Abstract
Objective
The goal of this study was to evaluate the biomechanical features of human cadaveric spines implanted with the Activ L prosthesis.
Methods
Five cadaveric human lumbosacral spines (L2-S2) were tested for different motion modes, i.e. extension and flexion, right and left lateral bending and rotation. Baseline measurements of the range of motion (ROM), disc pressure (DP), and facet strain (FS) were performed in six modes of motion by applying loads up to 8 Nm, with a loading rate of 0.3 Nm/second. A constant 400 N axial follower preload was applied throughout the loading. After the Activ L was implanted at the L4-L5 disc space, measurements were repeated in the same manner.
Results
The Activ L arthroplasty showed statistically significant decrease of ROM during rotation, increase of ROM during flexion and lateral bending at the operative segment and increase of ROM at the inferior segment during flexion. The DP of the superior disc of the operative site was comparable to those of intact spine and the DP of the inferior disc decreased in all motion modes, but these were not statistically significant. For FS, statistically significant decrease was detected at the operative facet during flexion and at the inferior facet during rotation.
Conclusion
In vitro physiologic preload setting, the Activ L arthroplasty showed less restoration of ROM at the operative and adjacent levels as compared with intact spine. However, results of this study revealed that there are several possible theoretical useful results to reduce the incidence of adjacent segment disease.
Keywords: Biomechanics, Lumbar spinal arthroplasty, Activ L, Range of motion, Disc pressure, Facet strain
INTRODUCTION
Lumbar degenerative disc disease (DDD) is one of the most commonly encountered disorders in spine surgery practices. If conservative treatments were failed, surgical treatment of symptomatic disc degeneration is considered based on the severity of disc pathology and stability of the motion segment. The current gold standard treatment for painful DDD of the lumbar spine that has failed nonsurgical management is arthrodesis of painful motion segments. Short-term clinical success rates as high as 80% have been reported with fusion surgeries11,15). Furthermore, a randomized, prospective trial found that the clinical success rate of surgical fusion was superior to that of nonsurgical management5). However, the pseudoarthrosis, which is estimated to be less than 10%2,10,17), and the need for postoperative orthoses after fusion surgery are distinct disadvantages. Also, long-term elimination of segmental motion leads to increase of strain at the adjacent levels, and result in adjacent segment hypertrophic facet arthropathy, dynamic instability, spinal stenosis, osteophyte formation and disc degeneration1,12-14,21). This accelerated process of degeneration adjacent to fused segments has been reported in both cervical and lumbar spine9,14,18,22) and these arthrodesis-related limitations were responsible for the development of total disc replacement arthroplasty.
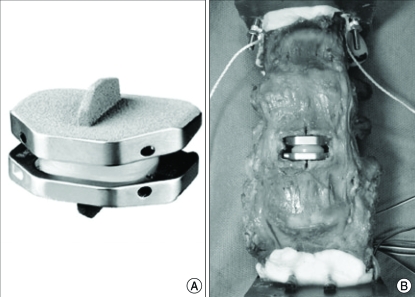
The ideal artificial disc prosthesis should replicate the biomechanical performance of the intact healthy disc. It has to restore the caliber of the neural foramina, disc height and lordosis. It also has to provide an acceptable range of spine motion and generate physiologic kinematics at the spinal triple joint complex without overloading the structural element. Moreover, it should possess long-term durability, long-term stability and function as an effective shock absorbing device. Finally, it has to be composed of material that is safe and nontoxic, not cause injury to normal tissue and be visible by standard imaging tests. For these goals, many artificial disc implants and prosthetic nuclei have been developed over the last years. Recently, Activ L (Aesculap, Inc., San Francisco, CA) (Fig. 1A) has been developed for the purpose of preventing facet joint degeneration, offering the lowest profile and sagittal translational movement of the center of rotation (COR) from the center to posterior depending on the shear forces.
Fig. 1.
Photographs showing the Activ L (Aesculap, Inc., San Francisco, CA) alone (A) and implanted in the human cadaveric lumbosacral spine (B).
The object of this study was to evaluate the biomechanical features, i.e. range of motion (ROM), disc pressure (DP) and facet strain (FS) of the preloaded human cadaveric spines implanted with the Activ L prosthesis. To our knowledge, this is the first report on a biomechanical study of the Activ L prosthesis.
MATERIALS AND METHODS
Cadaveric specimen preparation and fixation
Five human cadaveric lumbosacral spines (L2-S2) were obtained from Science Care Anatomical (Phoenix, AZ), and International Biological, Inc. (Grosse Pointe Farms, MI). Specimens containing osseous abnormalities were excluded based on anteroposterior and lateral radiographs. Bone mineral density (BMD) was measured by using dual-energy X-ray absorptiometry with a bone densitometer (Hologic QDR 4500A; Hologic, Inc., Waltham, MA).
For biomechanical testing, en bloc specimens were stored at -20 degrees centigrade until thawed at room temperature prior to manipulation, and were kept moist during all procedures. The attached paravertebral musculature was adequately removed to expose the facet surfaces of the vertebrae, avoiding disruption of the joint capsules, ligaments, discs, and bone structures.
Each spine was fixed by drilling and fixing screws to the most superior and most inferior segments. The end segments and screws were cast into two potting fixtures with polymethylmethacrylate (PMMA, COE tray plastic, GC America, Alsip, IL), and the PMMA-covered ends were potted in polyester resin (Bondo, Atlanta, GA).
Discectomy and artificial disc implantation
Load testing was performed with the intact spine prior to any surgical procedure. Throughout testing cycle, specimens were kept moist with warm saline. Activ L was placed in a 36 degrees centigrade saline bath for 72 hours prior to implantation to keep the discs maintained near a biophysiological temperature. The device was implanted into the discectomy defect according to manufacturer's specifications. The midline of the spinal column was confirmed radiographically using fluoroscopy before discectomy. A complete anterior discectomy was performed at the L4-L5 level with appropriate ring and cup curettes. Cartilage was removed from the vertebral endplates. Posterior osteophytes and the posterior longitudinal ligament were excised while maintaining the integrity of the lateral annulus fibrosus. After parallel distraction and restoration of the normal intervertebral disc height was accomplished, trial implant was placed to help select the proper artificial disc size, angle, and height. A sagittal groove was then cut in the vertebral endplates in the exact midline, using an arthroscopy chisel placed over the trial implant. This groove accepted the central keel of the implant. After the trial implant was removed, both the superior and inferior plates with ultra-high molecular weight polyethylene (UHMWPE) insert were impacted altogether into place with an insertion tool. Gross inspection was made to ensure the UHMWPE liner was properly flush against the inferior endplate after the insertion tool was removed (Fig. 1B). Fluoroscopy was used throughout the procedure to verify the correct position of the prostheses.
Biomechanical testing
The potting fixtures for L2 and S2 were attached to the upper and lower spine-loading fixtures of a biomechanical loading frame (MTS 858; Materials Testing Systems, Mini Bionix®, Eden Prairie, MN), respectively.
Range of motion was defined as the maximum displacement under the maximum applied load. Three infrared reflective markers were placed on the L4 and L5 vertebrae, and the vertebral ROM was tracked using a video-based motion-capturing system (MacReflex; Qualisys Medical AB, Gottenburg, Sweden).
Pressure transducer needle tips (Model 6377, Robert A. Denton, Inc., Rochester Hills, MI) were inserted into the L3-L4 and L5-S1 discs. Each needle had a pressure sensor, and the needle tips were inserted from anterior to posterior approximately two centimeters into the disc, so as to place the pressure sensor in the center of each disc. The "central DP" was defined as the value measured from the pressure sensor. In lateral bending, needle tips were inserted from left to right, so as to place the pressure sensor in the center of each disc.
Six facet strain gauges (Model CEA-06-062UW-350, Vishay Micro-Measurements, Raleigh, NC) were fixed on the lamina near the facet joints of L3-L4, L4-L5, and L5-S1, bilaterally. Each gauge measured the amount of strain over the specific facet where it was attached to.
Baseline measurements of the ROM, DP and FS were performed for each intact spine in six modes of motion i.e. flexion, extension, right/left lateral bending, and right/left axial rotation under the physiologic compressive follower preload.
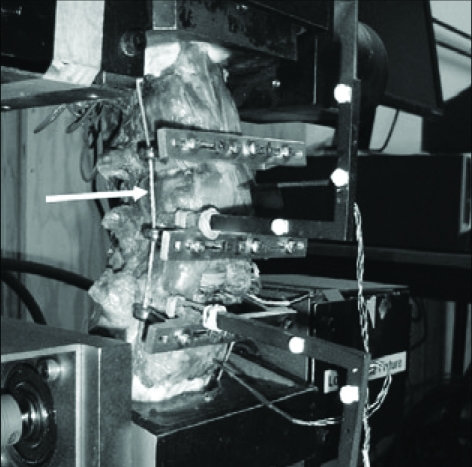
The method for applying compressive follower preload to a multi-segmented spine specimen (L2-S2) was adopted from a published method so that its path approximated the tangent of the curve of the lumbar spine (Fig. 2)16). The load was applied bilaterally by cables and dead weights. The loading cables were firmly anchored to the cup holding the L2 vertebral body and passed freely through cable guides attached to the bodies of L2-L5. The cable path approximated the tangent to the curve of the lumbar spine. Because the cable guides move with the vertebrae, the cable arrangement assures that the load path approximately passes through the COR of each vertebra as the spine is deformed under loading16). Thus, the compressive preload was applied along a follower load path rather than vertical load path.
Fig. 2.
Photographs showing the method for applying a compressive follower preload to a multi-segmented spine specimen (L2-S2) so that its path approximated the tangent of the curve of the lumbar spine. The load was applied bilaterally by cables (arrow) and dead weights.
The moments were applied to both L2 and S2 up to 8 Nm, with a loading rate of 0.3 Nm/second, and a constant 400 N axial follower preload was applied throughout the loading. These moments were selected as safe loads on the human cadaveric spine based on published data of biomechanical testing7,10). Axial rotation was determined by the upper spine fixator, whereas flexion, extension, and lateral bending were determined by the rotation of both spine fixators in the respective coronal and sagittal planes. To stabilize the viscoelastic effect for each mode of testing, the loading was applied three times and only the result of the third loading was used.
After the prosthesis was implanted at the L4-L5 disc, measurements of the ROM, DP and FS were repeated in the same manner under the preload condition.
The ROMs at the operative level, and the level above and below the operative site were compared to those of the intact spine. The DP and FS at the levels above and below the operative site and the FS additionally at the operative level were compared to those of the intact spine.
Statistical analysis
The mean ROM was determined and normalized according to the intact spine by dividing them by those of the intact spine. The values of right/left lateral bending and right/left axial rotation were added and termed lateral bending and axial rotation, respectively, thus making four biomechanical modes of motion i.e. flexion, extension, lateral bending and axial rotation.
Because the number of specimens was small and the data could not be assumed to be normally distributed, nonparametric statistical methods were used to ascertain the statistically-significant intergroup differences, and were compared to the intact spines. Paired comparisons between different treatment groups were made by using Wilcoxon paired tests, and statistical significance was established at a probability value of 0.05. Values are presented as the mean±SE.
RESULTS
The mean age of the two male and three female specimens at the time of death was 69.7±14.0 years (±SD) (range 54-81 years). The mean BMD value (±SD) of the spines was 0.82±0.14 g/cm2.
Range of motion (ROM)
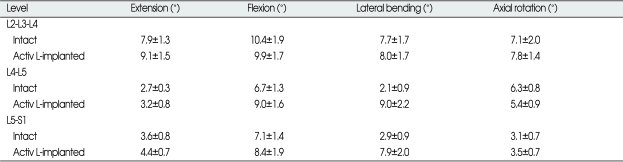
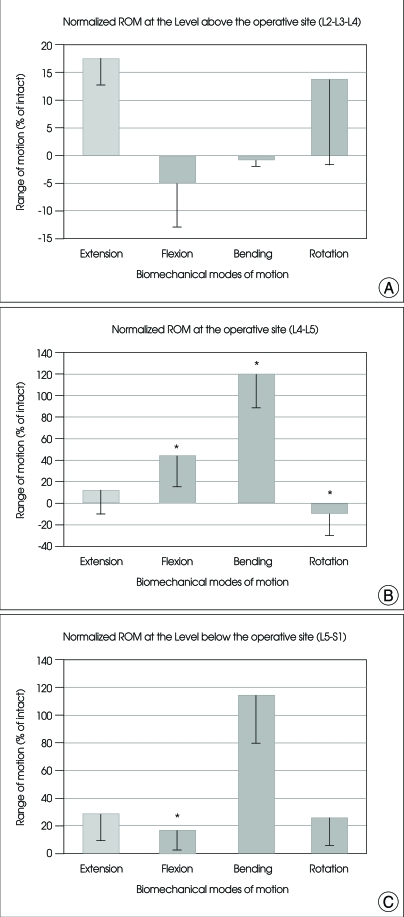
The values (mean±SE) of ROM at the operative level (L4-L5), at the level above (L2-L3-L4) and below (L5-S1) the operative level for all specimens are shown in Table 1. The values for each specimen were normalized in relation to that of the intact spine, as shown in Fig. 3.
Table 1.
Mean ROM values* at the superior, operative, and inferior segments of arthroplasty with Activ L at L4-L5 under a physiologic compressive follower preload of 400 N.
*Values are presented as the mean±SE
Fig. 3.
Graphs showing the mean and SE of normalized ROM values at the superior (A), operative (B), and inferior (C) segments of arthroplasty with Activ L at L4-L5 under physiologic compressive follower preload. For the abbreviation, bending : right/left lateral bending; rotation : right/left axial rotation. *p<0.05 versus intact.
At the level above the operative disc (L2-L3-L4)
As compared with that of intact spine, the ROM of the superior segment was increased during extension and rotation, decreased during flexion and bending, but a statistical significance was not observed (Fig. 3A).
At the operative level (L4-L5)
The ROM of the operative segment was increased for all motion modes except rotation, as compared with the intact spine, and statistical significances were observed in flexion, bending and rotation (p<0.05) (Fig. 3B).
At the level below the operative disc (L5-S1)
The ROM of the inferior segment was increased for all motion modes as compared with the intact spine, but a statistical significance was demonstrated only in flexion (p<0.05) (Fig. 3C).
Central disc pressure (DP)
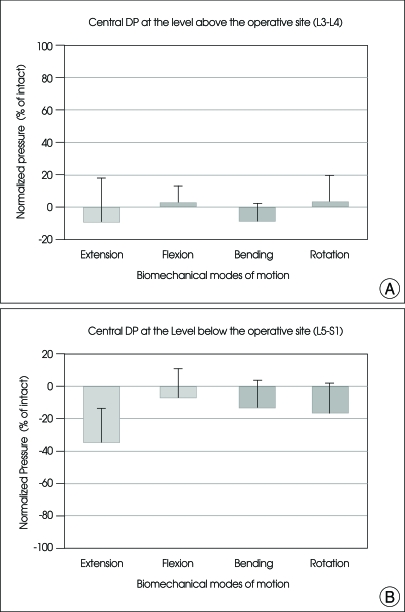
The values (mean±SE) of central DP at the level above (L3-L4) and below (L5-S1) the operative level for all the specimens were normalized in relation to that of the intact spine, as shown in Fig. 4.
Fig. 4.
Graphs showing the mean and SE of normalized disc pressure in the center of disc at the L3-L4 (A) and L5-S1 (B) levels after arthroplasty with Activ L at L4-L5 under physiologic compressive follower preload. For the abbreviation, bending : right/left lateral bending; rotation : right/left axial rotation.
At the disc level above the operation (L3-L4)
The central DP of the segment superior to the operative site was comparable to those of intact spine without a statistical significance (Fig. 4A).
At the disc level below the operation (L5-S1)
The central DP of the inferior segment was decreased during all the motion modes as compared with intact spine, but a statistical significance was not observed (Fig. 4B).
Facet strain
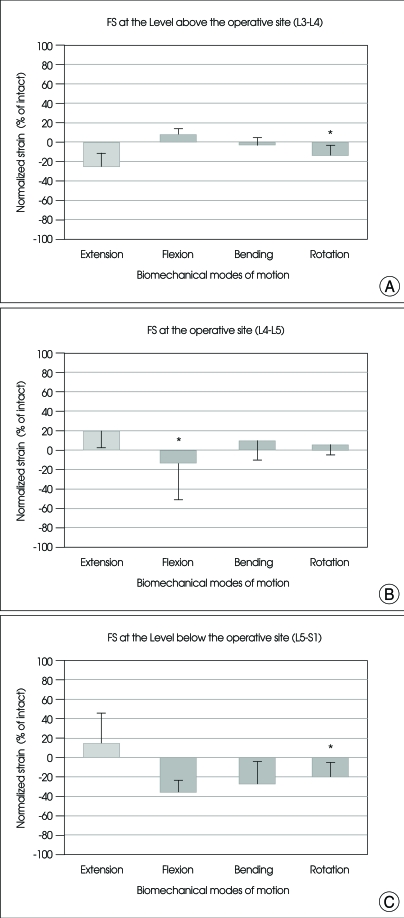
The values (mean±SE) of FS at the operative level (L4-L5), at the levels above and below the operative level for all the specimens were also normalized in relation to that of the intact spine, as shown in Fig. 5.
Fig. 5.
Graphs showing the mean and SE of normalized facet strain at L3-L4 (A), L4-L5 (B), and L5-S1 (C) facets after arthroplasty with Activ L at L4-L5 under physiologic compressive follower preload. For the abbreviation, bending : right/left lateral bending; rotation : right/left axial rotation. *p<0.05 versus intact.
At the facet joints above the operative level (L3-L4 facet)
The FS of the upper segment was decreased in extension, bending, and rotation as compared with intact spine, but a statistical significance was not observed (Fig. 5A).
At the operative facet joints (L4-L5 facet)
The FS of the operative segment was increased in all motion modes except flexion where the FS was decreased with statistical significance, as compared with intact spine (p<0.05) (Fig. 5B).
At the Facet Joints below the Operative Level (L5-S1 facet)
The FS of the lower segment was decreased in flexion, bending and rotation as compared with intact spine, but the statistical significance was observed only in rotation (p<0.05) (Fig. 5C).
DISCUSSION
The first described attempt of disc arthroplasty was performed with a steel-ball endoprosthesis by Fernstrom in the late 1950s4). Since that time a nonlinear progression of technology in lumbar disc arthroplasty has resulted in an extensive and diverse list of implant designs. Such a long list of implants, based on highly varied principles, has been proposed, and that only very few have reached the level of animal models, let alone human implantation, clearly demonstrates how challenging the task of designing an intervertebral disc replacement is20). The problems in designing a successful intervertebral implant are linked to the three-column structure of the spine, which involves three separate joints at each level. Furthermore, the intervertebral disc is not a true joint, and rather it serves a double function of mobility and damping, with load repartition properties. To add to the complexity, the COR constantly moves along the three axes8). Therefore, there are only a few designs that are in commercial production and current human use. In the lumbar spine, there are four major arthroplasty devices in the United States currently. The first two prostheses each involve metal-on-plastic devices. The Charité Artificial Disc (DePuy Spine Inc., Raynham, MA) and the ProDisc (Synthes Spine, Paoli, PA) lumbar prosthesis have both finished enrollment in Unites States clinical trials. The second two have metal-on metal (Co-Cr) bearing surfaces. These are the Maverick lumbar prosthesis (Medtronic Sofamor Danek Inc., Memphis, TN) and the Flexi-Core lumbar prosthesis (Stryker Spine, Allendale, NJ). Among these prostheses, 2 lumbar total disc replacement prostheses were Food & Drug Association (FDA) approved for general distribution : the Charité Artificial Disc and the Prodisc-L Disc.
Recently, the Activ L prosthesis has been developed for the purpose of preventing facet joint degeneration and offering the lowest profile of 8.5 mm. This prosthesis is a metal-on-plastic device, composed of three modular components : the inferior and the superior cobalt-chrome-molybdenum (CoCrMo) plates with either a large central keel or anterior spikes, and a UHMWPE inlay lying inside the inferior plate as Prodisc-L. This convex polyethylene core acts as a bearing surface and shock absorber. For Activ L arthroplasty, there can be a choice of end-plate combination according to the anatomical requirements between an end plate with spikes and an end plate with keel for anchorage to the vertebral bodies offering a tailored solution for multilevel implantation. The combination of Plasmapore® coating with a thin layer of dicalcium phosphate dehydrate stimulates and accelerates postoperative bone formation at the implant-bone interface.
The distinctive characteristic of Activ L is a sagittal translational movement of the COR from the center to posterior i.e. the COR in Activ L can move from the center to two mm dorsally depending on the shear forces. The physiologic COR is located posteriorly in the disc space6,19). Such a sagittal translational movement of the COR can unload the facet joints, increase the range of intersegmental movement, and provide for shear stability3). Whereas, the Prodisc-L has a fixed COR in the center and SB Charité has an unconstrained COR.
In this study, the principal biomechanical features of the human cadaveric spines implanted with the Activ L prosthesis was evaluated under a physiologic compressive follower preload. Theoretically, for reducing the incidence of adjacent segment disease and the maintenance of the biomechanical performance of the intact disc, the compensatory decrease of the adjacent segment motion, DP, and FS should be made by the use of an artificial disc. However, a statistically significant decrease of ROM was demonstrated at the operative segment during rotation, not in the adjacent segment, compared with the intact spine. Statistically-significant increase of ROM was found at the operative segment during flexion and bending and at the inferior segment during flexion. For central DP, the results were not consistent at the superior disc, but the central DP decreased in all the motion modes at the inferior disc, though a statistical significance was not observed. For FS, a statistically significant decrease was detected at the operative facets during flexion and at the inferior segment facets during rotation. The theoretical background to reduce the incidence of adjacent segment disease was noted, with own experiment, even though it is only partial, and further investigation using a larger series of specimens is needed in the future studies to make clear conclusion.
CONCLUSION
This study has several disadvantages like other biomechanical studies. These limitations include small sample size, in vitro experimentation, spine specimens without muscles, and no evaluation of wear and tear property. Despite these factors an analysis of our results indicates that the Activ L arthroplasty show less restoration of ROM at the operative and the adjacent levels as compared with intact spine. On the other side, our results revealed a few theoretical results to reduce the incidence of adjacent segment disease such as the decrease of central DP at the inferior disc without statistical significance, and the statistically significant decrease of FS at the operative facet during flexion and at the inferior facet during rotation. For the complete study of Activ L arthroplasty, further investigation using a larger series of specimens is needed in the future studies.
Footnotes
The Aesculap, Inc. provided instrument support. The authors received partial grant support from the Aesculap, Inc. for the study reported here.
References
- 1.Danielsson AJ, Cederlund CG, Ekholm S, Nachemson AL. The prevalence of disc aging and back pain after fusion extending into the lower lumbar spine. A matched MR study twenty-five years after surgery for adolescent idiopathic scoliosis. Acta Radiol. 2001;42:187–197. doi: 10.1080/028418501127346495. [DOI] [PubMed] [Google Scholar]
- 2.Dickman CA, Yahiro MA, Lu HT, Melkerson MN. Surgical treatment alternatives for fixation of unstable fractures of the thoracic and lumbar spine : a meta-analysis. Spine. 1994;19(20 Suppl):2266S–2273S. doi: 10.1097/00007632-199410151-00003. [DOI] [PubMed] [Google Scholar]
- 3.Dooris AP, Goel VK, Grosland NM, Gilbertson LG, Wilder DG. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine. 2001;26:E122–E129. doi: 10.1097/00007632-200103150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Fernstrom U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154–159. [PubMed] [Google Scholar]
- 5.Fritzell P, Hägg O, Wessberg P, Nordwall A Swedish Lumbar Spine Study Group. 2001 Volvo Award Winner in Clinical Studies : Lumbar fusion versus nonsurgical treatment for chronic low back pain a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521–2532. doi: 10.1097/00007632-200112010-00002. discussion 2532-2534. [DOI] [PubMed] [Google Scholar]
- 6.Gertzbein SD, Holtby R, Tile M, Kapasouri A, Chan KW, Cruickshank B. Determination of a locus of instantaneous centers of rotation of the lumbar disc by moire fringes. A new technique. Spine. 1984;9:409–413. doi: 10.1097/00007632-198405000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Coel VK, Weinstein JN, Patwardhan AG. Biomechanics of intact ligamentous spine. In: Coel VK, Weinstein JN, editors. Biomechanics of the Spine : Clinical and Surgical Perspectives. FL: CRC Press; 1990. pp. 97–156. [Google Scholar]
- 8.Gunzburg R, Mayer HM, Szpalski M, Aebi M. Arthroplasty of the spine : the long quest for mobility. Introduction. Eur Spine J. 2002;11 Suppl 2:S63–S64. doi: 10.1007/s00586-002-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hitchon PW, Eichholz K, Barry C, Rubenbauer P, Ingalhalikar A, Nakamura S, et al. Biomechanical studies of an artificial disc implant in the human cadaveric spine. J Neurosurg Spine. 2005;2:339–343. doi: 10.3171/spi.2005.2.3.0339. [DOI] [PubMed] [Google Scholar]
- 11.Kozak JA, O'Brien JP. Simultaneous combined anterior and posterior fusion. An independent analysis of a treatment for the disabled low-back pain patient. Spine. 1990;15:322–328. doi: 10.1097/00007632-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314–319. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J. 2001;10:309–313. doi: 10.1007/s005860000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Moore KR, Pinto MR, Butler LM. Degenerative disc disease treated with combined anterior and posterior arthrodesis and posterior instrumentation. Spine. 2002;27:1680–1686. doi: 10.1097/00007632-200208010-00018. [DOI] [PubMed] [Google Scholar]
- 16.Patwardhan AG, Havey RM, Meade KP, Lee B, Dunlap B. A follower load increases the load-carrying capacity of the lumbar spine in compression. Spine. 1999;24:1003–1009. doi: 10.1097/00007632-199905150-00014. [DOI] [PubMed] [Google Scholar]
- 17.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine. 1997;22:667. doi: 10.1097/00007632-199703150-00019. discussion 679-680. [DOI] [PubMed] [Google Scholar]
- 18.Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21:970–981. doi: 10.1097/00007632-199604150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Seligman JV, Gertzbein SD, Tile M, Kapasouri A. Computer analysis of spinal segment motion in degenerative disc disease with and without axial loading. Spine. 1984;9:566–573. doi: 10.1097/00007632-198409000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Szpalski M, Gunzburg R, Mayer M. Spine arthroplasty: a historical review. Eur Spine J. 2002;11 Suppl 2:S65–S84. doi: 10.1007/s00586-002-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tropiano P, Huang RC, Girardi FP, Cammisa FP, Jr, Marnay T. Lumbar total disc replacement. Seven to eleven-year follow-up. J Bone Joint Surg Am. 2005;87:490–496. doi: 10.2106/JBJS.C.01345. [DOI] [PubMed] [Google Scholar]
- 22.Whitecloud TSI, 3rd, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine. 1994;19:531–536. doi: 10.1097/00007632-199403000-00007. [DOI] [PubMed] [Google Scholar]