Abstract
To help develop better diagnostic tests for pertussis, we examined the serologic response to whole-cell proteins of Bordetella pertussis after natural infection or vaccination with diphtheria-tetanus-pertussis vaccine. Serum specimens collected during a pertussis outbreak investigation and from uninfected persons were used in Western blot (immunoblot) analyses to determine the presence of immunoglobulin G (IgG) and IgA antibodies to specific B. pertussis proteins. IgG antibodies to proteins of molecular masses 220 and 210 kilodaltons (kDa) were detected in 14 of 18 serum samples obtained from patients with culture-confirmed pertussis greater than or equal to 40 days after the onset of coughing. IgA antibodies were detected in 15 of the 18 samples. Of 19 serum samples obtained from patients who had not been ill with pertussis, 6 contained IgG antibodies to these proteins and 1 contained IgA antibodies. The two proteins bound antiserum specific for filamentous hemagglutinin and comigrated with purified filamentous hemagglutinin. IgG antibodies to two additional protein bands of molecular masses 84 and 75 kDa were associated with previous vaccination. Antibody to the 84-kDa protein was detected in 15 of 17 vaccinated, never-infected persons, and antibody to the 75-kDa protein was detected in 16 of the 17. None of 11 nonvaccinated, never-infected persons tested had antibodies to either protein. All seven fully vaccinated persons with culture-documented infection had antibodies to both proteins. Antibodies to the 84-kDa protein were detected in 6 of 22 nonvaccinated and infected persons, and antibodies to the 75-kDa protein were detected in 8 of the 22. Use of Western blot analysis in this study allowed us to distinguish antibody responses to infection and immunization.
Full text
PDF
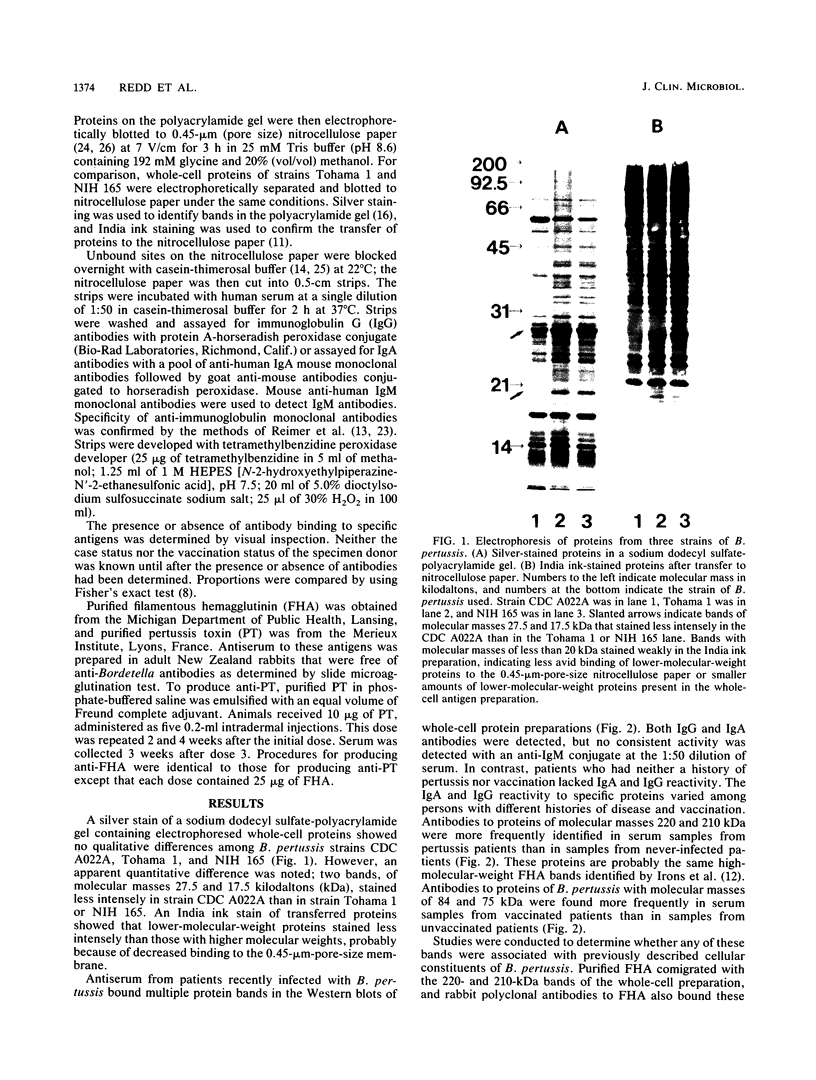
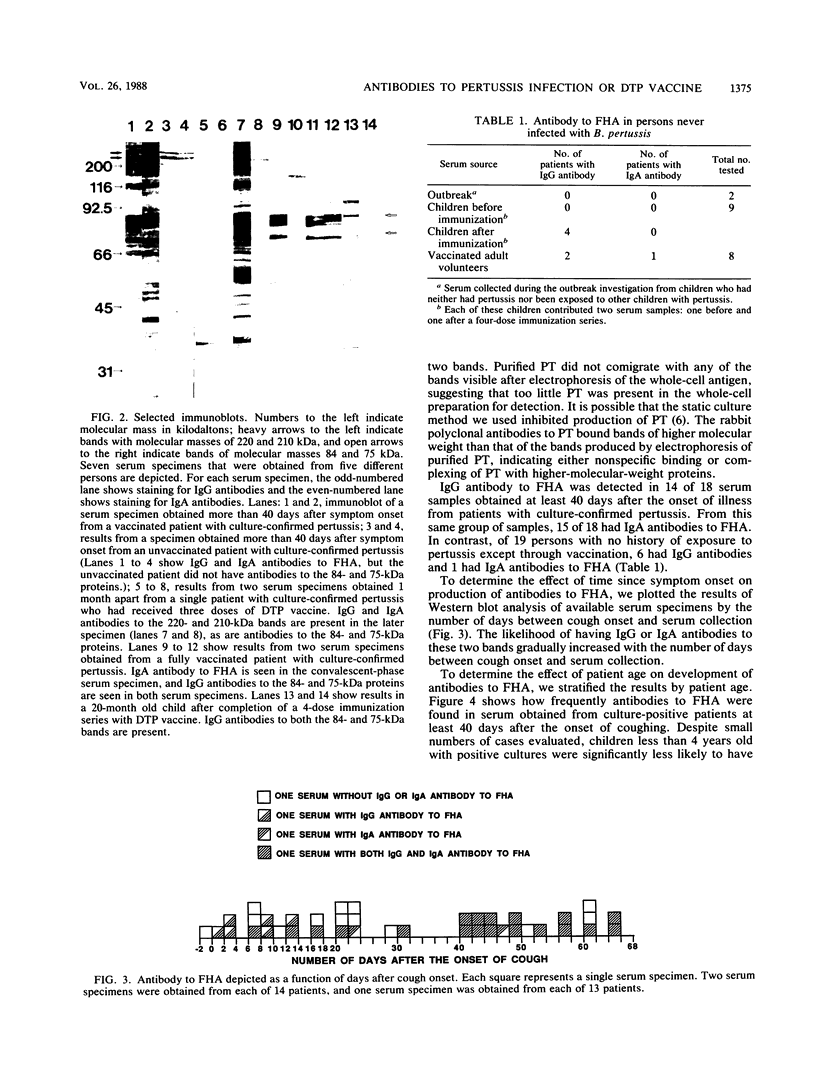


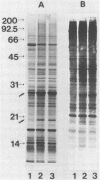
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstyn D. G., Baraff L. J., Peppler M. S., Leake R. D., St Geme J., Jr, Manclark C. R. Serological response to filamentous hemagglutinin and lymphocytosis-promoting toxin of Bordetella pertussis. Infect Immun. 1983 Sep;41(3):1150–1156. doi: 10.1128/iai.41.3.1150-1156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger H., Wirsing von Koenig C. H. Serological diagnosis of whooping cough. Dev Biol Stand. 1985;61:331–335. [PubMed] [Google Scholar]
- Goodman Y. E., Wort A. J., Jackson F. L. Enzyme-linked immunosorbent assay for detection of pertussis immunoglobulin A in nasopharyngeal secretions as an indicator of recent infection. J Clin Microbiol. 1981 Feb;13(2):286–292. doi: 10.1128/jcm.13.2.286-292.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granström M., Granström G., Lindfors A., Askelöf P. Serologic diagnosis of whooping cough by an enzyme-linked immunosorbent assay using fimbrial hemagglutinin as antigen. J Infect Dis. 1982 Dec;146(6):741–745. doi: 10.1093/infdis/146.6.741. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Irons L. I., Ashworth L. A., Wilton-Smith P. Heterogeneity of the filamentous haemagglutinin of Bordetella pertussis studied with monoclonal antibodies. J Gen Microbiol. 1983 Sep;129(9):2769–2778. doi: 10.1099/00221287-129-9-2769. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Reimer C. B., Skvaril F., de Lange G., Ling N. R., Lowe J., Walker M. R., Phillips D. J., Aloisio C. H., Wells T. W. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10(3-4):223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Kenna J. G., Major G. N., Williams R. S. Methods for reducing non-specific antibody binding in enzyme-linked immunosorbent assays. J Immunol Methods. 1985 Dec 27;85(2):409–419. doi: 10.1016/0022-1759(85)90150-4. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nagel J., Poot-Scholtens E. J. Serum IgA antibody to Bordetella pertussis as an indicator of infection. J Med Microbiol. 1983 Nov;16(4):417–426. doi: 10.1099/00222615-16-4-417. [DOI] [PubMed] [Google Scholar]
- Nagel J., de Graaf S., Schijf-Evers D. Improved serodiagnosis of whooping cough caused by Bordetella pertussis by determination of IgG anti-LPF antibody levels. Dev Biol Stand. 1985;61:325–330. [PubMed] [Google Scholar]
- Nkowane B. M., Wassilak S. G., McKee P. A., O'Mara D. J., Dellaportas G., Istre G. R., Orenstein W. A., Bart K. J. Pertussis epidemic in Oklahoma. Difficulties in preventing transmission. Am J Dis Child. 1986 May;140(5):433–437. doi: 10.1001/archpedi.1986.02140190043021. [DOI] [PubMed] [Google Scholar]
- Pittman M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev Infect Dis. 1979 May-Jun;1(3):401–412. doi: 10.1093/clinids/1.3.401. [DOI] [PubMed] [Google Scholar]
- Redhead K. Serum antibody responses to the outer membrane proteins of Bordetella pertussis. Infect Immun. 1984 Jun;44(3):724–729. doi: 10.1128/iai.44.3.724-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J., Lowe F. Enrichment medium for the isolation of Bordetella. J Clin Microbiol. 1977 Sep;6(3):303–309. doi: 10.1128/jcm.6.3.303-309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer C. B., Phillips D. J., Aloisio C. H., Moore D. D., Galland G. G., Wells T. W., Black C. M., McDougal J. S. Evaluation of thirty-one mouse monoclonal antibodies to human IgG epitopes. Hybridoma. 1984 Fall;3(3):263–275. doi: 10.1089/hyb.1984.3.263. [DOI] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinola S. M., Cannon J. G. Different blocking agents cause variation in the immunologic detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1985 Jul 16;81(1):161–165. doi: 10.1016/0022-1759(85)90132-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen M. K., Mertsola J., Kuronen T., Ruuskanen O. Class-specific antibody response to lymphocytosis promoting factor (LPF) and fimbriae (F) in pertussis. Dev Biol Stand. 1985;61:337–340. [PubMed] [Google Scholar]
- Zackrisson G., Lagergård T., Lönnroth I. An enzyme-linked-immunosorbent assay method for detection of immunoglobulins to pertussis toxin. Acta Pathol Microbiol Immunol Scand C. 1986 Dec;94(6):227–231. doi: 10.1111/j.1699-0463.1986.tb02116.x. [DOI] [PubMed] [Google Scholar]