699050410001671865. [DOI] [PubMed] [Google Scholar]
- 35.Thorngren-Jerneck K, Alling C, Herbst A, Amer-Wahlin I, Marsal K. S100 protein in serum as a prognostic marker for cerebral injury in term newborn infants with hypoxic ischemic encephalopathy. Pediatr Res. 2004;55:406–412. doi: 10.1203/01.PDR.0000106806.75086.D3. [DOI] [PubMed] [Google Scholar]
- 36.Watt SE, Shores EA, Baguley IJ, Dorsch N, Fearnside MR. Protein S-100 and neuropsychological functioning following severe traumatic brain injury. Brain Inj. 2006;20:1007–1017. doi: 10.1080/02699050600909698. [DOI] [PubMed] [Google Scholar]
- 37.Yasargil MG. Microneurosurgery. ed 1. Vol 4A. Stuttgart New York: Georg Thieme Verlag; 1994. pp. 2–114.pp. 255–256. [Google Scholar]
- 38.Yasargil MG. Microneurosurgery. ed 1. Vol 4B. Stuttgart: Georg Thieme Verlag; 1996. pp. 71–91. [Google Scholar]