Abstract
Amiodarone hydrochloride is a potent anti-arrhythmic agent, known as a multiple ion-channel blocker in the heart. Although it has been detected in the rat brain, there are no data related to its central nervous system (CNS) effects. In this study, we evaluated anticonvulsant and hypnotic effects of amiodarone. Convulsions were induced by phentylenetetrazole (PTZ) (100 mg/kg) or caffeine (300 mg/kg) in mice. In both models, amiodarone prolonged both latency period and time to death, and acted as an anticonvulsant drug. It was found to be more effective in the PTZ model than in the caffeine model; none of the animals treated with 150 mg/kg dose amiodarone had died in the PTZ model. For hypnotic effect, sleeping was induced with pentobarbital (35 mg/kg) in rats. Amiodarone dose-dependently increased the sleeping time (677.7%~725.9%). In the sleeping test, all rats in 200 mg/kg amiodarone group died. In conclusion, anticonvulsant and hypnotic effects of amiodarone have shown the depressant effects on CNS. These effects may be dependent on its pharmacological properties.
Keywords: Amiodarone, Phentylenetetrazole (PTZ), Caffeine, Convulsion, Sleeping
INTRODUCTION
Amiodarone is a multiple ion-channel blocker drug, inhibiting sodium and calcium inward currents and potassium outward current, and having non-competitive adrenergic blocking effect (Kodama et al., 1999; Herbette et al., 1988; Roden, 2006). It is the most promising drug in the treatment of life-threatening ventricular tachyarrhythmias in patients with significant structural heart diseases (Kodama et al., 1999). It is highly lipid-soluble, is concentrated in many tissues, and has been shown to be detectable in the rat brain and to reach the highest concentration 20~30 min after intravenous administration (Wyss et al., 1990; Riva et al., 1982). However, studies related with its central nervous system (CNS) effects are still limited. Only one study reported that it increased the concentrations of γ-aminobutyric acid (GABA) and glycine and decreased those of aspartate and glutamate in rat medulla oblongata (Turovaya et al., 2005).
Inhibition or excitation of a neuron depends on concentrations of intracellular Ca2+ and Na+ and extracellular K+, and also on balance between GABAergic and adenosinergic inhibitory transmissions and glutamatergic excitatory transmission. In epilepsy and sleeping, ion channels and neurotransmitters have important roles. Since amiodarone has multiple ion-channel blocker properties and increases the inhibitory neurotransmitters, in this study we wanted to investigate whether amiodarone has possible anticonvulsant effects in phentylenetetrazole (PTZ)- and caffeine-induced generalized convulsion models and whether it has possible hypnotic effect in pentobarbital-induced sleeping model.
MATERIALS AND METHODS
Animals
In the present study, male Swiss albino mice (25~35 g) and Sprague-Dawley rats (150~200 g) were used (Department of Pharmacology, Medical Faculty, Atatürk University, Erzurum, Turkey). Lighting operated on a 12-h dark:12-h light cycle, and temperature was maintained at 20~23 °C. The animals were left for 24 h to be accustomed to laboratory conditions and were maintained on standard pellet diet and water ad libitum. Experimental procedures were induced between 09:00 and 12:00 a.m. to minimize the effect of circadian rhythm.
Experiments were performed in accordance with the recommendations from the Declaration of Helsinki (National Institutes of Health, 1986) and the internationally accepted principles in the care and use of experimental animals.
Drugs
Amiodarone hydrochloride (Sanofi, Turkey), PTZ (Sigma, USA), caffeine (Sigma, USA), diazepam (Deva, Turkey), and pentobarbital (Abbott, Turkey) used in the study were dissolved in distilled water, in a volume of 0.1 ml/10 g, intraperitoneally (i.p.).
PTZ- and caffeine-induced generalized convulsion models
Animals were separated as control, diazepam, and amiodarone groups. Distilled water was administrated i.p. to the control group, and diazepam and several doses (50, 100, 150 mg/kg) of amiodarone to the diazepam and the amiodarone groups, respectively. Then 30 min later PTZ (100 mg/kg) and caffeine (300 mg/kg) injections were given i.p. in all the groups. The doses of PTZ and caffeine, determined by preliminary study, caused the seizures beginning with the loss of righting reflex followed by a long tonus and the death in all animals. Soon after the PTZ and caffeine injections, the animals were individually placed in plastic cages for observation during 30 min for latency (the time period until the onset of the loss of righting reflex followed by a long tonus) and during 24 h for the time to death (the time period until the death) (Dhanabal et al., 2007), and these periods were measured as second(s). The convulsion rates and the mortality were also evaluated for each group.
Pentobarbital-induced sleeping time
Rat sleep was induced by the intraperitoneal administration of 35 mg/kg of pentobarbital. The animals received distilled water, diazepam, and 50, 100 and 200 mg/kg of amiodarone i.p. 30 min before pentobarbital administration. Latency time of sleep (time to loose the righting reflex) and sleeping time (duration of loss of the righting reflex) were recorded. Latency time as second (s) and sleeping time as minute (min) were measured (Dos Santos et al., 2005).
Statistical analysis
Data are presented as mean±SEM for each group in all figures. Results were evaluated by using post-hoc LSD test. For the analyses of the convulsion rates and the mortality, Fisher’s exact test was used. P<0.05 was considered statistically significant.
RESULTS
Effects of amiodarone on the latency periods and the convulsion rates
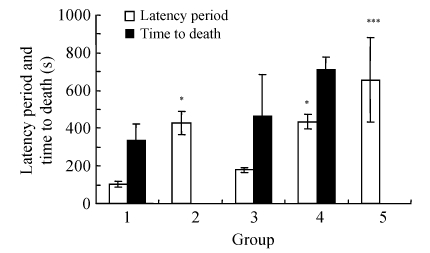
For PTZ-induced convulsions, diazepam and amiodarone dose-dependently prolonged the latency periods, and these effects of both diazepam and 100~150 mg/kg amiodarone were statistically significant (P<0.05), as shown in Fig.1. While the convulsion rates were 100% in the control, diazepam, and 50 mg/kg amiodarone groups, these rates were 66.7% and 83.3% in 100 mg/kg and 150 mg/kg amiodarone groups, respectively; these results were not significant statistically (P>0.05), as shown in Table 1.
Fig. 1.
Effects of amiodarone on the latency periods and the time to death in the PTZ-induced convulsion model
Groups: 1: control; 2: 0.5 mg/kg diazepam; 3: 50 mg/kg amiodarone; 4: 100 mg/kg amiodarone; 5: 150 mg/kg amiodarone. For each group, n=6. * P<0.05, ** P<0.01, *** P<0.005 as compared with the control group (PTZ alone). Post-hoc LSD test was performed for the latency period and the time to death. All data are shown as mean±SEM
Table 1.
Effects of amiodarone on the convulsion rate and the mortality in the PTZ-induced convulsion model
| Group | Convulsion rate | Mortality |
| Control | 6/6 | 6/6 |
| 0.5 mg/kg diazepam | 6/6 | 0/6*** |
| 50 mg/kg amiodarone | 6/6 | 3/6 |
| 100 mg/kg amiodarone | 4/6 | 2/6* |
| 150 mg/kg amiodarone | 5/6 | 0/6*** |
Fisher’s exact test was performed for the convulsion rate and the mortality. For each group, n=6. ** P<0.01 as compared with the control group (PTZ alone)
P<0.05 as compared with the control group (PTZ alone)
P<0.005 as compared with the control group (PTZ alone)
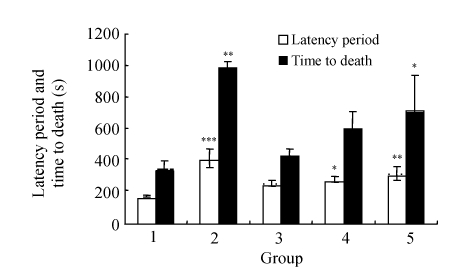
In the caffeine-induced convulsion model as shown in Fig.2, latency periods were prolonged by both diazepam and all doses of amiodarone, and these periods for diazepam and 100~150 mg/kg amiodarone were significantly different (P<0.05). In this model, the convulsion rates were 100% in all the groups (Table 2).
Fig. 2.
Effects of amiodarone on the latency period and the time to death in the caffeine-induced convulsion model
Groups: 1: control; 2: 5 mg/kg diazepam; 3: 50 mg/kg amiodarone; 4: 100 mg/kg amiodarone; 5: 150 mg/kg amiodarone. For each group, n=6. * P<0.05, ** P<0.01, *** P<0.005 as compared with the control group (caffeine alone). Post-hoc LSD test was performed for the latency period and the time to death
Table 2.
Effects of amiodarone on the convulsion rate and the mortality in the caffeine-induced convulsion model
| Group | Convulsion rate | Mortality |
| Control | 6/6 | 6/6 |
| 5 mg/kg diazepam | 6/6 | 2/6* |
| 50 mg/kg amiodarone | 6/6 | 6/6 |
| 100 mg/kg amiodarone | 6/6 | 6/6 |
| 150 mg/kg amiodarone | 6/6 | 6/6 |
Fisher’s exact test was performed for the convulsion rate and the mortality. For each group, n=6. ** P<0.01, *** P<0.005 as compared with the control group (caffeine alone)
P<0.05 as compared with the control group (caffeine alone)
Effects of amiodarone on the time to death and the mortality
Following the PTZ injections, since all mice in the diazepam and the 150 mg/kg amiodarone groups survived for 24 h, time to death of these groups was not presented in Fig.1. Amiodarone at 50 and 100 mg/kg doses had prolonged the time to death, but the difference was not statistically significant (P>0.05) in comparison to the control group. Following the PTZ injections, all mice in the control group had died, but the mortality in the amiodarone groups was reduced (Table 1), and this decrease for 100~150 mg/kg amiodarone groups was statistically significant (P<0.05). In this study, it was an interesting result that all animals in the 150 mg/kg amiodarone group remained alive in the 24 h observation period, similar to the diazepam group.
In the caffeine-induced convulsions (Fig.2), amiodarone dose-dependently prolonged the time to death in comparison to the control, but the effect at 150 mg/kg dose was statistically significant (P=0.038), and all of the mice in the three amiodarone groups died, as in the control group (Table 2).
Effects of amiodarone on the pentobarbital-induced sleeping time test
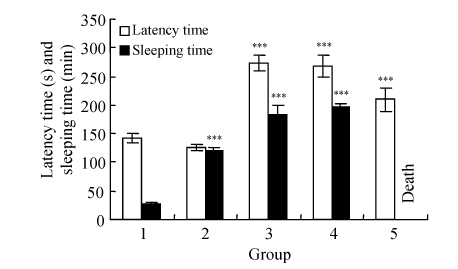
In this study, diazepam shortened the latency time, and prolonged the sleeping time (444.4%). All doses of amiodarone had prolonged the latency time in comparison to the control and the diazepam groups (P<0.005), but dose-dependently shortened latency time. Amiodarone at doses of 50 and 100 mg/kg prolonged the sleeping time (677.7% and 725.9%, respectively, P=0.000) and all rats in the 200 mg/kg amiodarone group could not be awaken up and were all dead eventually (Fig.3).
Fig. 3.
Effect of amiodarone on pentobarbital (35 mg/kg)-induced sleeping time test in rats
Groups: 1: control; 2: 1 mg/kg diazepam; 3: 50 mg/kg amiodarone; 4: 100 mg/kg amiodarone; 5: 200 mg/kg amiodarone. *** P<0.005 as compared with the control group (Post-hoc LSD test)
DISCUSSION
In the present study, amiodarone showed anticonvulsant and hypnotic effects, indicating that this drug can behave as a CNS depressant. We induced the seizures with PTZ and caffeine in mice and sleep with pentobarbital in rats. PTZ-induced model, the most popular and widely used animal seizure model, represents a valid model for human generalized myoclonic and also absence seizures, and this test has been used primarily to evaluate anticonvulsant drugs (Loscher and Schmidt, 1988). Although convulsive activity of PTZ is not fully understood, it has been reported that PTZ has induced seizures by inhibiting GABA pathway in CNS (Corda et al., 1990; Macdonald and Baker, 1977), acting as an antagonist at GABA-A receptor complex (Yesilyurt et al., 2005), increasing the central noradrenergic activity (Corda et al., 1990; de Potter et al., 1980), and increasing the intracellular calcium and extracellular potassium ion concentrations (Heinemann et al., 1977; Onozuko et al., 1989; Piredda et al., 1985). As shown in the Fig.1, in the PTZ-induced model, amiodarone administrations prolonged the latency time and the time to death, and decreased the convulsion rates and the mortality. Especially, at 150 mg/kg dose, none of the animals died. Until now, amiodarone has been known and used as only an anti-arrhythmic drug. Recently, in addition to its anti-arrhythmic effect, we have shown its gastro-protective (Ozbakis-Dengiz et al., 2007a) and anti-inflammatory effects in histamine- and carrageenan-induced paw oedema models (Ozbakis-Dengiz et al., 2007b; Halici et al., 2007). In these reports (carrageenan-induced paw inflammation (Halici et al., 2007) and indomethacine-induced gastric ulcer models (Ozbakis-Dengiz et al., 2007a)), this drug had also presented an antioxidant activity and had caused a decrease in the catalase activity. It has been suggested that catalase stimulates the expressions of mRNA and the protein for cyclooxygenase-2 (COX-2) in rats’ aortic smooth muscle cells, despite not affecting the expression of either mRNA or the protein for COX-1 (Ribeiro et al., 1997; Chen et al., 1998). We suggest that amiodarone may show the anti-inflammatory activity by inhibiting the COX-2 enzyme. Some researchers reported that some anti-inflammatory drugs (especially COX-2 inhibitors rofecoxib) had exhibited anticonvulsant effect (Kunz and Oliw, 2001; Gobbo and O′Mara, 2004; Dhir et al., 2008; Akula et al., 2008). Following PTZ-induced seizures, Takemiya et al.(2003) showed that COX-2 had induced prostaglandins and had enhanced levels of prostaglandin D2 and prostaglandin E2. In addition, Turovaya et al.(2005) reported that amiodarone had increased the concentrations of inhibitory GABA and glycine and had decreased those of excitatory aspartate and glutamate in rat medulla oblongata. In our study, the anticonvulsant effect of amiodarone in PTZ-induced seizures may be partially due to blockage of ion channels (Na+, K+ and Ca2+) and/or its involvement in noradrenergic pathways and/or in GABAergic pathway and/or its anti-oxidant and anti-inflammatory effects (COX-2 inhibition).
Some anticonvulsant drugs act by means of ion channels. Anticonvulsant activities of calcium channel blockers had been shown in in vivo and in vitro experiments (Fischer, 1988; Kaminski et al., 2001). However, it has been reported that K+ channel blockers precipitated seizure, and K+ channel activators had anticonvulsant effects in some experimental seizure models (Kwan et al., 2001). In our study, amiodarone, known as a K+ channel blocker, showed an anticonvulsant activity and we speculated that this drug showed this activity by inhibiting the outward K+ currents at the neuron-like cardiac cells.
In this study, generalized convulsions were created with high dose caffeine, too. The mechanism of seizures is, however, still unclear. Most relevant to the pharmacological and toxicological effects of caffeine are (1) the blockade of adenosine receptors, (2) the inhibition of cyclic nucleotide phosphodiesterases, (3) the sensitization to calcium of the cyclic adenosine diphosphate ribose-modulated calcium-release channel associated with certain intracellular stores of calcium, (4) the inhibition of inhibitory GABA-A and glycine receptors, and (5) the enhancement of N-methyl-D-aspartic acid (NMDA) receptor neurotransmission (Daly, 2000; Harinath and Sikdar, 2005). As shown in Fig.2 and Table 2, in the caffeine model, although amiodarone had no effect on the convulsion rates and the mortality, it had prolonged the latency time and the time to death; amiodarone was more effective in the PTZ model than in the caffeine model. Since caffeine-induced seizure is not mainly related to ion channels, we speculate that amiodarone may prevent the adenosine receptor blockage and/or the GABA-A receptor blockage and/or the enhancement of NMDA receptor neurotransmission.
We have also shown that amiodarone prolonged the sleeping time and behaved as CNS depressant drug in pentobarbital-induced sleeping model (Fig.3). This drug is highly lipid-soluble, and had been shown to pass into the brain (Wyss et al., 1990; Riva et al., 1982) and also to increase the concentrations of GABA and glycine (Turovaya et al., 2005); thus we may speculate that this drug had shown synergic effect with pentobarbital. Ohtsuka et al.(2006) reported that barbiturates used as anticonvulsant drug at high concentrations but not at clinically relevant concentrations inhibited ATP-sensitive K+ channel (KATP) channels activated by intracellular ATP depletion in rat substantia nigra, and Holmes et al.(2000) reported that amiodarone inhibited the KATP channels. In this section, all animals in the high dose amiodarone group had died; thus, we may suggest that amiodarone has potentiated the effect of pentobarbital on the KATP channels.
CONCLUSION
In this study, we have presented that amiodarone had an anticonvulsant and hypnotic effects, that none of the animals died at 150 mg/kg dose in the PTZ-induced seizure model, and that all of the animals at 200 mg/kg dose of amiodarone died in sleeping test. These results indicate that amiodarone has CNS effects, especially central depressant effects; however, further studies are necessary to investigate its anticonvulsant and hypnotic profiles, and other effects on CNS. In addition we also suggest that clinicians be cautious while using amiodarone in combination with other CNS depressants.
References
- 1.Akula KK, Dhir A, Kulkarni SK. Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor increases pentylenetetrazol seizure threshold in mice: possible involvement of adenosinergic mechanism. Epilepsy Res. 2008;78:60–70 . doi: 10.1016/j.eplepsyres.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Kamal M, Hannon R, Warner TD. Regulation of cyclooxygenase gene expression in rat smooth muscle cells by catalase. Biochemical Pharmacology. 1998;55(10):1621–1631. doi: 10.1016/S0006-2952(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 3.Corda MG, Giorgi O, Longoni B, Orlandi M, Biggio G. Decrease in the function of the γ-aminobutyric acid-coupled chloride channel produced by the repeated administration of pentylenetetrazol to rats. J Neurochem. 1990;55(4):1216–1221. doi: 10.1111/j.1471-4159.1990.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 4.Daly JW. Alkylxanthines as research tools. J Auton Nerv Syst. 2000;81(1-3):44–52. doi: 10.1016/S0165-1838(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 5.de Potter WP, de Potter RW, de Smett FH, de Schaepdryver AF. The effect of drugs on the concentration of dopamine β-hydroxylase in the cerebrospinal fluid of rabbits. Neuroscience. 1980;5(11):1969–1977. doi: 10.1016/0306-4522(80)90042-1. [DOI] [PubMed] [Google Scholar]
- 6.Dhanabal SP, Paramakrishnan N, Manimaran S, Suresh B. Anticonvulsant potential of essential oil of artemisia abrotanum. Curr Trends Biotechnol Pharm. 2007;1(1):112–116. [Google Scholar]
- 7.Dhir A, Akula KK, Kulkarni SK. Rofecoxib potentiates the anticonvulsant effect of topiramate. Inflammopharmacology. 2008;16(2):83–86. doi: 10.1007/s10787-007-7007-6. [DOI] [PubMed] [Google Scholar]
- 8.Dos Santos JGJr, Blanco MM, Do Monte FHM, Russi M, Lanziotti VMNB, Leal LKAM, Cunha GM. Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense . Fitoterapia. 2005;76(6):508–513. doi: 10.1016/j.fitote.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Fischer W. Antiepileptic effect of calcium antagonist. Drugs Today. 1988;24:167–174. [Google Scholar]
- 10.Gobbo OL, O′Mara SM. Post-treatment, but not pre-treatment, with the selective cyclooxygenase-2 inhibitor celecoxib markedly enhances functional recovery from kainic acid-induced neurodegeneration. Neuroscience. 2004;125(2):317–327. doi: 10.1016/j.neuroscience.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 11.Halici Z, Ozbakis-Dengiz G, Odabasoglu F, Suleyman H, Cadirci E, Halici M. Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw edema. Eur J Pharmacol. 2007;566(1-3):215–221. doi: 10.1016/j.ejphar.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 12.Harinath S, Sikdar SK. Inhibition of human TREK-1 channels by caffeine and theophylline. Epilepsy Res. 2005;64(3):127–135. doi: 10.1016/j.eplepsyres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Heinemann U, Lux HD, Gutnick MJ. Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977;27(3-4):237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- 14.Herbette LG, Trumbore M, Chester DW, Katz AM. Possible molecular basis for the pharmacokinetics and pharmacodynamics of three membrane-active drugs: propranolol, nimodipine and amiodarone. J Mol Cell Cardiol. 1988;20(5):373–378. doi: 10.1016/S0022-2828(88)80128-7. [DOI] [PubMed] [Google Scholar]
- 15.Holmes DS, Sun ZQ, Porter LM, Bernstein NE, Chinitz LA, Artman M, Coetzee WA. Amiodarone inhibits cardiac ATP-sensitive potassium channels. J Cardiovasc Electrophysiol. 2000;11(10):1152–1158. doi: 10.1111/j.1540-8167.2000.tb01762.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaminski RM, Mazurek M, Turski WA, Kleinrok Z, Czuczwar SJ. Amlodipine enhances the activity of antiepileptic drugs against penthylenetetrazole-induced seizures. Pharmacol Biochem Behav. 2001;68(4):661–668. doi: 10.1016/S0091-3057(01)00468-3. [DOI] [PubMed] [Google Scholar]
- 17.Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol. 1999;84(9):20R–28R. doi: 10.1016/S0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- 18.Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13(3):569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 19.Kwan P, Sills GJ, Brodie MJ. The mechanisms of action of commonly used antiepileptic drugs. Pharmacology & Therapeutics. 2001;90(1):21–34. doi: 10.1016/S0163-7258(01)00122-X. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2(3):145–181. doi: 10.1016/0920-1211(88)90054-X. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald RL, Baker JL. Pentylelnetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurons. Nature. 1977;267(5613):720–721. doi: 10.1038/267720a0. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Washington, DC: US Government Printing Office; 1986. DHEW Publication No. 86-23. [Google Scholar]
- 23.Ohtsuka T, Ishiwa D, Kamiya Y, Itoh H, Nagata I, Saito Y, Yamada Y, Sumitomo M, Andoh T. Effects of barbiturates on ATP-sensitive K+ channels in rat substantia nigra. Neuroscience. 2006;137(2):573–581. doi: 10.1016/j.neuroscience.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 24.Onozuko M, Nakagaki I, Sasaki S. Petylenetetrazole-induced seizure activity produces an increased release of calcium from endoplasmic reticulum by mediating cyclic AMP-dependent protein phosphorylation in rat cerebral cortex. Gen Pharmacol. 1989;20:627–634. doi: 10.1016/0306-3623(89)90098-0. [DOI] [PubMed] [Google Scholar]
- 25.Ozbakis-Dengiz G, Odabasoglu F, Halici Z, Suleyman H, Cadirci E, Bayir Y. Gastroprotective and antioxidant effects of amiodarone on indomethacin-induced gastric ulcers in rats. Arch Pharm Res. 2007;30(11):1426–1434. doi: 10.1007/BF02977367. [DOI] [PubMed] [Google Scholar]
- 26.Ozbakis-Dengiz G, Halici Z, Akpinar E, Cadirci E, Bilici D, Gursan N. Role of polymorphonuclear leukocyte infiltration in the mechanism of anti-inflammatory effect of amiodarone. Pharmacol Rep. 2007;59(5):538–544. [PubMed] [Google Scholar]
- 27.Piredda S, Yonekawa W, Whittingham TS, Kupferberg HJ. Potassium, pentylenetetrazole, and anticonvulsants in mouse hippocampal slices. Epilepsia. 1985;26(2):167–174. doi: 10.1111/j.1528-1157.1985.tb05401.x. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro SMR, Campello AP, Nascimento AJ, Kluppel LW. Effect of amiodarone (AMD) on the antioxidant enzymes, lipid peroxidation and mitochondrial metabolism. Cell Biochem Funct. 1997;15(3):145–152. doi: 10.1002/(SICI)1099-0844(199709)15:3<145::AID-CBF728>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 29.Riva E, Gerna M, Neyroz P, Urso R, Bartosek I, Guaitani A. Pharmacokinetics of amiodarone in rats. J Cardiovasc Pharmacol. 1982;4(2):270–275. doi: 10.1097/00005344-198203000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Roden DM. Antiarrhytmic Drugs. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. New York, USA: The McGraw-Hill Companies; 2006. pp. 899–932. [Google Scholar]
- 31.Takemiya T, Suzuki K, Sugiura H, Yasuda S, Yamagata K, Kawakami Y, Maru E. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins & Other Lipid Mediators. 2003;71(3-4):205–216. doi: 10.1016/S1098-8823(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 32.Turovaya AY, Galenko-Yaroshevskii PA, Kade AK, Uvarov AE, Kiguradze MI, Khvitiya NG, Tatulashvili DR. Effects of verapamil and amiodarone on sympathoadrenal system and balance of excitatory and inhibitory amino acids in rat medulla oblongata. Bull Exp Biol Med. 2005;139(6):665–667. doi: 10.1007/s10517-005-0372-5. [DOI] [PubMed] [Google Scholar]
- 33.Wyss PA, Moor MJ, Bickel MH. Single-dose kinetics of tissue distribution, excretion and metabolism of amiodarone in rats. J Pharmacol Exp Ther. 1990;254:502–507. [PubMed] [Google Scholar]
- 34.Yesilyurt O, Dogrul A, Uzbay T. Differential effects of systemic versus central routes of administration of L-type calcium channel blockers on pentylenetetrazole-induced seizures in mice. Bull Clin Psychopharmacol. 2005;15(4):153–157. [Google Scholar]