Abstract
An antifungal protein was isolated from a culture of Bacillus subtilis strain B29. The isolation procedure comprised ion exchange chromatography on diethylaminoethyl (DEAE)-52 cellulose and gel filtration chromatography on Bio-Gel® P-100. The protein was absorbed on DEAE-cellulose and Bio-Gel® P-100. The purified antifungal fraction was designated as B29I, with a molecular mass of 42.3 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), pI value 5.69 by isoelectric focusing (IEF)-PAGE, and 97.81% purity by high performance liquid chromatography (HPLC). B29I exhibited inhibitory activity on mycelial growth in Fusarium oxysporum, Rhizoctonia solani, Fusarium moniliforme, and Sclerotinia sclerotiorum. The 50% inhibitory concentrations (IC50) of its antifungal activity toward Fusarium oxysporum and Rhizoctonia solani were 45 and 112 μmol/L, respectively. B29I also demonstrated an inhibitory effect on conidial spore germination of Fusarium oxysporum and suppression of germ-tube elongation, and induced distortion, tumescence, and rupture of a portion of the germinated spores.
Keywords: Bacillus subtilis, Antifungal protein, Purification
INTRODUCTION
Antifungal proteins and peptides have been isolated from a great number of animals (Iijima et al., 1993; Wang and Ng, 2002), plants (Benhamou et al., 1993; Joshi et al., 1998; Lam et al., 2000), bacteria (Delcambe et al., 1977; Kim and Chung, 2004; Moyne et al., 2004), and fungi (Lam and Ng, 2001b; Wang and Ng, 2004; Theis et al., 2005). Based on their structures or functions, antifungal proteins and peptides can be classified into many different classes, such as thaumatin-like proteins (Pressey, 1997; Wurms et al., 1999; Ye et al., 1999), glucanases (Vogelsang and Barz, 1993), chitinases and chitinase-like proteins (Benhamou et al., 1993; Lam et al., 2000; Lam and Ng, 2001c), peroxidases (Ye and Ng, 2002b), ribonucleases (Ng and Wang, 2001; Liu et al., 2007), protease inhibitors (Joshi et al., 1998), ribosome inactivating proteins (Leah et al., 1991; Lam and Ng, 2001a), lectins (Broekaert et al., 1989; Ye et al., 2001), cyclophilin-like proteins (Ye and Ng, 2000; 2002a), lipid transfer protein-like proteins (Molina et al., 1993; Wang et al., 2004), and miraculin-like proteins (Ye et al., 2000).
Recently, Bacillus subtilis strains have been applied to control plant diseases (Hwang and Chakravarty, 1992; Asaka and Shoda, 1996; Wulff et al., 2002; Okigbo, 2005), as they are ubiquitous in soils, have high thermal tolerance, grow quickly in liquid culture, and readily form resistant spores. Additionally, B. subtilis non-ribosomally synthesizes several kinds of small antibiotic peptides (<2000 Da) that have antifungal activities, such as iturin (Delcambe et al., 1977; Tsuge et al., 2001; Stein, 2005), surfactin (Peypoux et al., 1999; Carrillo et al., 2003), fengymycin, bacilysin (Loeffler et al., 1986), bacillomycin (Peypoux et al., 1980), mycosubtilin (Peypoux et al., 1986), and mycobacillin (Majumdar and Bose, 1960; Sengupta et al., 1971). B. subtilis also secretes an abundance of proteins (Moyne et al., 2004; Liu et al., 2007). However, little is known about the antifungal activity of these proteins. In our previous study, we have isolated an antagonistic strain B. subtilis B29, which strongly inhibits pathogens such as Fusarium, Pythium, and Rhizoctonia on potato dextrose agar (PDA) plates, and controls vegetable and melon wilt diseases in field (Li and Yang, 2005). We have also found that B29 secretes antifungal proteins and obtained the optimum fermentation conditions to secrete antifungal proteins (Li et al., 2008). This study reports the isolation, purification, and preliminary characterization of a novel antifungal protein secreted by B. subtilis B29.
MATERIALS AND METHODS
Chemicals
All chemicals were of analytical grade. Bovine serum albumin (BSA) was obtained from Sigma (USA), diethylaminoethyl (DEAE)-52 cellulose from Whatman (USA), and Bio-Gel® P-100 from Bio-Rad (USA). Low-molecular weight protein standards were purchased from TaKaRa Biotechnology (Dalin) Co., Ltd. (China).
Organism and growth conditions
The strain of Bacillus subtilis B29 was isolated in the Institute of Microbiology of Heilongjiang Academy of Sciences (IMHAS) from a sample of soil collected at the suburb of Harbin City (China), and identified as Bacillus subtilis on the basis of its morphological, biochemical and physiological characteristics, and 16S rDNA analysis (Li and Yang, 2005). The strain was deposited in the own microbiology strain collection in the IMHAS. B29 was cultured in 250-ml Erlenmeyer flasks containing 50 ml of NYD medium (10 g glucose, 8 g beef extract, and 5 g yeast extract) in a rotary shaker at 150 r/min at 30 °C for 5 d. Its pH was adjusted to 7.5 before autoclaving at 121 °C for 15 min. On completion of the culture, the bacteria were removed by centrifugation at 6000 r/min for 30 min at 4 °C, and the supernatant was used for crude proteins isolation.
Fusarium oxysporum f. cucumerinum was isolated from wilted cucumber roots and stored at 4 °C in PDA. It was incubated on Petri plates containing 15 ml PDA under sterile conditions at room temperature [(28±2) °C] for 5 d to prepare a conidial suspension for later use.
Isolation and purification of antifungal protein
The precipitate from cell-free culture broth supernatants by ammonium sulphate at 30% (w/v) saturation was collected by centrifugation at 6000 r/min for 30 min and discarded. The crude proteins were precipitated from the supernatant at 70% (w/v) (NH4)2SO4 saturation. The latter was added in small portions with constant stirring for 30 min. The stirring was continued for 1 h, and the mixture was kept overnight at 4 °C. The precipitate was collected by centrifugation at 6000 r/min for 30 min, dissolved in a 1/15 (v/v) phosphate buffer (0.02 mol/L, pH 6.8), and dialyzed for 24 h with 4 changes in the same buffer (500 ml each) to remove ammonium sulphate. The dialysates were condensed to yield crude proteins, which were further purified by column chromatography. A part of the crude proteins were dissolved in 5 ml of phosphate buffer (0.02 mol/L, pH 6.8), and the antifungal activity of the crude proteins was tested against Fusarium oxysporum.
Chromatography was carried out on a DEAE-52 cellulose ion exchange column (2.5 cm×50 cm; Whatman) previously equilibrated with 0.02 mol/L phosphate buffer (pH 6.8). The column was first eluted with 500 ml of the same buffer (pH 6.8) to yield unadsorbed proteins, and subsequently with a linear gradient of 0~1 mol/L NaCl in phosphate buffer (0.02 mol/L, pH 6.8) to desorb the adsorbed proteins (fractions I, II, III, IV). Each fraction was dialyzed in distilled water to remove NaCl, and then adjusted to the same concentration with phosphate buffer. Antifungal activity was tested against Fusarium oxysporum. Fraction I was subsequently chromatographed on Bio-Gel® P-100 column (1.6 cm×50 cm; Bio-Rad). The column was eluted with 0.02 mol/L phosphate buffer (pH 6.8) to collect one main protein peak (P1) and another small protein peak (P2). The antifungal activity of the two fractions was tested. All purification processes were carried out with a Nucleic acid-Protein Detective System (8823A-UV MONITOR, Beijing, China). P1 representing the purified antifungal protein was designated as B29I.
Electrophoresis, molecular mass, pI value and protein concentration determination
The molecular mass of the purified antifungal protein from B. subtilis strain B29 was conducted by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), according to the procedure of Laemmli and Favre (1973) using a 12% (w/v) gel and 5% (w/v) stacking gel. The gel was stained with Coomassie brilliant blue after electrophoresis. The molecular mass of B29I was determined by comparison of its electrophoretic mobility with those of molecular mass marker proteins (TaKaRa Biotechnology (Dalin) Co., Ltd., China). The pI value of the purified antifungal protein was determined by isoelectric focusing (IEF)-PAGE. The protein concentration was determined using the method of Bradford (1976), with bovine serum albumin as a standard.
High performance liquid chromatography (HPLC) and mass spectrum analysis
The purity of the purified antifungal protein from B. subtilis strain B29 was analyzed by the C18 reversed phase column HPLC system. The HPLC was conducted on an Agilent zorbax 300SB-C18 column (4.6 mm×150 mm; 3.5 µm; 30 nm): the mobile phases A 0.1% (v/v) trifluoroacetic acid (TFA)-water solution and B 0.1% (v/v) TFA-acetonitrile solution, with gradient elution. The detection wavelength was 280 nm and column temperature 25 °C.
The antifungal protein B29I separated by SDS-PAGE was analyzed after in-gel digestion by National Center of Biomedical Analysis (NCBA), Beijing, China. The peptide-mass fingerprinting (PMF) was obtained by matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry and searched in Mascot.
N-terminal sequence analysis
The N-terminal amino acid sequence of the antifungal protein B29I was determined by means of automated Edman degradation using an ABI Procise™ 492cLC protein sequencer equipped with an HPLC system.
Assay of antifungal activity
The assay of the purified protein B29I for antifungal activity toward Fusarium oxysporum f. cucumerinum and Rhizoctonia solani was carried out on 100 mm×15 mm Petri plates each containing 10 ml of PDA. After the mycelial colony had developed, sterile blank paper disks (0.65 cm in diameter) were placed at a distance of 0.5 cm away from the rim of the mycelial colony. An aliquot (10 μl) containing either 9 or 36 μg of the purified protein B29I was added to each disk. The plates were sealed with parafilm, and incubated at 28 °C until mycelial growth had enveloped disks containing the control and had formed crescents of inhibition around disks containing samples with antifungal activity (Lam et al., 2000).
To determine the 50% inhibitory concentrations (IC50) for the antifungal activity, three doses of the antifungal protein B29I were added separately to three aliquots each containing 4 ml PDA at 45 °C, mixed rapidly, and poured into three separate small Petri dishes. After the agar had cooled down, a small amount of mycelia, the same amount to each plate, was added. Buffer without B29I served only as control, and all the plates were cultured at 28 °C. When the mycelia colony of the control had grown to almost fill the plate, the area of the mycelia colony was measured, and the inhibition of fungal growth in the other plates was determined by calculating the percent reduction in the area of the mycelia colony (Wang and Ng, 2004).
Bioassay of the antifungal protein B29I on Fusarium oxysporum conidial spore germination
The inhibitory effect of the purified antifungal protein B29I on conidial spore germination of Fusarium oxysporum f. cucumerinum was examined. 80 μl of 0.2% (w/v) sterile glucose, 80 μl of purified antifungal protein B29I (0.9 μg/μl) and 40 μl of Fusarium oxysporum conidial suspension (1×104 spore/ml) were mixed completely in an Eppendorf tube. Sterile phosphate buffer (0.02 mol/L, pH 6.8) was used as a control. 100 μl of the mixture was added into a hollow-ground slide with a cover, and then incubated in the dark at room temperature [(28±2) °C] in humidified Petri dishes with three replicates. The sample was observed under a microscope at regular intervals, and the percentage spore germination was determined.
RESULTS
Isolation and purification of antifungal protein
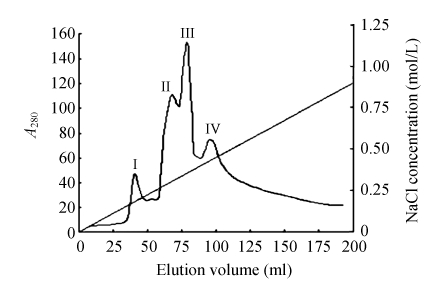
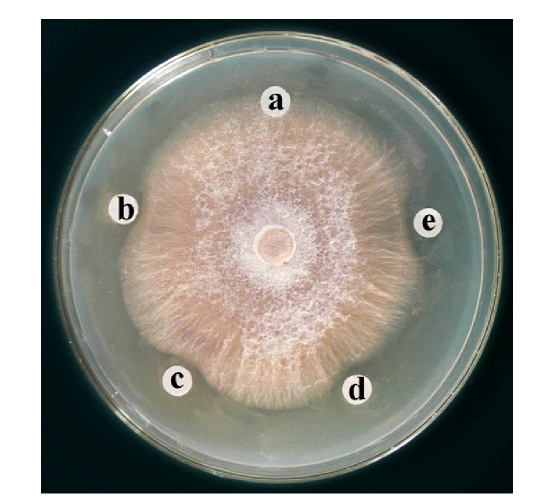
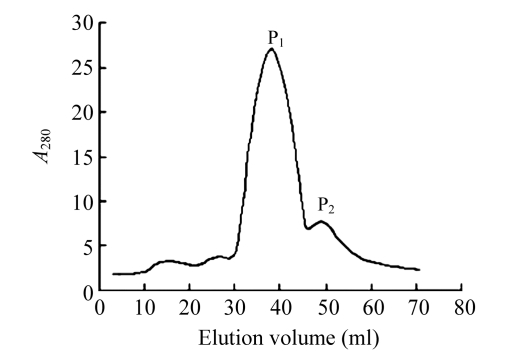
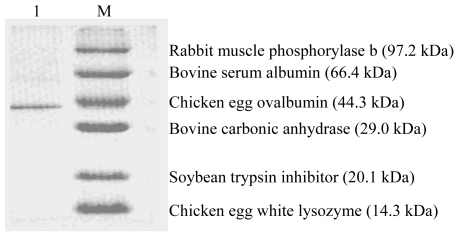
Ion exchange chromatography of B. subtilis strain B29 culture extract on a DEAE-52 column contained one unadsorbed fraction and four adsorbed fractions (I, II, III, and IV) (Fig.1). Of these fractions, only fraction I showed strong antifungal activity (Fig.2), and fraction II showed a less antifungal activity. Fraction I was resolved on the Bio-Gel® P-100 column into two fractions (P1 and P2) (Fig.3). Only fraction P1 exhibited antifungal activity (Fig.2) and showed a single band with a molecular mass of 42.3 kDa in SDS-PAGE (Fig.4). This single-band antifungal protein was designated as B29I. Its purity was 97.81% as detected by HPLC and its pI value was 5.69 as determined by IEF-PAGE. The protein yields at various stages of chromatographic purification are shown in Table 1.
Fig. 1.
Ion exchange chromatography on a DEAE-52 column (2.5 cm×50 cm)
Sample: crude proteins from the supernatant in Na2HPO4/NaH2PO4 buffer (20 mmol/L, pH 6.8). Slanting line across the chromatography indicates linear NaCl concentration gradient (0~1 mol/L) employed to elute the absorbed fractions I, II, III, and IV
Fig. 2.
Antifungal activity of fractions from DEAE-52 and Bio-Gel® P-100 chromatographies toward Fusarium oxysporum
A: control (10 μl 20 mmol/L Na2HPO4/NaH2PO4 buffer (PB buffer), pH 6.8); B, C: fraction I from DEAE-52 column chromatography; D: 9 μg antifungal protein B29I (fraction P1) in 10 μl PB buffer; E: 36 μg antifungal protein B29I (fraction P1) in 10 μl PB buffer
Fig. 3.
Gel chromatography on a Bio-Gel® P-100 column (1.6 cm×50 cm)
Sample: fraction I from DEAE-52 column chromatography. Buffer: 20 mmol/L Na2HPO4/NaH2PO4 (pH 6.8). Flow rate: 0.4 ml/min
Fig. 4.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of fraction P1 from Bio-Gel® P-100 column chromatography
Lane 1: purified antifungal protein B29I; Lane M: low molecular mass markers from TaKaRa Biotechnology (China)
Table 1.
Protein yields of chromatographic fractions with antifungal activity obtained at different stages of purification
| Fraction | Total protein (mg) | Recovery of protein (%) |
| Crude extract | 224.1 | 100.0 |
| DEAE-52 fraction I | 17.0 | 7.6 |
| Bio-Gel® P-100 fraction P1 | 14.3 | 84.1 |
Amino acid sequence analysis
The PMF of antifungal protein B29I was obtained by MALDI-TOF mass spectrometry, and was an unreported protein as searched in the Mascot database. Subsequently, the digestion products of B29I were detected by ESI-QUADRUPOLE-OA-TOF (Q-TOF2), and three random double electric charge segments were chosen to be detected by collision-induced dissociation (CID). The amino acid sequences of three peptide segments were KTHVLEDEFK, KGYQTGDFGAYLH, and RTYEVAEESPVLGL respectively.
The N-terminal amino acid sequence of the antifungal protein B29I was different from those of other antifungal proteins from Bacillus subtilis (Table 2). However, a random amino acid sequence (RTY EVAEESPVLGL) from B29I showed slight resemblance to a small portion of those antifungal proteins from Bacillus subtilis (Table 2).
Table 2.
Comparison of N-terminal and a random sequence of B29I with other antifungal proteins
| Antifungal protein | Sequence* | Origin |
| B29I | GRIWHN…RTYEVAEESPVLGL | Bacillus subtilis |
| Bacisubin | AQPGIQQI | B. subtilisa |
| YxjF (fragment) | RIGFHGKAAYNSAKHG…VIGLT | B. subtilisb |
| Endo-1,4-β-glucanase | MMRRRK…VIYEIANE…PD…VG | B. subtilisb |
| YnfF | MMSSVK…ISDPILNDP…LGTHLY | B. subtilisb |
| BamD | MNNLA…RPY…EVIEMGP…AGLL | B. subtilisb |
| Putative sensor kinase | MNWRLT…YSYFRGFKNPSLGK…L | B. subtilisb |
| Bacillomycin D synthetase C | MSEFKQ…TYQVVNE…SP…VIGIL | B. subtilisb |
… is space introduced to maximize sequence similarity and identical amino acid residues were underlined;
Data taken from (Liu et al., 2007);
Data searched from National Center for Biotechnology Information (NCBI)
Antifungal activity of B29I
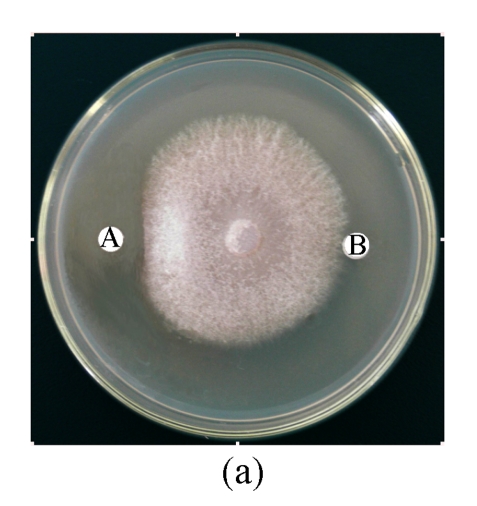
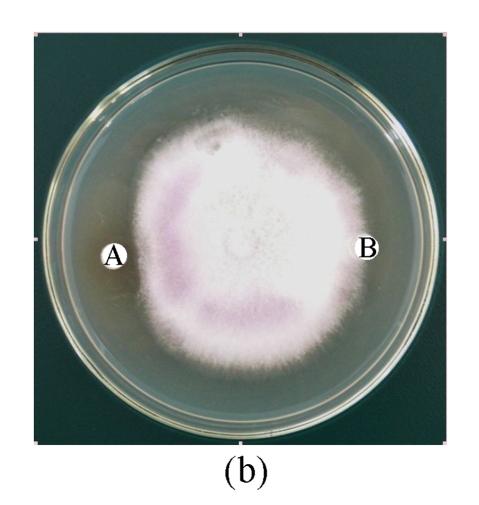
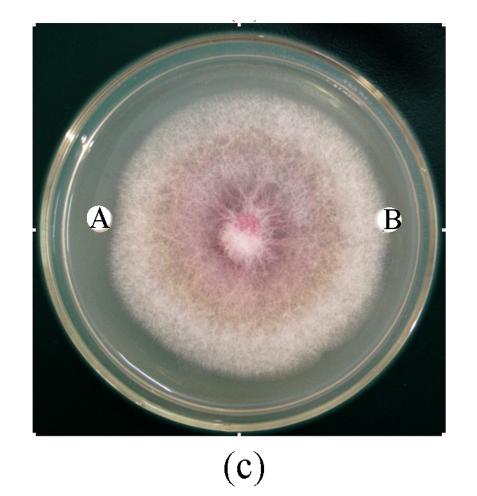
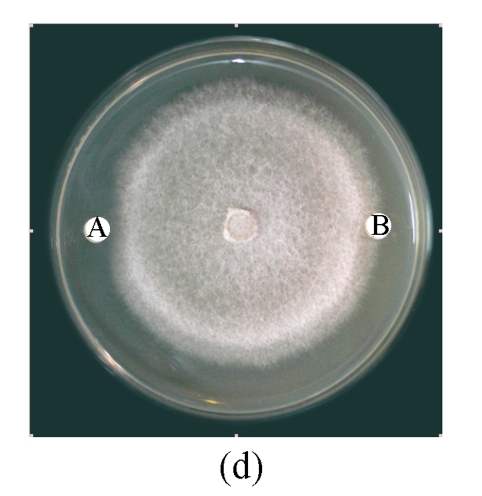
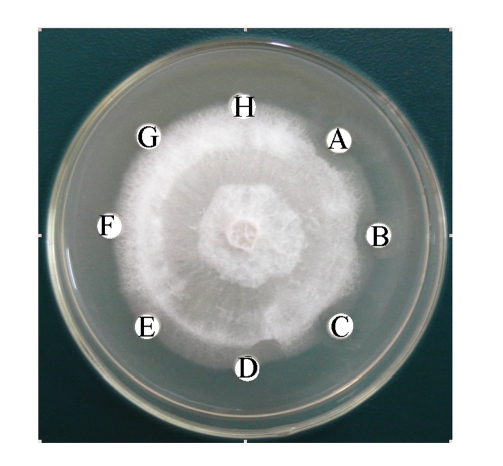
Antifungal activity of B29I toward Fusarium oxysporum, Rhizoctonia solani, Fusarium moniliforme, and Sclerotinia sclerotiorum was observed (Fig.5). Of the various fungal species, Fusarium oxysporum and Rhizoctonia solani showed the highest sensitivity to the antifungal protein B29I. The IC50 values of the inhibitory activity of the antifungal protein B29I on hyphal growth in Fusarium oxysporum and Rhizoctonia solani were 45 and 112 μmol/L, respectively. Antifungal activity was discernible at various temperatures up to 80 °C, but disappeared at 100 °C (Fig.6).
Fig. 5.
Antifungal activity of antifungal protein B29I toward Fusarium oxysporum (a), Rhizoctonia solani (b), Fusarium moniliforme (c), and Sclerotinia sclerotiorum (d)
A: 10 μl of 85.2 μmol/L (36 μg) B29I; B: 10 μl of 20 mmol/L Na2HPO4/NaH2PO4 buffer (pH 6.8)
Fig. 6.
Thermal stability test of antifungal protein B29I (36 μg) on Fusarium oxysporum
A: 0 °C; B: 20 °C; C: 40 °C; D: 60 °C; E: 80 °C; F: 100 °C; G: 120 °C; H: 10 μl of 20 mmol/L Na2HPO4/NaH2PO4 buffer (pH 6.8). Disks (A~G): 36 μg B29I exposed to various stipulated temperatures for 10 min prior to assay for antifungal activity
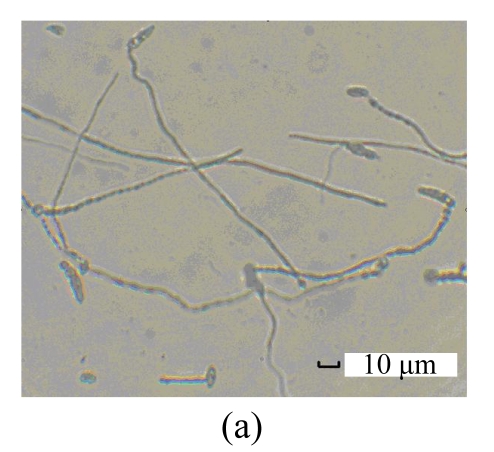
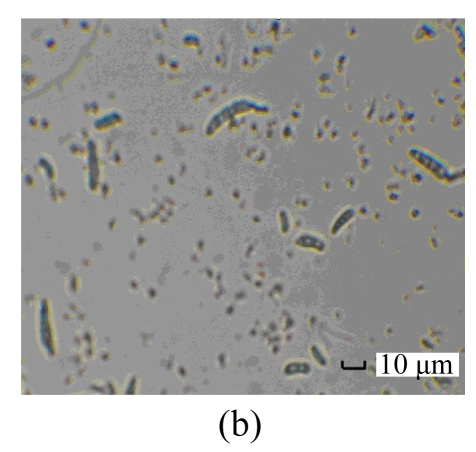
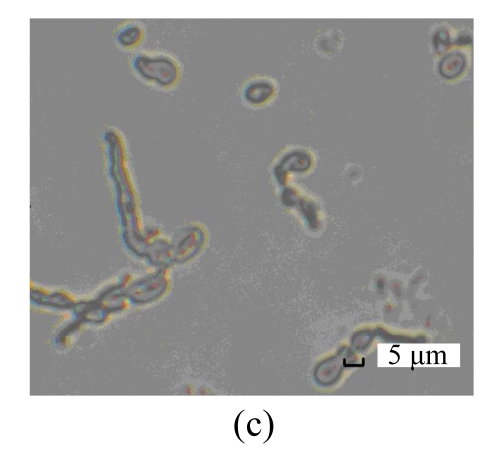
The purified antifungal protein B29I was strongly inhibitory to conidial spore germination of F. oxysporum, and suppressive to germ-tube elongation (Table 3). Percentage spore germination of the control increased with time from 18% at 8 h to 97.5% at 16 h. At that time, mycelia were growing after germ-tube emergence, growth and elongation. However, no spore germination was observed after treatment with antifungal protein B29I (72 μg). When incubation time was increased to 24 h, 22.7% spore germination and germ-tube emergence were detected after treatment with B29I. In addition, a portion of germinated spores of F. oxysporum appeared distorted, tumescent, and ruptured (Fig.7), and the germ-tube was unable to elongate anymore, while percentage spore germination was 100%, and mycelial extension also occurred in the control. At 36 h and 72 h, 60% of spores incubated with antifungal protein B29I germinated, but less mycelial growth was observed compared with the control. The purified antifungal protein B29I showed an inhibitory effect on conidial spore germination of F. oxysporum, germ-tube elongation, and mycelial development.
Table 3.
The inhibitory effect of the antifungal protein B29I on Fusarium oxysporum spore germination
| Incubation time (h) | Control |
B29I |
||
| Spore germination (%) | Morphology | Spore germination (%) | Morphology | |
| 8 | 18.0 | Germ-tube emergence | 0 | − |
| 10 | 27.0 | Germ-tube growth | 0 | − |
| 12 | 53.0 | Germ-tube elongation | 0 | − |
| 16 | 97.5 | Mycelial growth | 0 | − |
| 24 | 100.0 | Mycelial extension | 22.7 | Germ-tube emergence |
| 36 | 100.0 | Compact mycelia | 60.0 | Germ-tube elongation |
| 72 | 100.0 | Compact mycelia | 60.0 | Limited mycelial growth |
Fig. 7.
Inhibitory effect of B29I on Fusarium oxysporum spore germination as seen under the light microscope
(a) Normal spore germination of F. oxysporum; (b) Ungerminated spores of F. oxysporum after treatment with B29I (72 μg) for 24 h; (c) Abnormal germinated spores of F. oxysporum appeared distorted, tumescent, and ruptured after treatment with B29I (72 μg) for 24 h
DISCUSSION
This paper reports a novel antifungal protein, designated as B29I, isolated from Bacillus subtilis strain B29. The molecular mass of the antifungal protein B29I was different from those of other reported antifungal proteins from B. subtilis, such as bacisubin (41.9 kDa) (Liu et al., 2007), YxjF (fragment) (12.23 kDa), endo-1-4-β-glucanase (46.60 kDa), YnfF (45.39 kDa), BamD (44.94 kDa), putative sensor kinase (53.38 kDa), bacillomycin D synthetase C (309.04 kDa) (NCBI search), and X98III (59.0 kDa ) (Xie et al., 1998). The N-terminal amino acid sequence of the antifungal protein B29I was distinctly different from those of the abovementioned antifungal proteins. However, a random amino acid sequence (RTYEVAEESPVLGL) from B29I was slightly similar to a small portion of the sequences in BamD, bacillomycin D synthetase C, endo-1-4-β-glucanase, YxjF (fragment), putative sensor kinase, and YnfF (Table 2), and those antifungal proteins from Bacillus subtilis demonstrated antifungal activity toward Aspergillus flavus (Moyne et al., 2004).
The antifungal spectra of those reported antifungal proteins from B. subtilis were different. Bacisubin secreted from B. subtilis strain B-916 strongly inhibited mycelial growth in some species of Alternaria and Botrytis, while the antifungal protein B29I inhibited mycelial growth in Fusarium oxysporum, Rhizoctonia solani, Fusarium moniliforme, and Sclerotinia sclerotiorum. The IC50 values of antifungal activity of B29I toward F. oxysporum and R. solani were as low as 45 and 112 μmol/L, respectively. The antifungal protein B29I was also strongly inhibitory to conidial spore germination of F. oxysporum, suppressive to germ-tube elongation, and induced distortion, tumescence, and rupture of a portion of germinated spores of F. oxysporum. The fairly high thermostability of antifungal protein B29I was noteworthy.
The antifungal protein B29I was absorbed on DEAE-cellulose, unlike most of the antifungal proteins and peptides reported earlier (Wang and Ng, 2002; 2004; Ngai et al., 2005). Its chromatographic behaviors on DEAE-cellulose and Bio-Gel® P-100 were distinct from those of other antifungal proteins (Lam et al., 2000; Ng and Wang, 2001; Liu et al., 2007).
Bacillus subtilis can produce more than two dozens of antibiotics with an amazing variety of structures. Most of the anti-microbial active compounds produced include peptides that are either ribosomally synthesized and post-translationally modified (lantibiotics and lantibiotic-like peptides), or are non-ribosomally generated. Some non-peptidic compounds such as polyketides, an aminosugar, and a phospholipid are also produced (Stein, 2005). The lipoheptapeptide (surfactin) is the most powerful biosurfactant known; it exerts a detergent-like action on biological membranes (Carrillo et al., 2003), and is distinguished by its exceptional emulsifying, foaming, anti-viral and anti-mycoplasma activities. The iturines and the bacillomycins possess strong anti-fungal and haemolytic properties, but exhibit only limited anti-bacterial activity. The properties, activities, and genes for biosynthesis of lipopeptide antibiotics have been intensively investigated (Stein, 2005; Tsuge et al., 2001). The differences of antifungal protein B29I in biochemical and biological characteristics from known antifungal molecules produced by Bacillus subtilis indicate that the antifungal protein B29I is a novel antifungal protein. Additionally, the report of antifungal protein B29I constitutes an addition to the scanty literature on Bacillus subtilis antifungal proteins.
Acknowledgments
The authors thank Dr. Sheau-fang Hwang (Crop Diversification Centre North, Canada), Dr. Ying Ma (Food Science and Engineering, Harbin Institute of Technology, China), and Dr. Shu-xia Bai (Chapel Hill School of Medicine, University of North Carolina, USA) for their critical review of the manuscript, National Center of Biomedical Analysis (Beijing, China) for mass spectrum analysis and Shanghai GeneCore BioTechnologies Co. Ltd. (Shanghai, China) for N-terminal amino acid sequence analysis of the purified protein.
Footnotes
Project supported by the Hi-Tech Research and Development Program (863) of China (No. 2003AA241140) and the Natural Science Foundation of Heilongjiang Province, China (No. C200522)
References
- 1.Asaka O, Shoda M. Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol. 1996;62(11):4081–4085. doi: 10.1128/aem.62.11.4081-4085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benhamou N, Broglie K, Broglie R, Chet I. Antifungal effect of bean endochitinase on Rhizoctonia solani: ultrastructural changes and cytochemical aspect of chitin breakdown. Can J Microbiol. 1993;39(3):318–328. doi: 10.1139/m93-045. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantification of milligram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Broekaert WF, van Parijs J, Leyns F, Joos H, Peumans WJ. A chitin-binding lectin from stinging nettle rhizomes with antifungal properties. Science. 1989;245(4922):1100–1102. doi: 10.1126/science.245.4922.1100. [DOI] [PubMed] [Google Scholar]
- 5.Carrillo C, Teruel JA, Aranda FJ, Ortiz A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta. 2003;1611(1-2):91–97. doi: 10.1016/S0005-2736(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 6.Delcambe L, Peypoux F, Besson F, Guinand M, Michel G. Structure of iturin and iturin-like substance. Biochem Society Transac. 1977;5(4):1122–1124. doi: 10.1042/bst0051122. [DOI] [PubMed] [Google Scholar]
- 7.Hwang SF, Chakravarty P. Potential for the integrated control of Rhizoctonia root-rot of Pisum sativum using Bacillus subtilis and a fungicide. Z PflKrankh PflSchutz. 1992;99:626–636. [Google Scholar]
- 8.Iijima R, Kurata S, Natori S. Purification, characterization and cDNA cloning of an antifungal protein from the hemolymph of Sarcophaga peregrine (flesh fly) J Biol Chem. 1993;268(16):12055–12062. [PubMed] [Google Scholar]
- 9.Joshi BN, Sainani MN, Bastawade KB, Gupta VS, Ranjekar PK. Cysteine protease inhibitor from pearl millet: a new class of antifungal protein. Biochem Biophys Res Commun. 1998;246(2):382–387. doi: 10.1006/bbrc.1998.8625. [DOI] [PubMed] [Google Scholar]
- 10.Kim PI, Chung KC. Production of an antifungal protein for control of Colletotrichum lagenarium by Bacillus amyloliquefaciens MET0908. FEMS Microbiol Lett. 2004;234(1):177–183. doi: 10.1016/j.femsle.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Laemmli UK, Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 12.Lam SK, Ng TB. First simultaneous isolation of a ribosome inactivating protein and an antifungal protein from a mushroom (Lyophyllum shimeji) together with evidence for synergism of their antifungal effects. Arch Biochem Biophys. 2001;393(2):271–280. doi: 10.1006/abbi.2001.2506. [DOI] [PubMed] [Google Scholar]
- 13.Lam SK, Ng TB. Isolation of a novel thermolabile heterodimeric ribonuclease with antifungal and antiproliferative activities from roots of the sanchi ginseng Panax notoginseng . Biochem Biophys Res Commun. 2001;285(2):419–423. doi: 10.1006/bbrc.2001.5193. [DOI] [PubMed] [Google Scholar]
- 14.Lam SK, Ng TB. Isolation of a small chitinase-like antifungal protein from Panax notoginseng (sanchi ginseng) roots. Int J Biochem Cell Biol. 2001;33(3):287–292. doi: 10.1016/S1357-2725(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 15.Lam YW, Wang HX, Ng TB. A robust cysteine-deficient chitinase-like antifungal protein from inner shoots of the edible chive Allium tuberosum . Biochem Biophys Res Commun. 2000;279(1):74–80. doi: 10.1006/bbrc.2000.3821. [DOI] [PubMed] [Google Scholar]
- 16.Leah R, Tommerup H, Svendsen I, Mundy J. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem. 1991;266(3):1564–1573. [PubMed] [Google Scholar]
- 17.Li J, Yang Q. Biological Control of Fusarisum Wilt with an Antagonistic Strain of Bacillus subtilis . In: Yang Q, Yu ZN, editors. Study on Plant Pest and Diseases Biological Control and Bio-technology. The 3rd International Symposium on Bio-Control and Biotechnology. Harbin, China: Heilongjiang Science Technology Press; 2005. pp. 229–236. [Google Scholar]
- 18.Li J, Yang Q, Zhao L, Wang Y. Antifungal substance from biocontrol Bacillus subtilis B29 strain. China Biotechnology. 2008;28(2):59–65. (in Chinese) [Google Scholar]
- 19.Liu Y, Chen Z, Ng TB, Zhang J, Zhou M, Song F, Lu F, Liu Y. Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides. 2007;28(3):553–559. doi: 10.1016/j.peptides.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Loeffler W, Tschen JSM, Vanittanakom N, Kulger M, Knorpp E, Hsieh TF, Wu TG. Antifungal effects of bacilysin and fengycin from Bacillus subtilis F29-3. A comparison with activities of other Bacillus antibiotics. J Phytopathol. 1986;115(3):204–213. doi: 10.1111/j.1439-0434.1986.tb00878.x. [DOI] [Google Scholar]
- 21.Majumdar SK, Bose SK. Isolation and homogeneity of mycobacillin. Arch Biochem Biophy. 1960;90(1):154–158. doi: 10.1016/0003-9861(60)90626-3. [DOI] [PubMed] [Google Scholar]
- 22.Molina A, Segura A, Garia-Olmedo F. Lipid transfer proteins (nsLTPS) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett. 1993;316(2):119–122. doi: 10.1016/0014-5793(93)81198-9. [DOI] [PubMed] [Google Scholar]
- 23.Moyne AL, Cleveland TE, Tuzun S. Molecular characterization and analysis of operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol Lett. 2004;234(1):43–49. doi: 10.1111/j.1574-6968.2004.tb09511.x. [DOI] [PubMed] [Google Scholar]
- 24.Ng TB, Wang HX. Panaxagin, a new protein from Chinese ginseng possesses anti-fungal, anti-viral, translation-inhibiting and ribonuclease activities. Life Sci. 2001;68(7):739–749. doi: 10.1016/S0024-3205(00)00970-X. [DOI] [PubMed] [Google Scholar]
- 25.Ngai PHK, Zhao Z, Ng TB. Agrocybin, an antifungal peptide from the edible mushroom Agrocybe cylindracea . Peptides. 2005;26(2):191–196. doi: 10.1016/j.peptides.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Okigbo RN. Biological control of postharvest fungal rot of yam (Dioscorea spp.) with Bacillus subtilis . Mycopathologia. 2005;159(2):307–314. doi: 10.1007/s11046-004-2454-8. [DOI] [PubMed] [Google Scholar]
- 27.Peypoux F, Besson F, Michel G. Characterization of a new antibiotic of iturin group bacilloycin D. J Antibiot. 1980;33(10):1146–1149. doi: 10.7164/antibiotics.33.1146. [DOI] [PubMed] [Google Scholar]
- 28.Peypoux F, Pommier MT, Marion D, Ptak M, Das BC, Michel G. Revised structure of mycosubtilin, a lipidolipid antibiotic from B. subtilis . J Antibiot. 1986;39(5):636–641. doi: 10.7164/antibiotics.39.636. [DOI] [PubMed] [Google Scholar]
- 29.Peypoux F, Bonmatin JM, Wallach J. Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol. 1999;51(5):553–563. doi: 10.1007/s002530051432. [DOI] [PubMed] [Google Scholar]
- 30.Pressey R. Two isoforms of NP24: a thaumatin-like protein in tomato fruit. Phytochemistry. 1997;44(7):1241–1245. doi: 10.1016/S0031-9422(96)00667-X. [DOI] [PubMed] [Google Scholar]
- 31.Sengupta S, Banerjee AB, Bose SK. γ-Glutamyl and D- or L-peptide linkages in mycobacillin, a cyclic peptide antibiotic. Biochem J. 1971;121:839–846. doi: 10.1042/bj1210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56(4):845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 33.Theis T, Marax F, Salvenmoser W, Stahl U, Meyer V. New insight into the target site and mode of action of the antifungal protein of Aspergillus giganteus . Res. 2005;156(1):47–56. doi: 10.1016/j.resmic.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Tsuge K, Akiyama T, Shoda M. Cloning, sequencing, and characterization of the iturin A operon. J Bacteriol. 2001;183(21):6265–6273. doi: 10.1128/JB.183.21.6265-6273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelsang R, Barz W. Purification, characterization and differential hormonal regulation of a β-1,3-glucanase and two chitinases from chickpea (Cicer arietinum L.) Planta. 1993;189(1):60–69. doi: 10.1007/BF00201344. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Ng TB. Isolation of cicadin, a novel and potent antifungal peptide from juvenile cicadas. Peptides. 2002;23(1):7–11. doi: 10.1016/S0196-9781(01)00573-3. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Ng TB. Eryngin, a novel antifungal peptide from fruiting bodies of the edible mushroom Pleurotus eryngii . Peptides. 2004;25(1):1–5. doi: 10.1016/j.peptides.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Wang SY, Wu JH, Ng TB, Ye XY, Rao PF. A non-specific lipid transfer protein with antifungal and antibacterial activities from the mung bean. Peptides. 2004;25(8):1235–1242. doi: 10.1016/j.peptides.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Wulff EG, Mguni CM, Mortensen CN, Keswani CL, Hockenhull J. Biological control of black rot (Xanthomonas campestris pv. campestris) of brassicas with an antagonistic strain of Bacillus subtilis in Zimbabwe. Eur J Plant Pathol. 2002;108(4):317–325. doi: 10.1023/A:1015671031906. [DOI] [Google Scholar]
- 40.Wurms K, Greenwood D, Sharrock K, Long P. Thaumatinlike protein in kiwi fruit. J Sci Food Agric. 1999;79(11):1448–1452. doi: 10.1002/(SICI)1097-0010(199908)79:11<1448::AID-JSFA381>3.0.CO;2-3. [DOI] [Google Scholar]
- 41.Xie D, Peng J, Wang J, Hu J, Wang Y. Purification and properties of antifungal protein X98III from Bacillus subtilis . Acta Microbiologica Sinica. 1998;38(1):13–19. (in Chinese) [PubMed] [Google Scholar]
- 42.Ye XY, Ng TB. Mungin, a novel cyclophilin-like antifungal protein from the mung bean. Biochem Biophys Res Commun. 2000;273(3):1111–1115. doi: 10.1006/bbrc.2000.3067. [DOI] [PubMed] [Google Scholar]
- 43.Ye XY, Ng TB. Isolation of a new cyclophilin-like protein from chickpeas with mitogenic, antifungal and anti-HIV-1 reverse transcriptase activities. Life Sci. 2002;70(10):1129–1138. doi: 10.1016/S0024-3205(01)01473-4. [DOI] [PubMed] [Google Scholar]
- 44.Ye XY, Ng TB. Isolation of a novel peroxidase from French bean legumes and first demonstration of antifungal activity of a non-milk peroxidase. Life Sci. 2002;71(14):1667–1680. doi: 10.1016/S0024-3205(02)01925-2. [DOI] [PubMed] [Google Scholar]
- 45.Ye XY, Wang HX, Ng TB. First chromatographic isolation of an antifungal thaumatin-like protein from French bean legumes and demonstration of its antifungal activity. Biochem Biophys Res Comun. 1999;263(1):130–134. doi: 10.1006/bbrc.1999.1166. [DOI] [PubMed] [Google Scholar]
- 46.Ye XY, Wang HX, Ng TB. Sativin, a novel antifungal miraculin-like protein isolated from legumes of the sugar snap Pisum sativum var. macrocarpon Life Sci. 2000;67(7):775–781. doi: 10.1016/S0024-3205(00)00672-X. [DOI] [PubMed] [Google Scholar]
- 47.Ye XY, Ng TB, Tsang PWK, Wang J. Isolation of a homodimeric lectin with antifungal and antiviral activiral activities from red kidney bean (Phaseolus vulgaris) activities. J Protein Chem. 2001;20(5):367–375. doi: 10.1023/A:1012276619686. [DOI] [PubMed] [Google Scholar]