Abstract
Statistical shape analysis, a relatively a new method for biological research, compares body forms by using specific landmarks determined by anatomical prominences. In this study, we aimed to identify normal facial asymmetry between the right and the left sides of the face. Facial landmark data were collected from two-dimensional digital images of 321 young healthy subjects (150 males and 171 females). These data were analysed using Euclidean distance matrix analysis. The number of significantly asymmetric linear distances between the two halves of the face was greater in females than in males. We found that the left side of the face was most commonly dominant in both males and females. Such data may be useful in establishing a database for future similar studies.
Keywords: anthropometry, asymmetry, face, geometric morphometry, statistical shape analysis, symmetry
Introduction
Mild asymmetries occur in the normal growth and development of the body, including the face (Burke & Healy, 1993; Ferrario et al. 1995, 2001; Shaner et al. 2000). Interestingly, the human form is, generally, externally symmetrical and internally asymmetrical. However, in normal individuals, there are small differences between the dimensions of the left and right halves of the face, which alone do not normally result in an aesthetically unpleasing appearance (Ferrario et al. 1995; Ferrario et al. 2001). Facial symmetry has been reported as a factor in attractiveness (Baudouin & Tiberghien, 2004), and thus the role of symmetry in beauty has been questioned. Zaidel & Cohen (2005) have studied facial symmetry and reported an unexpected result in that certain asymmetrical dimensions were accepted as being traits of beauty. These authors concluded that beautiful faces may indeed have asymmetry between left and right sides.
Stedman's Medical Dictionary (2006) defines symmetry as the ‘equality or correspondence in form of parts distributed around a center or axis, at the two extremes or poles, or on the two opposite sides of the body’. When applied to facial morphology, symmetry and balance refer to the state of facial equilibrium, the correspondence in size, shape and arrangement of facial features on opposite sides of the median sagittal plane. Facial asymmetry can also be defined as simply one side being larger than the other (Smith, 2000).
Previously, direct and indirect measurements have been performed on both radiographs and photographs to discern for facial asymmetry. For such examinations, traditional morphometric measurements such as metric distances, areas, angles and ratios have been calculated for left and right sides (Burke & Healy, 1993; Shaner et al. 2000; Ferrario et al. 2001; Baudouin & Tiberghien, 2004). However, Ferrario et al. (1993, 1995) mentioned that such methods provide information, which only refers to local imbalances and does not allow full facial analysis and does not reflect shape differences between the two sides.
Statistical shape analysis, a relatively new method for biological research, compares body forms by using specific landmarks determined by anatomical prominences (Lele & Richtsmeier, 1991; Lele, 1993; Ferrario et al. 1995; McIntyre & Mossey, 2002; Hennessy et al. 2004, 2006; DeLeon, 2007; Mutsvangwa & Douglas, 2007). Several procedures for obtaining such shape information from anatomical landmark data have been proposed. For example, Euclidean Distance Matrix Analysis (EDMA) is a landmark-based method that uses landmark coordinate data to calculate all possible linear distances among landmarks, creating a form matrix for each object (Burrows et al. 1999). Difficulties in displaying EDMA results are actually related to the coordinate-system-invariant properties that make EDMA biologically and statistically advantageous (Cole & Richtsmeier, 1998).
The current study is aimed at determining asymmetry between the right and the left parts of the face and detecting landmarks that may contribute to such asymmetry in both sexes. Such data may be useful in establishing a database for future similar studies and may have application for studies in plastic surgery and facial nerve paralysis.
Materials and methods
Sample
The study group consisted of 321 young adult Turks (171 females and 150 males) 17–23 years of age (17–23; mean ± SD: 18.88 ± 1.24; 17–23; 19.76 ± 1.42, respectively), all from Uludag University. There was no noticeable nasal or facial disfigurement and no history of previous nasal or facial surgery in any subject. Participants gave informed consent regarding the investigation. All data were obtained from standardized digital photographic images taken anteriorly using a 5.1-mega pixel digital camera (from 2 m away). The same investigator took all photographs. Extreme care was taken to ensure that the best possible frontal photograph was obtained. Subjects were instructed to fixate on the camera lens, not to smile, and none was permitted to wear glasses.
Collection of two-dimensional facial landmarks
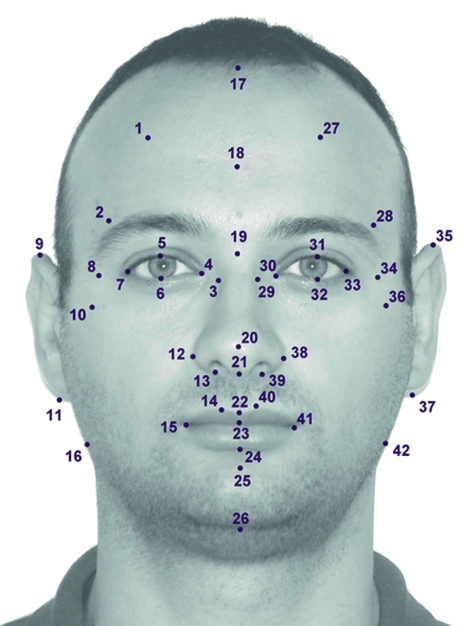
Standard anthropometric landmarks were chosen and marked on each digital image using TPSDIG 2.04 software. For each subject, 42 (10 midline landmarks, 16 right-sided landmarks, 16 left-sided landmarks) anthropometric landmarks on the anterior aspect of the face were defined. Midsagittal landmarks were defined by the trichion (tr), supraglabella (sg) nasion (n), pronasale (pr), subnasale (sn), labiale superior (ls), stomion (sto), labiale inferior (li), sublabiale (sl), and gnathion (gn). The landmarks were located on the forehead (trichion; supraglabella; frontotemporale, frontozygomaticus), eyes (exocanthion; endocanthion; palpebrale superior; palpebrale inferior), lateral facial region (zygion; gonion), nose (nasion; pronasale; columella; subnasale; alare; nasal alar crest; inferior and superior points of the nostril axis), lips and mouth (labiale superior; stomion; labiale inferior; sublabiale; crista philtri; cheilion), chin (pogonion; menton), and ears (superaurale; subaurale). The descriptions of these landmarks are found in Table 1 and shown in Fig. 1. Analysis of asymmetry then required identification of homologous landmarks on either side of the midsagittal plane of the face and deduction of their differences in a two-dimensional plane.
Table 1.
Landmarks used in this study
| No. | Name of landmark | Description of landmark |
|---|---|---|
| Midline landmarks used in this study | ||
| 17 | trichion | Midpoint of the hairline |
| 18 | supraglabella | Most anterior point on midline |
| 19 | nasion | The midpoint of the nasofrontal suture |
| 20 | pronasale | The most protruded point of the nasal tip |
| 21 | subnasale | The junction between the lower border of the nasal septum, the partition that divides the nostrils, and the cutaneous portion of the upper lip in the midline |
| 22 | labiale superius | The midpoint of the vermilion border of the upper lip |
| 23 | stomion | The midpoint of the labial fissure when the lips are closed naturally |
| 24 | labiale inferius | The midpoint of the vermillion border of the lower lip |
| 25 | sublabiale | The midpoint of the labiomental sulcus |
| 26 | gnathion | The lowest point in the midline on the lower border of the chin |
| Right- and left-side landmarks used in this study | ||
| 1, 27 | frontal eminence | Centered on eye pupil, most anterior point of the forehead |
| 2, 28 | frontotemporale | The most medial point on the temporal crest of the frontal bone |
| 3, 29 | maxillofrontale | The anterior lacrimal crest of the maxilla at the frontomaxillary suture |
| 4, 30 | endocanthion | The inner corner of the eye fissure where the eyelids meet, not the caruncles (the red eminences at the medial angles of the eyes) |
| 5, 31 | palpebrale superius | The highest point on the upper margin of the middle portion of the eyelid |
| 6, 32 | palpebrale inferius | The lowest point in the middle of the margin of the lower eyelid |
| 7, 33 | exocanthion | The outer corner of the eye fissure where the eyelids meet |
| 8, 34 | frontozygomaticus | The most lateral point on the frontozygomatic suture |
| 9, 35 | supraaurale | The highest point of the free margin of the ear |
| 10, 36 | zygion | The most lateral point on the zygomatic arch |
| 11, 37 | subaurale | The lowest point of the ear lobe |
| 12, 38 | alare | The most lateral point on the nasal ala |
| 13, 39 | subalare | The point on the lower margin of the base of the nasal ala where the ala disappears into the upper lip skin |
| 14, 40 | crista philtre | The point on the crest of the philtrum, the vertical groove in the median portion of the upper lip, just above the vermilion border |
| 15, 41 | cheilion | The outer corner of the mouth where the outer edges of the upper and lower vermilions meet |
| 16, 42 | gonion | The most lateral point at the angle of the mandible |
Fig. 1. Used landmarks in this study.
Landmark reliability
We calculated the intra-rater reliability coefficient for a two-facet crossed design (‘landmark pairs-by-rater-by-subject’, l × r × s) based on the generalizability theory (GT) (Ercan et al. 2008). In GT, the reliability for relative (norm-referenced) interpretations is referred to as the generalizability (G) coefficient. (Dimitrov, 2006).
In this study, 42 facial landmarks (10 midline, 16 right side of face, 16 left side of face) were marked by same investigator. After 1 month, this same investigator marked the landmarks on the 20 individuals (10 male, 10 female) who were selected randomly from the study population. Analysis was performed to obtain a G reliability coefficient. As a result, the analysis of the rating indicated good repeatability for both female and male subject (G = 0.99).
Morphometric asymmetry analysis (MAA)
The MAA was used to evaluate shape-related asymmetry as shape is independent of size, location and orientation (Dryden & Mardia, 1998). EDMA software was used to analyse shape asymmetry (Cole, 2002). This program generates a matrix for right and left landmark configurations by calculating all possible Euclidean distances between the landmark pairs. Each corresponding pair of Euclidean distances is systematically compared as a ratio to produce the form-difference matrix (FDM). These are subsequently sorted to rank the elements according to increasing value. The test statistic ‘T’ was calculated as the ratio of the largest/smallest elements of the FDM. The null distribution of T was calculated using a nonparametric bootstrap technique based on 1000 resamples (pseudosamples) and the proportion of bootstrapped Ts greater than T are represented as a P-value (Lele & Richtsmeier, 2001).
Results
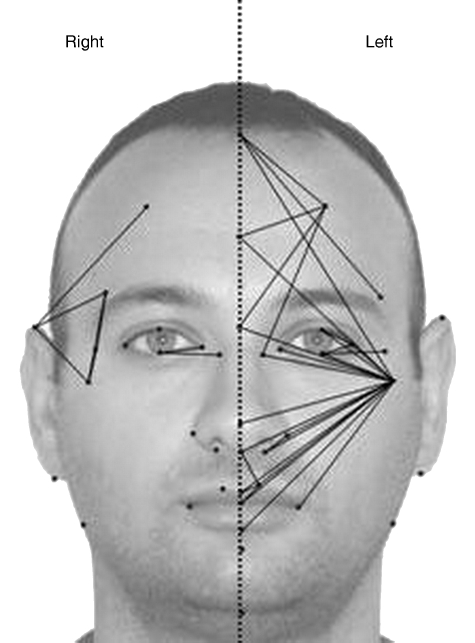
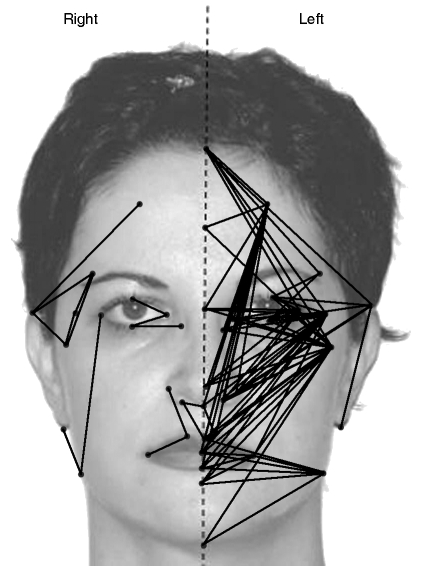
According to statistical shape analysis, some differences between two parts of the face were found in both sexes. (In females EDMA T = 1.368, P = 0.001, in males EDMA T = 1.319, P = 0.001). The number of significantly asymmetric linear distances between the two halves of the face was greater in females than in males. The results from the asymmetry analysis are shown in Tables 2–3 and Figs 2–3. In all, 280 possible linear distances were used for the asymmetry analysis to evaluate the differences between the two vertical parts of face. In females, 33% (91/280) of distances demonstrated asymmetry regarding the differences between the left and right sides of the face. In this group, 86% (78/91) of asymmetric linear distances were larger on the left side and 14% (13/91) were larger on the right side (Table 3). In females, among all significantly asymmetric linear distances, the number of linear distances involving the zygion was the highest (24%, 21/91). Distances important for mandibular width 42–24 (labiale inferius–gonion), 42–25 (sublabiale–gonion), 42–26 (gnathion–gonion) were found to be wider on the left side of the face than on the right side in females. The supraaurale–subaurale (35–37) distance, which indicates ear length, was greater on the left in females. In males, 13% (36/280) of these distances demonstrated asymmetry regarding the differences between right and left sides of the face. In this group, 81% (29/36) of asymmetric linear distances were larger on the left, and 19% (7/36) were larger on the right (Table 3). In males, among all significantly asymmetric linear distances, the number of linear distances involving the zygion was the highest (56%, 20/36). Asymmetric linear distances between the two sides of face were found more commonly at the middle third of the face (maxillary bone, zygomatic corner and lower orbital border) in both sexes (Figs 2 and 3).
Table 2.
Asymmetric values for males and females. (Left/Right ratios) If > 1, the distance between two landmarks is greater for left vs. right sides
| Euclidean distance (landmark) | Female | Male | Euclidean distance (landmark) | Female | Male |
|---|---|---|---|---|---|
| 40–22 | 1.234 | 1.085 | 36–17 | 1.029 | 1.038 |
| 27–17 | 1.153 | 1.176 | 41–25 | 1.028 | – |
| 33–32 | 1.150 | 1.116 | 32–28 | 1.028 | – |
| 36–32 | 1.143 | 1.136 | 42–22 | 1.027 | – |
| 27–18 | 1.134 | 1.101 | 34–20 | 1.027 | – |
| 40–23 | 1.124 | 1.056 | 35–31 | 1.027 | – |
| 38–36 | 1.113 | 1.068 | 31–28 | 1.027 | – |
| 33–31 | 1.109 | 1.059 | 27–20 | 1.027 | – |
| 39–36 | 1.103 | 1.066 | 39–27 | 1.026 | – |
| 36–20 | 1.081 | 1.054 | 34–23 | 1.026 | – |
| 34–32 | 1.077 | 1.041 | 39–35 | 1.025 | – |
| 36–21 | 1.076 | 1.051 | 34–21 | 1.025 | – |
| 36–30 | 1.075 | 1.074 | 34–29 | 1.025 | – |
| 36–29 | 1.073 | 1.066 | 37–35 | 1.025 | – |
| 36–31 | 1.071 | 1.093 | 36–18 | 1.025 | 1.034 |
| 36–23 | 1.069 | 1.043 | 34–17 | 1.024 | – |
| 36–22 | 1.068 | 1.044 | 34–24 | 1.023 | – |
| 40–21 | 1.066 | – | 34–22 | 1.023 | – |
| 41–36 | 1.065 | 1.038 | 38–33 | 1.023 | – |
| 36–24 | 1.061 | 1.036 | 27–21 | 1.023 | – |
| 27–19 | 1.058 | 1.040 | 33–20 | 1.022 | – |
| 40–36 | 1.057 | 1.040 | 34–30 | 1.022 | – |
| 39–38 | 1.057 | 1.065 | 34–25 | 1.022 | – |
| 36–25 | 1.055 | – | 38–35 | 1.021 | – |
| 36–19 | 1.054 | 1.047 | 38–27 | 1.021 | – |
| 42–25 | 1.052 | – | 27–22 | 1.019 | – |
| 42–24 | 1.048 | – | 27–23 | 1.019 | – |
| 34–31 | 1.045 | – | 34–19 | 1.019 | – |
| 42–41 | 1.045 | – | 41–34 | 1.018 | – |
| 42–23 | 1.042 | – | 40–27 | 1.018 | – |
| 42–26 | 1.042 | – | 27–24 | 1.017 | – |
| 29–27 | 1.040 | 1.029 | 40–38 | 0.976 | – |
| 39–34 | 1.040 | – | 36–28 | 0.973 | 0.962 |
| 28–17 | 1.039 | 1.056 | 42–33 | 0.973 | – |
| 36–33 | 1.038 | 1.111 | 32–29 | 0.971 | 0.966 |
| 36–26 | 1.038 | – | 39–22 | 0.970 | – |
| 33–29 | 1.037 | – | 31–30 | 0.970 | – |
| 38–34 | 1.037 | – | 40–39 | 0.966 | – |
| 35–32 | 1.036 | – | 34–28 | 0.962 | 0.892 |
| 33–30 | 1.034 | – | 32–30 | 0.949 | 0.954 |
| 33–17 | 1.032 | – | 39–21 | 0.929 | – |
| 41–24 | 1.032 | – | 41–40 | 0.916 | – |
| 41–23 | 1.032 | – | 42–37 | 0.907 | – |
| 35–17 | 1.032 | – | 36–35 | 0.902 | 0.892 |
| 30–27 | 1.032 | – | 36–34 | – | 1.067 |
| 33–19 | 1.031 | – | 35–27 | – | 0.965 |
| 39–33 | 1.030 | – | 35–28 | – | 0.910 |
Table 3.
The ratio of asymmetry
| Female, % (n) | Male, % (n) | |
|---|---|---|
| The ratio of asymmetric distances between two sides to all of the measured distances | 33 (91/280) | 13 (36/280) |
| In asymmetric cases, the ratio of larger left-sided faces | 86 (78/91) | 81 (29/36) |
| In asymmetric cases, the ratio of larger right-sided faces | 14 (13/91) | 19 (7/36) |
Fig. 2. Asymmetry in males: the lines show bigger inter-landmark distances than on the other side.
Fig. 3. Asymmetry in females: the lines show bigger inter-landmark distances than on the other side.
Discussion
In evolutionary psychology, facial symmetry is one of a number of personal traits, including healthiness, physical attractiveness and overall beauty (Baudouin & Tiberghien, 2004; Zaidel & Cohen, 2005; Sengupta & Karmakar, 2007). Facial asymmetry is not uncommon in normal healthy individuals. Normal individuals are most often the focus of facial symmetry studies which attempt to quantify the amount of normal variability. Asymmetric development has to be evaluated with regard to many factors, e.g. developmental rates of the facial muscles, contralateral hemispheric control, genetic factors, prenatal stress, and environmental factors such as temperature (Smith, 1998; Rossi et al. 2003).
An analysis of facial asymmetry should take into account both the relative dimensions of the left and right hemifaces (size) and the arrangement of structures (shape) (Ferrario et al. 1995). In recent years, studies have used craniofacial shape differences and asymmetry of the craniofacial skeleton using statistical shape analysis methods, especially EDMA (Kane et al. 2007; Weinberg et al. 2008). In this study, the two-dimensional coordinates of selected facial landmarks of young healthy adults were collected using photography and EDMA was used to quantify their global facial asymmetry. Sixteen landmarks on both sides of the midline were used to achieve several inter-landmark distances to determine asymmetry.
Although many investigators have also found asymmetry as a normal facial feature, there is no consensus in the literature regarding the degree, side and spatial localization of facial asymmetry (Ferrario et al. 1995, 2001; Shaner et al. 2000). Shaner et al. (2000) have pointed out that the normal limits of soft tissue asymmetry in the measurements taken from the upper and middle regions of the face did not exceed 5 mm in males and 6 mm in females as a general rule. In the same study, it was stated that measurements that involved lower regions of the face had a much higher normal variability, with the differences between the two parts being 6 mm or greater. Ferrario et al. (2001) indicated that the differences between the most symmetric and the most asymmetric groups were less than 2.5 mm.
In all investigations, a significant facial asymmetry has been demonstrated even in aesthetically pleasing faces, but no agreement exists regarding the side of dominance. The left side of the face has been found to be more dominant in some studies (Vig & Hewitt, 1975; McIntyre & Mossey, 2002) and the right side more dominant in other studies (Farkas & Cheung, 1981; Ferrario et al. 1994, 1995,2001; Shaner et al. 2000). McIntyre & Mossey (2002) evaluated facial asymmetry using eight linear, nine angular, and three midfacial area measurements with conventional cephalometric analysis; their results showed that three linear distances, nine angles, and two areas differed between right and left sides of the craniofacial complex, indicating size asymmetry characterized by a wider left side of the face and a shorter vertical dimension on the right side. Ferrario et al. (1994) developed a new method for quantification of facial asymmetry and applied it to a group of 80 young healthy people. For each subject, 16 standardized soft tissue facial landmarks were selected. Results showed that the right side of the face was larger than the left side. These differing results could be due to the use of different methodologies or different age groups and not using standardized measurement techniques. Also, variable head positioning has been reported to alter linear and angular measurements (Ferrario et al. 1995). In our study, we found that the left side of the face was most commonly dominant in both sexes.
Different results have been reported related to sex and age regarding the dominant part of the face. Smith (2000) has stated that whereas the right side of the face was larger in females than the males, the left sides of the face is larger in males than the females. In another study where three different age groups were evaluated, maximum normal asymmetry was found slightly more often in females than in males (Ferrario et al. 2001). In their study, they indicated that the right side of the face was larger than the left side except for adolescent females. As the growth stage proceeds, right-sided dominance becomes less frequent, whereas left-side dominance becomes more frequent (Haraguchi et al. 2008). In our study, conducted with young adults, the number of significantly asymmetric linear distances between the two halves of the face was greater in females than in males.
In the literature, significant differences regarding the degree of facial asymmetry between the different regions of the face have been reported. Farkas & Cheung (1981) carried out a study on 308 normal Caucasian children to evaluate the degree of subtle asymmetry using anthropometrics. In their study, the most asymmetric part of the face (69.2%) was the upper third. Ferrario et al. (1994) showed that there was a certain degree of soft tissue facial asymmetry both in individuals and in global populations and that this was especially evident in the middle (tragus) and lower (gonion) thirds of the face. Shaner et al. (2000) have stated that measurements that involve tragion and gonion to the mouth and chin regions had a much greater normal variability. In several studies, it has been reported that asymmetry in the lower third of the face was greater than in the middle and the upper thirds (Severt & Proffit, 1997; Shaner et al. 2000; Haraguchi et al. 2002). The response of functional adaptation to asymmetrical masticatory activity is mentioned in the literature as the main cause of asymmetries in the lower part of the face (Vig & Hewitt, 1975). In the present study, the most asymmetric part of the face in both sexes was the middle third of the face (maxillary bone, zygomatic corner and lower orbital border). However, asymmetry in the lower facial third, which is seen especially in females, has been accepted to be a result of functional adaptation of this part.
Ferrario et al. (2001) reported that the tragion, gonion and zygion were the most asymmetrical landmarks to use. In the present study, linear distances involving the zygion were highly asymmetric between the two sides of the face in both sexes. Ferrario et al. (1995) has been stated that understanding of the form characteristics of the face could be improved by adding more landmarks belonging to soft tissue. Therefore, in this study, we aimed to evaluate facial asymmetry more extensively by increasing the number of soft-tissue landmarks.
The facial hemi-sides, as with the cerebral hemispheres, are functionally asymmetric, which is not surprising given the morphogenetic link between the brain and craniofacial appearance. Differential activity of the two hemifaces in relation to the contralateral hemispheres was thought to result in differential muscular development of the two hemifaces, hence, facial asymmetry (Smith, 2000). The control of the facial musculature is complex, with different patterns of neural innervations present for the upper vs. the lower face, depending on the nature of neurological control of the two sides of the face by the two cerebral hemispheres. Mobility of facial expression also exhibits facial asymmetry (Haraguchi et al. 2008). Most studies suggested that the left side of the face is more expressive of emotions: an asymmetry that probably stems from the right hemisphere dominance for emotional expression (Borod et al. 1998; Haraguchi et al. 2002; Nicholls et al. 2004). Such a functional asymmetry in facial expression may have some relationship to the dimensional balance between the left and the right hemiface (Haraguchi et al. 2008).
Differential hemispheric cognitive activity may influence the two sides of the face (Smith, 2000). Smith et al. (2000) reported that humanities faculty members were predominantly right-face dominant due to their dominant verbal activity. However, mathematicians and physicists were predominantly left-face dominant due to their visuospatial activities. We cannot comment on such a relationship as this aspect of our study population was not examined. In addiction, Hennessy et al. (2004) indicated that variation in facial shape and asymmetry reflected variation in adult brain function.
Conclusions
Facial asymmetry is common and can be easily underappreciated with subjective evaluations. In the preoperative evaluation of facial surgery and orthodontic work, asymmetry of the face should be considered and may only be noticed with detailed morphometric analysis. Our data may be of use for future clinical studies.
References
- Baudouin JY, Tiberghien G. Symmetry, averageness, and feature size in the facial attractiveness of women. Acta Psychol (Amst) 2004;117:313–332. doi: 10.1016/j.actpsy.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Borod JC, Koff E, Yecker S, Santschi C, Schmidt JM. Facial asymmetry during emotional expression: gender, valence, and measurement technique. Neuropsychologia. 1998;36:1209–1215. doi: 10.1016/s0028-3932(97)00166-8. [DOI] [PubMed] [Google Scholar]
- Burke PH, Healy MJ. A serial study of normal facial asymmetry in monozygotic twins. Ann Hum Biol. 1993;20:527–534. doi: 10.1080/03014469300002932. [DOI] [PubMed] [Google Scholar]
- Burrows AM, Richtsmeier JT, Mooney MP, Smith TD, Losken HW, Siegel MI. Three-dimensional analysis of craniofacial form in a familial rabbit model of nonsyndromic coronal synostosis using Euclidean distance matrix analysis. Cleft Palate Craniofac J. 1999;36:196–206. doi: 10.1597/1545-1569_1999_036_0196_taocfi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Cole TM III. Win EDMA: Software for Euclidean Distance Matrix Analysis. Kansas City: University of Missouri–Kansas City School of Medicine; 2002. Version 1.0.1 beta. [Google Scholar]
- Cole TM III, Richtsmeier JT. A simple method for visualization of influential landmarks when using Euclidean distance matrix analysis. Am J Phys Anthropol. 1998;107:273–283. doi: 10.1002/(SICI)1096-8644(199811)107:3<273::AID-AJPA4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- DeLeon VB. Fluctuating asymmetry and stress in a medieval Nubian population. Am J Phys Anthropol. 2007;132:520–534. doi: 10.1002/ajpa.20549. [DOI] [PubMed] [Google Scholar]
- Dimitrov DM. Reliability. In: Erford BT, editor. Assessment for Counselors. Boston: Houghton-Mifflin/Lahaska Press; 2006. pp. 99–122. Chapter 3. [Google Scholar]
- Dryden IL, Mardia KV. Statistical Shape Analysis. Chichester: John Wiley & Sons; 1998. pp. 1–3. [Google Scholar]
- Ercan I, Ocakoglu G, Guney I, Yazici B. Adaptation of generalizability theory for inter-rater reliability for landmark localization. Int J Tomogr Stat. 2008;9(S08):51–58. [Google Scholar]
- Farkas LG, Cheung G. Facial asymmetry in healthy North American caucasians. Angle Orthod. 1981;51:70–77. doi: 10.1043/0003-3219(1981)051<0070:FAIHNA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Pizzini G, Vogel G. Sexual dimorphism in the human face assessed by euclidean distance matrix analysis. J Anat. 1993;183:593–600. [PMC free article] [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Poggio CE, Tartaglia G. Distance from symmetry: a three-dimensional evaluation of facial asymmetry. J Oral Maxillofac Surg. 1994;52:1126–1132. doi: 10.1016/0278-2391(94)90528-2. [DOI] [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Miani A, Serrao G. A three-dimensional evaluation of human facial asymmetry. J Anat. 1995;186(Pt 1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Ciusa V, Dellavia C, Tartaglia GM. The effect of sex and age on facial asymmetry in healthy subjects: a cross-sectional study from adolescence to mid-adulthood. J Oral Maxillofac Surg. 2001;59:382–388. doi: 10.1053/joms.2001.21872. [DOI] [PubMed] [Google Scholar]
- Haraguchi S, Takada K, Yasuda Y. Facial asymmetry in subjects with skeletal Class III deformity. Angle Orthod. 2002;72:28–35. doi: 10.1043/0003-3219(2002)072<0028:FAISWS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Haraguchi S, Iguchi Y, Takada K. Asymmetry of the face in orthodontic patients. Angle Orthod. 2008;78:421–426. doi: 10.2319/022107-85.1. [DOI] [PubMed] [Google Scholar]
- Hennessy RJ, Lane A, Kinsella A, Larkin C, O’Callaghan E, Waddington JL. 3D morphometrics of craniofacial dysmorphology reveals sex-specific asymmetries in schizophrenia. Schizophr Res. 2004;67:261–268. doi: 10.1016/j.schres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hennessy RJ, McLearie S, Kinsella A, Waddington JL. Facial shape and asymmetry by three-dimensional laser surface scanning covary with cognition in a sexually dimorphic manner. J Neuropsychiatry Clin Neurosci. 2006;18:73–80. doi: 10.1176/jnp.18.1.73. [DOI] [PubMed] [Google Scholar]
- Kane AA, DeLeon VB, Valeri C, Becker DB, Richtsmeier JT, Lo LJ. Preoperative osseous dysmorphology in unilateral complete cleft lip and palate: A quantitative analysis of computed tomography data. Plast Reconstr Surg. 2007;119:1295–1301. doi: 10.1097/01.prs.0000258519.88178.c4. [DOI] [PubMed] [Google Scholar]
- Lele S. Euclidean distance matrix analysis (EDMA): estimation of mean form and mean form difference. Math Geol. 1993;25:573–602. [Google Scholar]
- Lele S, Richtsmeier JT. Euclidean distance matrix analysis: a coordinate free approach to comparing biological shapes using landmark data. Am J Phys Anthropol. 1991;86:415–428. doi: 10.1002/ajpa.1330860307. [DOI] [PubMed] [Google Scholar]
- Lele S, Richtsmeier JT. An Invariant Approach to Statistical Analysis of Shapes. New York: Chapman and Hall/CRC Press; 2001. [Google Scholar]
- McIntyre GT, Mossey PA. Asymmetry of the parental craniofacial skeleton in orofacial clefting. J Orthod. 2002;29:299–305. doi: 10.1093/ortho/29.4.299. [DOI] [PubMed] [Google Scholar]
- Mutsvangwa T, Douglas T. Morphometric analysis of facial landmark data to characterize the facial phenotype associated with fetal alcohol syndrome. J Anat. 2007;210:209–220. doi: 10.1111/j.1469-7580.2006.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls ME, Ellis BE, Clement JG, Yoshino M. Detecting hemifacial asymmetries in emotional expression with three-dimensional computerized image analysis. Proc Biol Sci. 2004;271:663–668. doi: 10.1098/rspb.2003.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Ribeiro E, Smith R. Craniofacial asymmetry in development: An anatomical study. Angle Orthod. 2003;73:381–385. doi: 10.1043/0003-3219(2003)073<0381:CAIDAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sengupta M, Karmakar B. Genetics of anthropometric asymmetry in an Indian endogamous population – Vaidyas. Am J Hum Biol. 2007;19:399–408. doi: 10.1002/ajhb.20601. [DOI] [PubMed] [Google Scholar]
- Severt TR, Proffit WR. The prevalence of facial asymmetry in the dentofacial deformities population at the University of North Carolina. Int J Adult Orthod Orthognath. 1997;12:171–176. [PubMed] [Google Scholar]
- Shaner DJ, Peterson AE, Beattie OB, Bamforth JS. Assessment of soft tissue facial asymmetry in medically normal and syndrome-affected individuals by analysis of landmarks and measurements. Am J Med Genet. 2000;93:143–154. doi: 10.1002/1096-8628(20000717)93:2<143::aid-ajmg12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Smith WM. Hemispheric and facial asymmetry: Faces of Academe. J Cogn Neurosci. 1998;10:663–665. doi: 10.1162/089892998563077. [DOI] [PubMed] [Google Scholar]
- Smith WM. Hemispheric and facial asymmetry: Gender differences. Laterality. 2000;5:251–258. doi: 10.1080/713754376. [DOI] [PubMed] [Google Scholar]
- Stedman JK. Stedman's Medical Dictionary. 28th edn. 2006. http://www.stedmans.com/
- Vig PS, Hewitt AB. Asymmetry of the human facial skeleton. Angle Orthod. 1975;45:125–129. doi: 10.1043/0003-3219(1975)045<0125:AOTHFS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Weinberg SM, Neiswanger K, Richtsmeier JT, et al. Three-dimensional morphometric analysis of craniofacial shape in the unaffected relatives of individuals with nonsyndromic orofacial clefts: A possible marker for genetic susceptibility. Am J Med Genet Part A. 2008;146:409–420. doi: 10.1002/ajmg.a.32177. [DOI] [PubMed] [Google Scholar]
- Zaidel DW, Cohen JA. The face, beauty, and symmetry: perceiving asymmetry in beautiful faces. Int J Neurosci. 2005;115:1165–1173. doi: 10.1080/00207450590914464. [DOI] [PubMed] [Google Scholar]