Abstract
We present reconstructions of petrosal anatomy based on high-resolution X-ray computed tomography scans for the fossil mammal Necrolestes and for the marsupial mole Notoryctes sp. Compared with other mammals, Necrolestes exhibits a mosaic of plesiomorphic and derived characters, but most of the evidence supports its metatherian status. We revised previous descriptions and report on features of phylogenetic or functional significance. Necrolestes exhibits features that support metatherian affinities, such as the presence of a short and lateral prootic canal, and the loss of the stapedial artery in adults. A deep groove at the anterior pole of the promontorium is present in front of the cochlear housing, a variant on the extrabullar pathway of the internal carotid artery. The promontorium is laterally bordered by a large bony projection resembling the eutherian tegmen tympani [De Beer GR (1937) The Development of the Vertebrate Skull, Oxford, Clarendon Press, p. 391]. Posteromedial to the secondary facial foramen and anterolateral to the fenestra vestibuli is a pronounced fossa for the tensor tympani muscle. On the medial part of the pars canalicularis there is a great inflation of the medial side of the caudal tympanic process, a structure of unknown function. The internal acoustic meatus exhibits a broad transverse septum and is bordered laterally by a broad prefacial commissure. The cochleae of Necrolestes and of Notoryctes have fewer spiral turns (1.1 and 1.6, respectively) than most marsupials. The lateral semicircular canal is more expanded than the posterior semicircular canal in Necrolestes but not in Notoryctes. Both Necrolestes and Notoryctes have a second crus commune, i.e. the lateral semicircular canal opens into the ampulla of the posterior semicircular canal. A stylomastoid foramen enclosed anterodorsally by both the pars cochlearis and pars canalicularis is present in Dasyuridae, Dromiciops gliroides and Notoryctes.
Keywords: cochlea, computed tomography, Marsupialia, Notoryctes, periotic, skull
Introduction
Necrolestes patagonensis is an enigmatic fossil mammal from the early Miocene of Argentina. It has eluded confident assignment to a high-level clade since its discovery in the late 19th century (Ameghino, 1891). Much of the skeleton of this fossil is known, but the mosaic of features of Necrolestes, many correlated with fossoriality, do not provide an unequivocal signal for the affinities of this genus (Asher et al. 2007). The presence of a coiled cochlea of the inner ear, a scapular spine, an astragalar neck, and the absence of a septomaxilla are features of Necrolestes that support its status as a therian mammal. However, the molar pattern of Necrolestes is untypical for therians and cannot be linked easily to distinct occlusal patterns present among both marsupial and placental mammals (Asher & Sánchez-Villagra, 2005). Dental material representing a new species of Necrolestes shows an erupting premolar anterior to three more posterior cheek teeth (Goin et al. 2007), supporting a dental formula for the genus of 5.1.3.3/4.1.3.3. The presence of three premolars has been identified as a metatherian synapomorphy (Rougier et al. 1998), although the purported basal metatherian Sinodelphys szalayi described at a later date has four (Luo et al. 2003). Nonetheless, Necrolestes has only three molars, unlike the four reconstructed for the common ancestor of metatherians. Some other features favour metatherian affinities of Necrolestes, for example the lack of a stapedial artery sulcus and the presence of a palatal process of the premaxilla reaching the canine alveolus.
Here, we present reconstructions of petrosal anatomy based on high-resolution X-ray computed tomography (CT) scans and identify yet more of Necrolestes’ novel mosaic of anatomical features, as well as provide evidence that it is indeed more closely related to marsupial than to placental mammals.
The mammalian paired petrosal bones contain the organs of hearing and equilibrium. They provide attachment for the muscles and ligaments of the middle-ear ossicles, and contain grooves, canals, and foramina for cranial blood vessels and nerves (Hyrtl, 1845; van Kampen, 1905; Werner, 1960; Beck et al. 2008). CT scans of fossil and extant organisms permit access to the internal anatomy of the petrosal without conducting invasive sampling. With CT scans, it is possible to reconstruct the osseous labyrinth with its three parts: the cochlea (containing the cochlear duct), the vestibule (containing the utricle and saccule); and the semicircular canals (containing the semicircular ducts).
Given the fossorial adaptations of Necrolestes, we compare its petrosal anatomy to that of the extant marsupial mole Notoryctes, the affinities of which are contested. Many recent molecular studies place Notoryctes as the sister group of Dasyuromorphia (e.g. Amrine-Madsen et al. 2003; Asher et al. 2004; Nilsson et al. 2004). A more recent analysis of molecular data excluding ‘compositional biases’ supports the placement of Notoryctes adjacent to a peramelian-dasyuromorph clade (Phillips et al. 2006). Several morphological characters (see Asher et al. 2004, p. 248) support a dasyuromorphian-Notoryctes association, although this is not the most parsimonious hypothesis based on morphology alone (Horovitz & Sánchez-Villagra, 2003).
Materials and methods
A right petrosal and glenoid region and a left petrosal fragment (YPM-PU 15384, illustrated by Asher et al. 2007, Fig. 5) of Necrolestes were scanned with a microCT scanner in the Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology, Leipzig. A skull of Notoryctes typhlops (UMZC A5.1/5) was scanned using a microCT scanner in the Department of Engineering at the University of Cambridge. Data on stapedial and cochlear ratios are presented for a large sample of marsupials, based on the examination of museum collections. Institutional abbreviations used in the text are AMNH, American Museum of Natural History, New York; CM, Carnegie Museum of Natural History, Pittsburgh; KS, personal collection K. K. Smith, Duke University; MHNH, Muséum national d’Histoire Naturelle, Paris; MRSV, personal collection M. R. Sánchez-Villagra; SMF, Senckenberg Museum, Frankfurt; UMZC, University Museum of Zoology Cambridge; UWZM, University of Wisconsin Zoological Museum; WM, personal collection W. Maier; YPM-PU, Yale Peabody Museum-Princeton University collection.
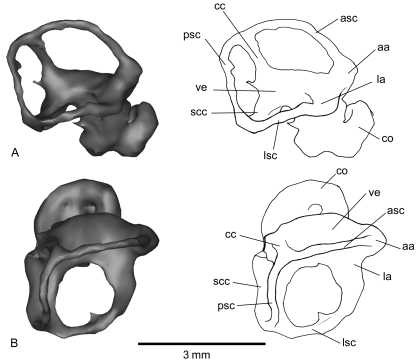
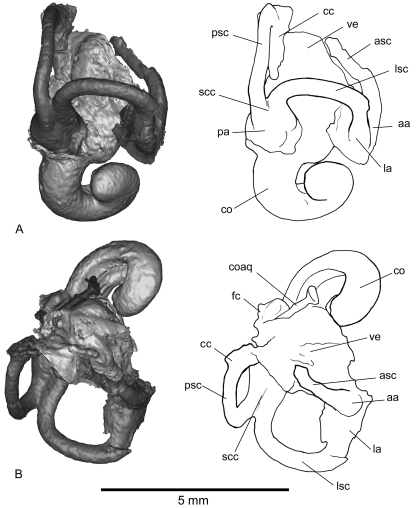
Fig. 5.
Inner-ear reconstruction of the right petrosal of Notoryctes typhlops(UMZC A5) in lateral (A) and dorsal (B) views. aa, anterior ampulla; asc, anterior semicircular canal; cc, crus commune; co, cochlear duct; la, lateral ampulla; lsc, lateral semicircular canal; pa, posterior ampulla; psc, posterior semicircular canal; scc, second crus commune; ve, vestibule.
To process the CT data and produce 3D rendering, we used MIMICS software 64 bits version running on a Dell 690 Windows XP 64 workstation with 16 GB of RAM (Materialise's Interactive Medical Image Control System®, Materialize Inc. NV, Leuven, Belgium; proprietary software at the Museum national d’Histoire Naturelle, Paris). MIMICS is useful to deal with such a large number of datasets generated from X-ray microtomography. Using MIMICS, regions of interest (i.e. petrosal bone and inner ear) can be selected with accuracy using the threshold method to create segmentation masks (e.g. Clack et al. 2003). With this method, selections depend on a range of defined grey values, not on manual outlining operations; 3D models were calculated from segmentation masks and combined through Boolean operations.
Measurements from macerated skulls are in millimetres and were taken with a Wild MMS 235 digital length-measuring system mounted on a stereomicroscope. The stapedial ratio, i.e. length/width of oval window or footplate (Segall, 1970) and the cochlear curvature (West, 1985) were measured.
It is worth stating that the reconstructed endocasts in this paper represent the bony labyrinth, i.e. osseous channels. Connections between canals (as in the second crus communae), refer then to connections between bony canals and not between the soft tissues they contain (as in Schmelzle et al. 2007).
For descriptive purposes, the petrosal is generally divided in two parts: the pars cochlearis, enclosing the cochlear duct and saccule of the inner ear, and the pars canalicularis, housing the utricle and semicircular canals. In the following descriptions we use the terminology of Wible (2003). The reconstruction of the major vessels and nerves associated with the petrosal is based on previous studies on extant mammals (e.g. MacPhee, 1981; Wible, 1990, 2003; Rougier et al. 1992; Wible & Hopson, 1995).
Ear anatomy of Necrolestes
Petrosal anatomy of Necrolestes
The pars cochlearis and the pars canalicularis are separated by a large breakage, which does not allow us to see the exact contours of the two openings lying in the outer border of the promontorium. Nonetheless, this part of the petrosal is better preserved in the isolated broken left petrosal. The rounded fenestra cochleae (Zeller, 1985) is evident posteriorly. Posterolaterally visible is the oval-shaped fenestra vestibuli, which was in life closed by the footplate of the stapes. The stapedial ratio is 1.5.
The promontorium of Necrolestes is nearly smooth, and except for its anterior pole does not exhibit any sulci for either a stapedial artery or a transpromontorial internal carotid artery together with the carotid plexus. These two features indicate an arterial pathway pattern that is typical for metatherian mammals (see Discussion). In metatherians, the internal carotid generally follows an extrabullar pathway, lying medially to the auditory bulla or near the basioccipital-petrosal suture (Wible, 1986). In Necrolestes, a deep groove at the anterior pole of the promontorium is evident in front of the cochlear housing, which may be for the internal carotid artery. However, this is not the equivalent of a transpromontorial course, which is within the middle ear, but a variant on the extrabullar pathway (Rougier & Wible, 2006).
Projecting anteromedially from the promontorium is a flat shelf of bone, similar to the ‘epitympanic wing’ (sensu MacPhee, 1981, p. 22). This feature is present in many therians but is wholly lacking in extinct non-therian mammals (Rougier et al. 1996, 1998).
The promontorium is laterally bordered by a large bony projection – the posterior part of which is missing – which resembles the eutherian tegmen tympani (MacPhee, 1981, p. 64). This bony flange holds a ventrally directed process that resembles the tuberculum tympani described by Wible (2003) for the opossum Monodelphis brevicaudata and which may be homologous to the eutherian tegmen tympani (Kuhn & Zeller, 1987; see also Maier, 1989; Schmelzle, 2003). The tegmen tympani of Necrolestes ventrally covers a deep sulcus, perhaps the sulcus facialis running posterolaterally to the promontorium. The sulcus facialis received the main or hyomandibular branch of the facial nerve that was transmitted by the secondary facial foramen into the middle-ear space. The tympanic opening of the facial canal (what in this context some authors would call the secondary facial foramen in spite of the lack of the osseous floor referred to below) is situated between the tegmen tympani and the promontorium and consists of a large and almost rounded aperture. As in all extant mammals, the facial nerve leaves the cranial cavity via the internal acoustic meatus, runs beneath the prefacial commissure (Wible, 1990), and enters into the cavum supracochleare by the primary facial foramen (Wible, 1990; Wible & Hopson, 1993). The cavum supracochleare, which encloses the geniculate ganglion of the facial nerve (Gaupp, 1908), is not ventrally closed by an osseous floor in Necrolestes. As a matter of fact, the aperture for the hyomandibular branch of the facial nerve is not differentiated from the aperture for the second branch of the facial nerve, the greater petrosal nerve, which is normally transmitted by the hiatus Fallopii in extant mammals. Therefore, and considering the probable breakage of the floor of the cavum, Necrolestes must have exhibited the following pattern of the facial nerve course: the two branches of the facial nerve leave the posterior aspect of the geniculate ganglion and the main branch or hyomandibular ramus of the facial nerve enters the middle-ear space via the tympanic aperture or secondary facial foramen. There it runs posteroventrally in the sulcus facialis and exits the skull by the stylomastoid notch or foramen (see discussion below). The greater petrosal nerve or palatine ramus of the facial nerve exits via the hiatus Fallopii, which was opened on the tympanic side of petrosal and anterior to the secondary facial foramen. The greater petrosal nerve there runs anteroventrally to the posterior opening of the pterygoid canal.
Posteromedial to the secondary facial foramen and anterolateral to the fenestra vestibuli is a pronounced fossa (fossa muscularis major) that was probably for the origin of the tensor tympani muscle.
On the ventral aspect of the tegmen tympani, a deep fossa is bordered laterally and medially by two distinct crests. This fossa, which is not complete as it lacks the posterior part, most likely housed the mallear-incudal articulation and is thus identified as a part of the epitympanic recess. This fossa is lateral to and at a level with the tympanic aperture for the facial canal. Only its anteriormost part is preserved on the tegmen tympani. This fossa is separated from the facial sulcus by a prominent ridge of bone.
Posteromedial to the epitympanic recess is a great excavation that creates a medial accessory cavity of the epitympanic recess (sensu Archer, 1976; MacPhee, 1981), perhaps a mastoid recess formed by pneumatization. This striking feature of the pars canalicularis is well visible in lateral view (Fig. 1C). This accessory cavity is posterolateral to the articulation between malleus and incus, normally housed within the epitympanic recess, but was nevertheless called ‘medial’ by Archer (1976) and MacPhee (1981) for peramelid marsupials and lorises.
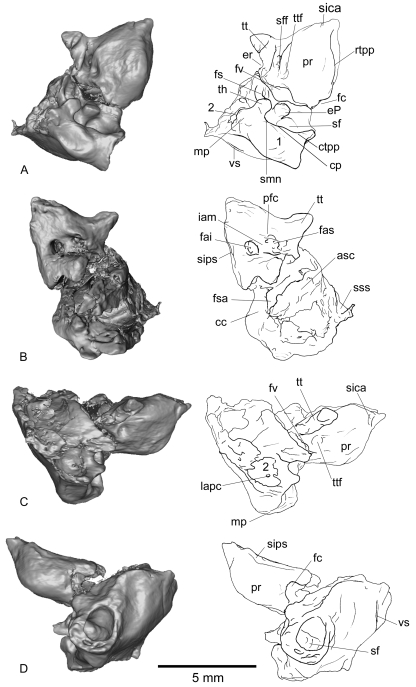
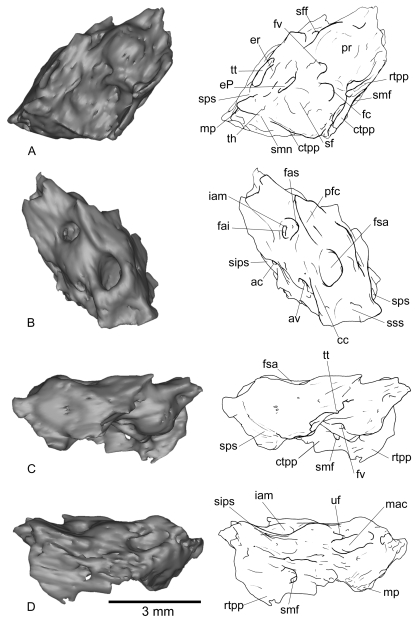
Fig. 1.
Right petrosal of Necrolestes patagonensis (YPM-PU 15384) in ventral (A), dorsal (B), lateral (C), and medial (D) views. cc, crus commune; cp, crista parotica; ctpp, caudal tympanic process of petrosal; eP, element of Paauw; er, epitympanic recess; fai, foramen acousticum inferius; fas, foramen acousticum superius; fc, fenestra cochleae; fs, facial sulcus; fsa, fossa subarcuata; fv, fenestra vestibuli; iam, internal auditory meatus; lapc, lateral aperture of the prootic canal; mp, mastoid process; pfc, prefacial commissure; pr, promontorium; rtpp, rostral tympanic process of petrosal; sf, stapedius fossa; sff, secondary facial foramen; sica, sulcus for the internal carotid artery; sips, sulcus for the inferior petrosal sinus; smn, stylomastoid notch; sss, sulcus for the sigmoid sinus; th, tympanohyal; tt, tegmen tympani; ttf, tensor tympani fossa; vs, vascular sulcus; 1, probable accessory auditory cavity formed by the inflation of the area containing the mastoid and caudal processes and the large excavation and aperture medial to it; 2, accessory cavity of the epitympanic recess (sensu Archer, 1976; MacPhee, 1981).
There is no evidence of a fossa for the crus breve of the incus (fossa incudis). It might have been in the missing part of the tegmen tympani, as it is usually situated on the pars canalicularis posterolateral to the promontorium and secondary facial foramen and posterior to the epitympanic recess.
A salient crista parotica posterolaterally borders the deep sulcus facialis and must have formed the medial wall of the fossa incudis. This crest supports a thick and prominent protuberance, the tympanohyal (the ossified proximal segment of Reichert's cartilage), posterior to which is the stylomastoid notch by which the facial nerve probably left the middle ear.
The stylomastoid notch is bounded medially by a ridge running posteromedially, the caudal tympanic process of petrosal (MacPhee, 1981). The latter extends medially from the stylomastoid notch to the jugular foramen (posterior lacerate foramen of Archer, 1976) showing a slight decrease in height. The medial end of the caudal tympanic process of the petrosal probably contacted the paracondylar process of the exoccipital bone.
Dorsal to the caudal tympanic process there is a deep depression, hidden in ventral view. The narrower medial part of this depression is the postpromontorial tympanic sinus, partially covered by the expansion of the caudal tympanic process; its broader and deeper lateral part is the fossa for the stapedius muscle. According to the size and depth of this fossa, the stapedius muscle must have been greatly developed. A prominent process lies anteromedially to the sulcus facialis and projects anteroventrally from the stapedius fossa. This could be interpreted as the ossified element of Paauw (the os quatrum of De Beer, 1937), this element being an ossification within the tendon of the stapedius muscle (Maier, 1987), although preservation of such a structure would be exceptional. The element of Paauw plays a functional role, concentrating and transmitting forces between the stapedius muscle and the stapes (Sánchez-Villagra et al. 2002). On the medial part of the pars canalicularis (Fig. 1D), just medial to the stapedius fossa and postpromontorial sulcus, a striking structure remains difficult to interpret as it has never been observed by the authors in any therian petrosal. This structure consists in a great inflation of the medial side of the caudal tympanic process that contains a large aperture on the medial side of the petrosal. It may represent a surface area for attachment of the stapedius muscle, part of an accessory auditory chamber (MacPhee, 1981), or facial nerve enclosure. If the last interpretation is right, the facial nerve of Necrolestes would have not exited the middle ear via the stylomastoid notch (as described above) but instead via a stylomastoid foramen (as it is the case of some metatherians and most eutherians). Nonetheless, we should also consider the hypothesis that this part of the petrosal is broken, as this specimen lacks the adjacent exoccipital and paroccipital process. The whole area containing the mastoid and caudal processes and the large excavation and aperture medial to it is clearly inflated and may have played the role of an accessory auditory cavity.
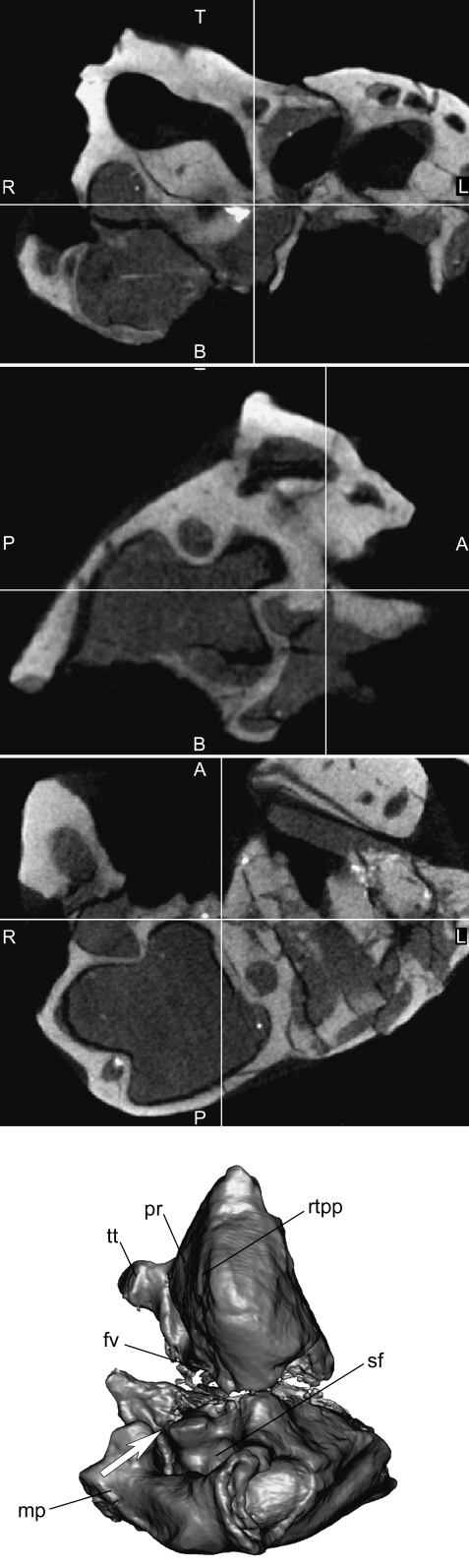
Just anterolateral to the ossified element of Paauw is a small foramen that we interpret as the tympanic opening of the prootic canal (contra Asher et al. 2007). This opening corresponds with a lateral one, contained in the accessory cavity of the epitympanic process, and shows the prootic canal was small and horizontal. The opening is quite small; nevertheless it is clear in our 3D reconstruction based on CT (Fig. 2). A tiny sulcus for the lateral head vein runs from the tympanic aperture of the prootic canal to the stylomastoid notch.
Fig. 2.
3D reconstruction and 2D slices of the left petrosal of Necrolestes patagonensis(YPM-PU 15384). The petrosal is oriented so that the tympanic aperture of the prootic canal is visible. The cross-sections in the slices show the tympanic opening of the prootic canal. A, anterior; B, bottom; L, left; P, posterior; R, right; T, top; fv, fenestra vestibuli; mp, mastoid process; pr, promontorium; rtpp, rostral tympanic process of petrosal; sf, stapedius fossa; tt, tegmen tympani. Not to scale.
The surface of the pars canalicularis posterolateral to the caudal tympanic process of the petrosal is the mastoid exposure, the petrosal surface exposed on the occiput. On its anteriormost part the mastoid exhibits a large transverse process, which is likely to be the mastoid process of petrosal, to which the sternocleidomastoideus muscle was attached. On the posteriormost aspect of the mastoid, two tiny sulci are observable but do not correspond to the trajectories of the diploetic vessels.
The dorsal or endocranial view of the petrosal of Necrolestes is poorly preserved; nonetheless, two dominant features of this view are evident (Fig. 1B). Anteromedially, the internal acoustic meatus for the facial and vestibulocochlear nerves lies on the roof of the pars cochlearis. The extent of the prefacial commissure in Necrolestes was underestimated by Asher et al. (2007, Fig. 5B) based on their emphasis of a left petrosal fragment of YPM-PU 15384 that exposed the region around the internal acoustic meatus. In fact, based on our CT reconstruction from the more complete right side (Fig. 1B), the internal acoustic meatus exhibits a broad transverse septum and is bordered laterally by a quite broad prefacial commissure, broader than that of Monodelphis brevicaudata(Wible, 2003; Fig. 7). The floor of the internal acoustic meatus has a rough depression, the medial and lateral extremities of which are larger than the centre.
Posterodorsally, the three semicircular canals (see the description of the inner ear below) constitute the boundary of the non-preserved fossa subarcuata, which accommodated the paraflocculus of the cerebellum. According to the respective positions of the three semicircular canals and to the depth of the space inside the bone, the fossa subarcuata is likely to have been conical and deep. The posterior semicircular canal joins the anterior semicircular canal at the crus commune that forms the posteromedial rim of the fossa subarcuata. The anterior semicircular canal, which can be evidenced by its preserved dorsolateral part and the anterior ampulla, forms the rim for the orifice into the fossa subarcuata.
On the dorsoposterolateral corner of the pars canalicularis, behind the crus commune and posterior semicircular canal, is the narrow groove for the sigmoid sinus. This sinus is the posterodorsal branch of the transverse sinus (the anteroventral branch being the prootic sinus and sphenoparietal emissary vein) (Dom et al. 1970). There is no clear sulcus to link with the prootic sinus pathway; however, a tiny sulcus on the posterolateral corner of the pars canalicularis is likely to be for the transverse sinus. At this point the transverse sinus bifurcates. Its anteroventral branch is the prootic sinus which must have passed through the foramen just anteroventral to the point of bifurcation of the transverse sinus. Anterior to the sigmoid sinus sulcus there is a sulcus running along the lateral border of the fossa subarcuata. This short, shallow sulcus for the superior petrosal sinus appears to enter a tiny foramen at the anterior rim of the fossa subarcuata.
A broad sulcus running along the dorsomedial edge of the promontorium received the inferior petrosal sinus, which originated at the cavernous sinus around the hypophysis and left the skull via its own foramen or via the jugular foramen (see Wible, 2003).
We were not able to reconstruct the aqueductus vestibuli for passage of the endolymphatic duct. The aqueductus cochleae for passage of the perilymphatic duct is posteromedial to the internal acoustic meatus, but hidden by a bony bar behind the foramen acusticum inferius in dorsal view. The aqueductus cochleae opens into the jugular foramen, which transmitted cranial nerves (presumably the glossopharyngeal, vagus, and accessory nerves as in didelphids; Wible, 2003), and occasionally also a very small branch of the sigmoid sinus to the internal jugular vein. In certain metatherians (e.g. Monodelphis, Didelphis, Dasyurus; Wible, 2003; Pucadelphys; Marshall & de Muizon, 1995) the jugular foramen has a separate foramen for the inferior petrosal sinus anterior to it.
On the ventroposterior part of the pars canalicularis there is a tiny sulcus that runs mediolaterally (Fig. 1D). This sulcus is difficult to identify but it is not for the diploetic vessels, as it has no connection with the pathways of the prootic sinus or sphenoparietal emissary vein.
Inner-ear anatomy of Necrolestes (Fig. 3A–B)
Fig. 3.
Inner-ear reconstruction of the right petrosal of Necrolestes patagonensis (YPM-PU 15384) in lateral (A) and dorsal (B) views. aa, anterior ampulla; asc, anterior semicircular canal; cc, crus commune; co, cochlear duct; coaq, cochlear aqueduct; fc, fenestra cochleae; la, lateral ampulla; lsc, lateral semicircular canal; pa, posterior ampulla; psc, posterior semicircular canal; scc, second crus commune; ve, vestibule.
The cochlea, a broad and hollow tube of uniform diameter, is coiled but with only 1.1 spiral turns. Just posteromedial to the fenestra cochleae is the long, narrow cochlear aqueduct of the perilymphatic duct. The connection between the cochlea and vestibule is at the posteromedial aspect of the pars cochlearis. The vestibule communicates with the cochlea anteriorly and the semicircular canals posteriorly. It is an irregular, oval, central space which is joined distally by the ampullae of the three semicircular canals. The spaces for the saccule and utricle are not clearly differentiated from one another, but the approximate dimensions of the two cavities can be inferred. As in most mammals, the semicircular canals join the utricle through five openings: one for the crus commune, the others for the medial entrance to the lateral semicircular canal and the three ampullae (anterior, lateral, posterior). Thus, the distance between the junctions of the semicircular canals with the vestibule delimits the utricle. The saccule is located at the anteroventral side of the utricle and is more inflated than the utricle.
The anterior ampulla is dorsolateral to the vestibule, the lateral ampulla ventrolateral, and the posterior ampulla ventromedial. The anterior semicircular canal is only preserved on its lateral part. The crus commune is dorsomedial and is only seen via the non-ampullated end of the posterior semicircular canal. The posterior arm of the lateral semicircular canal and the inferior arm of the posterior semicircular canal build a second crus commune (that is, the bony structures forming the endocast, not the unknown soft parts).
Ear anatomy of Notoryctes
The petrosal of Notoryctes has a wing-like rostral tympanic process that covers a large petrosal hypotympanic sinus (Fig. 4A). The rostral tympanic process extends posteriorly and is fused with the caudal tympanic process in a large wing-like petrosal plate. The stylomastoid foramen pierces the petrosal plate (Fig. 4C).
Fig. 4.
Right petrosal of Notoryctes typhlops(UMZC A5) in ventral (A), dorsal (B), lateral (C), and medial (D) views. ac, aqueductus cochleae; av, aqueductus vestibuli; cc, crus commune; ctpp, caudal tympanic process of petrosal; ?eP, probable element of Paauw; er, epitympanic recess; fai, foramen acousticum inferius; fas, foramen acousticum superius; fc, fenestra cochleae; fsa, fossa subarcuata; fv, fenestra vestibuli; iam, internal auditory meatus; mac, medial accessory cavity; mp, mastoid process; pfc, prefacial commissure; pr, promontorium; rtpp, rostral tympanic process of petrosal; sf, stapedius fossa; sff, secondary facial foramen; sips, sulcus for the inferior petrosal sinus; smf, stylomastoid foramen; smn, stylomastoid notch; sps, sulcus for prootic sinus; sss, sulcus for the sigmoid sinus; th, tympanohyal; tt, tegmen tympani; uf, unknown foramen.
The bulla of Notoryctes is mostly formed by the alisphenoid process, which ventrally covers a large alisphenoid hypotympanic sinus.
There is no evidence for a prootic canal in this 3D model of Notoryctes’ petrosal.
In Notoryctes, the facial nerve is enclosed in a canal that pierces the petrosal plate (i.e. the fused rostral and caudal tympanic processes), an aperture for the facial nerve known as the stylomastoid foramen. Wroe (1999) indicated that the morphology of elements surrounding the secondary facial nerve canal and stylomastoid foramen varies considerably among marsupials. He described a secondary facial sulcus oriented ventromedially and that exits the skull via the jugular foramen in derived borhyaenoids (Notogale mitis and Cladosictis patagonica); however, this structure was not recognized by Ladevèze & de Muizon (2007) in these taxa. Among extant didelphids, a stylomastoid foramen is present in Caluromys (Archer, 1976) and is bounded anteriorly by squamosal. A stylomastoid foramen enclosed anterodorsally by both the pars cochlearis and canalicularis is present in all modern Dasyuridae (Archer, 1976; Wroe, 1999), Dromiciops and Notoryctes.
Notoryctes exhibits a relatively large epitympanic recess (Fig. 4A), despite the fact that the malleus and other ear ossicles are not particularly large (cf. Segall, 1970, Fig. 3). The epitympanic recess of Notoryctes is large compared to that of didelphids, but not so large compared to that of peramelids. In peramelids, the epitympanic recess is large and dorsally excavated; this excavation forms the medial accessory cavity of the epitympanic recess (Archer, 1976).
Mason (2001), in his comparisons among middle-ear structures in fossorial mammals, concluded that Notoryctes typhlops has a series of reduced structures in that region and that hearing in this species may be ‘vestigial’. This conclusion was based mostly on the study of ear ossicles. The data presented here are not a test of Mason's hypothesis; however, possible specializations such as the relatively large epitympanic recess and stapedius fossa do not appear to be consistent with it.
The stapedial ratio in Notoryctes is similar to that of Necrolestes (1.57 for AMNH-15015). The internal carotid artery has an extrabullar course, as in all extant marsupials, and, contrary to Necrolestes, does not leave any sulcus on the anteriormost part of the promontorium.
Regarding the ossicular muscles, Notoryctes lacks noticeable scars for the tensor tympani muscle on the pars cochlearis of the petrosal, very much in contrast to the condition in Necrolestes. However, as in Necrolestes, Notoryctes exhibits a very large fossa for the stapedius muscle (Fig. 4A). As in Necrolestes(Fig. 1B), the dorsal view of the petrosal in Notoryctes is marked by a conical and deep fossa subarcuata (Fig. 4B) and a broad prefacial commissure of the internal acoustic meatus.
The cochlea of Notoryctes has fewer spiral turns (1.6) than most marsupials, but resembles Necrolestes in this regard. It is noteworthy that the lateral semicircular canal is more expanded than the posterior semicircular canal in Necrolestes but not in Notoryctes.
In his description of the Notoryctes inner ear, Gray (1908) identified a peculiar junction between the lateral and posterior semicircular canals at the point at which the latter passes under the former. This condition does not correspond to the formation of a bony second crus commune, as is present in Notoryctes(Fig. 5). According to Gray, the junction between the lateral and posterior semicircular canal at the point where the posterior semicircular canal passes under the lateral semicircular canal is very rare for mammals but does occur in true moles and many birds. We could not confirm this feature on the reconstructed inner ear of Notoryctes because of preservational biases.
Discussion
Use of a non-invasive technique on a unique fossil has provided us with new anatomical data which reveal a singular mosaic of features. A similar technique reveals several features of the ear anatomy of the marsupial mole which together with additional comparisons with other mammals provide the functional and phylogenetic information discussed below.
Middle-ear musculature
The apparent lack of the tensor tympani muscle in Notoryctes sp. is surprising and merits investigation based on histological investigation of soft tissues. The presence of both tensor tympani muscle and stapedius muscle in Marsupialia seems to be universal judging from the breadth of clades represented by the species so far studied, all of which have these muscles. They include koala, wombat, several macropodids and possums (Aplin, 1990; Sánchez-Villagra, 1998), several didelphids (Maier, 1987; Smith, 1994; Sánchez-Villagra, 1998; Schmelzle, 2003), Perameles sp., Dromicios gliroides, Sminthopsis sp. (Sánchez-Villagra, 1998) and, as reconstructed here, Necrolestes patagonensis.
The tensor tympani and stapedius muscles reportedly affect the frequency response of the middle ear by decreasing sensitivity to low-frequency sounds and increasing sensitivity to high-frequency sounds (Fleischer, 1978). Among placentals, one of the middle-ear specializations recorded in some fossorial groups is the reduction of middle-ear muscles. The lack of a tensor tympani muscle is reported for all adult golden moles examined to date (Simonetta, 1957; Burda et al. 1992; von Mayer et al. 1995; Mason, 2003) and in some strictly fossorial talpid moles (Mason, 2006). Some less fossorial talpid moles, including Neurotrichus, Parascalops, Condylura, which exhibit ‘some above ground activity’ (Mason, 2006), have a weakly developed tensor tympani muscle. However, the reduction or loss of the tensor tympani muscle is not restricted to fossorial species. Tree shrews (Zeller, 1986), the pangolin Manis (Fleischer, 1978) the pika Ochotona pusilla (Maier, 2008) and elephants (Fischer & Tassy, 1993; Court, 1994) lack a tensor tympani muscle. Maier (2008, p. 341) suggested that in the case of tree shrews, the reduction of the tensor tympani muscle is correlated to space and topological restrictions resulting from a ventral lamella of the tegmen tympani which covers the anterior portion of the stapedial artery in these animals. It appears likely then that functional aspects (e.g. optimal hearing for some frequencies) are not the only factors related to the development of middle-ear muscles.
In contrast to some fossorial eutherians, the fossa for the stapedius muscle is very large relative to skull size in both Necrolestes and Notoryctes, indicating that the stapedius muscle was large. In addition, an ossified element of Paauw characterizes these species. The stapedius muscle is present but relatively small in talpid moles (Mason, 2006). von Mayer et al. (1995) described the stapedius muscle as a ligament (containing no muscle fibers) in several golden moles (see also Mason, 2003). The loss of the stapedius muscle also occurs in monotremes (Fleischer, 1978) and in many heteromyids, but is otherwise generally present among rodents (Ruf et al. in press).
Cochlea and semicircular canals
The cochleae of both Necrolestes and Notoryctes have few spiral turns (1.1 and 1.6, respectively). Hedgehogs, sea cows, and wombats are among the only extant therians that also show (presumably) reduced cochlear coiling (i.e. ‘as few as one and a half’, Gray, 1908; Sánchez-Villagra & Schmelzle, 2007). Meng & Fox (1995) reported one and a half turns of the cochlea of basal metatherian petrosals from the Late Cretaceous of Montana. Wible et al. (2001) reported only one turn (360°) for the early Cretaceous eutherian Prokennalestes. The late Cretaceous eutherians ‘Zhelestidae’ exhibit a cochlea coiled at least 270° (but which most likely completed nearly one full 360° turn, Ekdale et al. 2004). The cochlear features reported here for Necrolestes (little coiled, broad) could have evolved convergently and do not represent necessarily the retention of the therian plesiomorphic condition.
The posterior arm of the lateral semicircular canal and the inferior arm of the posterior semicircular canal build a bony second crus commune. Among metatherians, this condition is found in Caluromys, Monodelphis, Dasyurus, Isoodon, Herpetotherium from the Oligocene of Wyoming (Sánchez-Villagra et al. 2007), and in a palaeothentid caenolestoid from the early Miocene of Patagonia (Sánchez-Villagra & Schmelzle, 2007).
Metatherian affinities of Necrolestes
Necrolestes has been identified as an undoubted therian (see Asher et al. 2007) and its metatherian allocation is consistent with ear region features reported here such as the presence of a reduced prootic canal between petrosal and squamosal; the location of the inferior petrosal sinus between petrosal, basisphenoid, and basioccipital; the extrabullar location of the internal carotid artery, the loss of the stapedial artery in adults, and the presence of a caudal tympanic process of petrosal. However, Necrolestes also exhibits characters that are not metatherian-like, such as the non-inflected mandibular angle, although this feature has been secondarily lost in several marsupials (Sánchez-Villagra & Smith, 1997) and is absent in the oldest metatherian, Sinodelphys szalayi (Luo et al. 2003). Asher et al. (2007) pointed out other evidence for a metatherian identification, such as a transverse canal foramina anteromedial to the carotid foramina and five upper incisors. Asher et al. (2007) also inferred from the presence of radial enamel, a coiled inner-ear cochlea, and a scapular spine (but see Luo et al. 2002) that Necrolestes is a therian mammal.
In extant adult marsupials, the development of the sphenoparietal emissary vein causes a reduction of the tympanic portion of the lateral head vein (Wible, 1990). The lateral head vein and the prootic canal are retained in adult monotremes (Wible & Hopson, 1995), the eutherian Prokennalestes (Wible et al. 2001) and ‘zhelestid’ eutherians (Ekdale et al. 2004), the metatherians Deltatheridium, Didelphodon, Pediomys (Rougier et al. 1998; Wible et al. 2001), Pucadelphys, and Andinodelphys (Marshall & Muizon, 1995; Ladevèze & Muizon, 2007), some extant marsupials (didelphids, some caenolestids, peramelinans, and dasyuromorphians (Wible, 1990; Sánchez-Villagra & Wible, 2002), and, we now report, Necrolestes(contra Asher et al. 2007). Among extant mammals, monotremes have a long, vertical prootic canal that transmits the prootic sinus from the cranial cavity to the middle ear where it joins the lateral head vein, and some metatherians (i.e. didelphids, some caenolestids, peramelinans, dasyuromorphians, and here Necrolestes) have a short, horizontal prootic canal that encloses the lateral head vein (Wible & Hopson, 1995). Prokennalestes and ‘zhelestid’ eutherians are the only eutherians reported to possess a prootic canal (Wible et al. 2001; Ekdale et al. 2004).
In Necrolestes, the posterodorsal branch of the transverse sinus, the sigmoid sinus, occupies a narrow sulcus, posteromedial to the fossa subarcuata, which is likely to have ended near the aqueductus vestibuli, thus suggesting the sigmoid sinus exited via the foramen magnum, as in monotremes (Hochstetter, 1896), Prokennalestes (Wible et al. 2001), and all adult marsupials (Dom et al. 1970; Archer, 1976; Wible, 1990; Wible et al. 2001).
The reconstructed position of the internal carotid artery described for Necrolestes was first observed by Muizon et al. (1997) in borhyaenids and in Pucadelphys, Andinodelphys and Mayulestes from Tiupampa, and is also found in some isolated petrosals from Itaboraí (Ladevèze, 2004, 2007).
The stapedial ratio of Necrolestes (1.5) is comparable to that of didelphid marsupials and Notoryctes (Horovitz et al. 2008). Monotremes and most metatherians have a slightly oval footplate (stapedial ratio less than 1.8), whereas it tends to be more elliptical in eutherians (stapedial ratio higher than 1.8), much less so in Prokennalestes (1.71; Wible et al. 2001) and ‘Zhelestidae’ (1.6; Ekdale et al. 2004), the only eutherians having a ratio lower than 1.8. The placental mole Talpa europea has a stapedial ratio of 1.8 (Segall, 1970).
Conclusion
The mosaic of characters in the ear anatomy of Necrolestes patagonensis is a further example of the anatomical singularity of this fossorial mammal. Necrolestes exhibits diagnostic characters for Metatheria, such as the presence of a short and vertical prootic canal between petrosal and squamosal, the location of the inferior petrosal sinus between petrosal, basisphenoid and basioccipital, the loss of stapedial artery in adults, and the presence of a caudal tympanic process of petrosal. Other metatherian features are the presence of transverse canal foramina, and the palatal process that reaches the canine alveolus. But the reported dental formula is more consistent with eutherian affinities. The comparison of Necrolestes with the extant marsupial mole Notoryctes highlighted some resemblances and differences. Both have a large stapedial fossa for the stapedius muscle, which is not the case in most fossorial eutherians where the stapedius muscle is weak or absent. The cochlea of both Necrolestes and Notoryctes is little coiled and broad. It would be worth looking for the significance of this plesiomorphic trait for therians, also found in hedgehogs, sea cows, and the wombat. Necrolestes differs from Notoryctes in having a prootic canal (lost in australidelphian marsupials) and a less developed rostral tympanic process of petrosal (forming a petrosal plate in Notoryctes). The phylogenetic position of Notoryctes among Metatheria is contested (Amrine-Madsen et al. 2003; Asher et al. 2004; Nilsson et al. 2004; Phillips et al. 2006) but some specialized features from the ear region (e.g. rostral and caudal tympanic processes fused in a petrosal plate, presence of a stylomastoid foramen) may link it to Dasyuromorphia. The phylogenetic position of Necrolestes remains unresolved, perhaps because of the singular ecomorphological specializations of its lineage during the splendid isolation that the South American biota experienced for much of the Cenozoic era.
Acknowledgments
We thank the UMR 5143 of the MNHN (Paris) for access to MIMICS software, G. Clement, D. Geffard, F. Goussard and A. Pradel for technical help with that software, D. Brinkmann, J. Gauthier, W. Joyce and M. Fox for access to material of Necrolestes, T. Smith, M. Skinner, and J.-J. Hublin for CT scanning in Leipzig, A. Heaver for CT scanning in Cambridge, W. Leis (Fachhochschule Aalen) for CT scanning in Aalen, R. Beck (Sydney) for discussions, and W. Maier (Tübingen) and an anonymous reviewer for useful suggestions that helped to improve the manuscript greatly.
References
- Ameghino F. Nuevos restos de mamíferos fósiles descubiertos por Carlos Ameghino en el Eoceno inferior de la Patagonia austral. Especies nuevas adiciones y correcciones. Rev Argent Hist Nat. 1891;1:289–328. [Google Scholar]
- Amrine-Madsen H, Scally M, Westerman M, Stanhope MJ, Krajewski C, Springer MS. Nuclear gene sequences provide evidence for the monophyly of australidelphian marsupials. Mol Phylogenet Evol. 2003;28:186–196. doi: 10.1016/s1055-7903(03)00122-2. [DOI] [PubMed] [Google Scholar]
- Aplin KP. Basicranial regions of diprotodontian marsupials: anatomy, ontogeny and phylogeny. Sydney: University of New South Wales; 1990. PhD Thesis. [Google Scholar]
- Archer M. The basicranial region of marsupicarnivores (Marsupialia), interrelationships of carnivorous mammals, and affinities of the insectivorous marsupial peramelids. Zool J Linn Soc. 1976;59:217–322. [Google Scholar]
- Asher RJ, Sánchez-Villagra MR. Locking yourself out: diversity among dentally zalambdodont therian mammals. J Mammal Evol. 2005;12:267–284. [Google Scholar]
- Asher RJ, Horovitz I, Sánchez-Villagra MR. First combined cladistic analysis of marsupial mammal interrelationships. Mol Phylogenet Evol. 2004;33:240–250. doi: 10.1016/j.ympev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Asher RJ, Horovitz I, Martin T, Sánchez-Villagra MR. Neither a rodent nor a platypus: a reexamination of Necrolestes patagonensis Ameghino. Am Mus Novit. 2007;3546:1–40. [Google Scholar]
- Beck RMD, Godthelp H, Weisbecker V, Archer M, Hand SJ. Australia's oldest marsupial fossils and their biogeographical implications. PLoS ONE. 2008;3(3):e1858. doi: 10.1371/journal.pone.0001858. doi: 10.1371/journal.pone.0001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda H, Bruns V, Hickman GC. The ear in subterranean Insectivora and Rodentia in comparison with ground-dwelling representatives. 1. Sound conducting system of the middle ear. J Morphol. 1992;214:49–61. doi: 10.1002/jmor.1052140104. [DOI] [PubMed] [Google Scholar]
- Clack JA, Ahlberg PE, Finney SM, Dominguez-Alonso P, Robinson J, Ketcham RA. A uniquely specialized ear in a very early tetrapod. Nature. 2003;425:65–69. doi: 10.1038/nature01904. [DOI] [PubMed] [Google Scholar]
- Court N. The periotic of Moeritherium(Mammalia, Proboscidea): homology or homoplasy in the ear region of Tethytheria McKenna, 1975? Zool J Linn Soc. 1994;112:13–28. [Google Scholar]
- De Beer GR. The Development of the Vertebrate Skull. Oxford: Clarendon Press; 1937. [Google Scholar]
- Dom R, Fisher BL, Martin GF. The venous system of the head and neck of the opossum (Didelphis virginiana) J Morphol. 1970;132:487–496. doi: 10.1002/jmor.1051320408. [DOI] [PubMed] [Google Scholar]
- Ekdale EG, Archibald JD, Averianov AO. Petrosal bones of placental mammals from the Late Cretaceous of Uzbekistan. Acta Palaeontol Pol. 2004;49:161–176. [Google Scholar]
- Fischer M, Tassy P. The interrelations between Proboscidea-Sirenia-Hyracoidea-Mesaxonia. In: Szalay FS, Novacek MJ, McKenna MC, editors. Mammal Phylogeny. New York: Springer-Verlag; 1993. pp. 217–234. [Google Scholar]
- Fleischer G. Evolutionary principles of the mammalian middle ear. Ergeb Anat Entwicklungsgesch (Adv Anat Embryol Cell Biol) 1978;55:1–70. doi: 10.1007/978-3-642-67143-2. [DOI] [PubMed] [Google Scholar]
- Gaupp E. Zur Entwicklungsgeschichte und vergleichenden Morphologie des Schädels von Echidna aculeata var. typica. Semon's Zool Forsch Australien. Denkschr Medicinischnaturwiss Ges Jena. 1908;6:539–788. [Google Scholar]
- Goin FJ, Abello A, Bellosi E, Kay RF, Madden R, Carlini AA. Los metatheria sudamericanos de comienzos del Neógeno (Mioceno temprano, Edad-mamífero Colhuehuapense). Parte I. Introducción, Didelphimorphia y Sparassodonta. Ameghiniana. 2007;44:29–71. [Google Scholar]
- Gray AA. The Labyrinth of Animals Including Mammals, Birds, Reptiles and Amphibians. London: J. & A. Churchill; 1908. [Google Scholar]
- Hochstetter F. Beitraïge zur Anatomie und Entwickelungsgeschichte des Blutgefaïsssystems der Monotremen. Semon's Zoologische Forschungsreisen in Australien. 1896;5:189–243. [Google Scholar]
- Horovitz I, Sánchez-Villagra MR. A morphological analysis of marsupial mammal higher-level phylogenetic relationships. Cladistics. 2003;19:181–212. [Google Scholar]
- Horovitz I, Ladevèze S, Argot C, et al. The anatomy of Herpetotherium fugax Cope 1873, a metatherian from the Oligocene of North America. Palaeontographica. 2008;284:109–141. [Google Scholar]
- Hyrtl J. Vergleichend-anatomische Untersuchungen über das innere Gehörorgan des Menschen und der Säugethiere. Prague: Friedrich Ehrlich.; 1845. [Google Scholar]
- van Kampen PN. Die Tympanalgegend des Säugetierschädels. Gegenbaurs Morphol Jahrb. 1905;34:321–722. [Google Scholar]
- Kuhn H-J, Zeller U. The cavum epiptericum in monotremes and therian mammals. In: Kuhn H-J, Zeller U, editors. Morphogenesis of the Mammalian Skull. Hamburg: Verlag Paul Varey; 1987. pp. 51–70. [Google Scholar]
- Ladevèze S. Metatherian petrosals from the Late Paleocene of Itaboraí (Brazil), and their phylogenetic implications. J Vertebr Paleontol. 2004;24:202–213. [Google Scholar]
- Ladevèze S. Petrosal bones of metatherian mammals from the Late Paleocene of Itaboraí (Brazil), and a cladistic analysis of petrosal features in metatherians. Zool J Linn Soc. 2007;150:85–115. [Google Scholar]
- Ladevèze S, Muizon C de. The auditory region of early Paleocene Pucadelphydae (Mammalia, Metatheria) from Tiupampa, Bolivia, with phylogenetic implications. Palaeontology. 2007;50:1123–1154. [Google Scholar]
- Luo Z-X, Kielan-Jaworowska Z, Cifelli RL. In quest for a phylogeny of Mesozoic mammals. Acta Palaeontol Pol. 2002;47:1–78. [Google Scholar]
- Luo Z-X, Ji Q, Wible JR, Yuan C-X. An early Cretaceous tribosphenic mammal and metatherian evolution. Science. 2003;302:1934–1940. doi: 10.1126/science.1090718. [DOI] [PubMed] [Google Scholar]
- MacPhee RDE. Auditory regions of primates and eutherian insectivores: morphology, ontogeny, and character analysis. Contrib Primatol. 1981;18:1–282. [Google Scholar]
- Maier W. Der Processus angularis bei Monodelphis domestica(Didelphidae; Marsupialia) und seine Beziehungen zum Mittelohr: Eine ontogenetische und evolutionsmorphologische Untersuchung. Gegenbaurs Morphol Jahrb. 1987;133:123–161. [PubMed] [Google Scholar]
- Maier W. Morphologische Untersuchungen am Mittelohr der Marsupialia. Z Zool Sys Evolutionsforsch. 1989;27:149–168. [Google Scholar]
- Maier W. Epitensoric position of the Chorda tympani in Anthropoidea: a new synapomorphic character, with remarks on the Fissura Glaseri in Primates. In: Sargis EJ, Dagosto M, editors. Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Dordrecht: Springer; 2008. pp. 339–352. [Google Scholar]
- Marshall LG, Muizon C de. Part II. The skull. In: Muizon C de., editor. Pucadelphys Andinus (Marsupialia, Mammalia) from the Early Paleocene of Bolivia. Paris: Mémoires du Muséum national d’Histoire naturelle; 1995. pp. 21–90. [Google Scholar]
- Mason MJ. Middle ear structures in fossorial mammals: a comparison with non-fossorial species. J Zool Lond. 2001;255:457–486. [Google Scholar]
- Mason MJ. Morphology of the middle ear of golden moles (Chrysochloridae) J Zool Lond. 2003;260:391–403. [Google Scholar]
- Mason MJ. Evolution of the middle ear apparatus in talpid moles. J Morphol. 2006;267:678–695. doi: 10.1002/jmor.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mayer A, O’Brien G, Sarmiento EE. Functional and systematic implications of the ear in golden moles (Chrysochloridae) J Zool Lond. 1995;236:417–430. [Google Scholar]
- Meng J, Fox RC. Osseous inner ear structures and hearing in early marsupials and placentals. Zool J Linn Soc. 1995;115:47–71. [Google Scholar]
- Muizon C de, Cifelli RL, Céspedes Paz R. The origin of the dog-like borhyaenoid marsupials of South America. Nature. 1997;389:486–489. doi: 10.1038/39029. [DOI] [PubMed] [Google Scholar]
- Nilsson MA, Arnason U, Spencer PBS, Janke A. Marsupial relationships and a timeline for marsupial radiation in South Gondwana. Gene. 2004;340:189–196. doi: 10.1016/j.gene.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, MacLenachan PA, Down C, Gibb GC, Penny D. Combined mitochondrial and nuclear protein-coding DNA sequences resolve the interrelations of the major Australasian marsupial radiations. Syst Biol. 2006;55:122–137. doi: 10.1080/10635150500481614. [DOI] [PubMed] [Google Scholar]
- Rougier GW, Wible JR. Major changes in the ear region and basicranium of early mammals. In: Carrano M, Gaudin TJ, Blob R, Wible JR, editors. Amniote Paleobiology: Phylogenetic and Functional Perspectives on the Evolution of Mammals, Birds and Reptiles. Chicago: University of Chicago Press; 2006. pp. 269–311. [Google Scholar]
- Rougier GW, Wible JR, Hopson JA. Reconstruction of the cranial vessels in the Early Cretaceous mammal Vincelestes neuquenianus: implications for the evolution of the mammalian cranial vascular system. J Vertebr Paleontol. 1992;12:188–216. [Google Scholar]
- Rougier GW, Wible JR, Hopson JA. Basicranial anatomy of Priacodon fruitaensis(Triconodontidae, Mammalia) from the Late Jurassic of Colorado, and a reappraisal of mammaliaform interrelationships. Am Mus Novit. 1996;3183:1–38. [Google Scholar]
- Rougier GW, Wible JR, Novacek MJ. Implications of Deltatheridium specimens for early marsupial history. Nature. 1998;396:459–463. doi: 10.1038/24856. [DOI] [PubMed] [Google Scholar]
- Ruf I, Frahnert S, Maier W. The chorda tympani and its significance for rodent phylogeny. Mammal Biol. DOI: 10.1016/j.mambio.2008.01.002. in press. [Google Scholar]
- Sánchez-Villagra MR. Patterns of morphological change in the ontogeny and phylogeny of the marsupial skull. Durham: Duke University; 1998. Doctoral Thesis. [Google Scholar]
- Sánchez-Villagra MR, Gemballa S, Nummela S, Smith KK, Maier W. Ontogenetic and phylogenetic transformations of the ear ossicles in marsupial mammals. J Morphol. 2002;251:219–238. doi: 10.1002/jmor.1085. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Ladevèze S, Horovitz I, et al. Exceptionally preserved North American Paleogene metatherians: adaptations and discovery of a major gap in the opossum fossil record. Proc R Soc, Biol Lett. 2007;3:318–322. doi: 10.1098/rsbl.2007.0090. DOI: 10.1098/rsbl.2007.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Schmelzle T. Anatomy and development of the bony inner ear in the woolly opossum, Caluromys philander(Didelphimorphia, Marsupialia) Mastozool Neotrop. 2007;14:53–60. [Google Scholar]
- Sánchez-Villagra MR, Smith KK. Diversity and evolution of the marsupial mandibular angular process. J Mammal Evol. 1997;4:119–144. [Google Scholar]
- Sánchez-Villagra MR, Wible JR. Patterns of evolutionary transformation in the petrosal bone and some basicranial features in marsupial mammals, with special reference to didelphids. J Zool Syst Evol Res. 2002;40:26–45. [Google Scholar]
- Schmelzle T. Ontogenetische Untersuchungen an der Ohrregion von Macropus eugenii (DESMAREST, 1817) (Macropodida; Marsupialia) Universität Tübingen; 2003. Diplomarbeit. [Google Scholar]
- Schmelzle T, Sánchez-Villagra MR, Maier W. Vestibular labyrinth evolution in diprotodontian marsupial mammals. Mammal Study. 2007;32:83–97. [Google Scholar]
- Segall W. Morphological parallelisms of the bulla and auditory ossicles in some insectivores and marsupials. Fieldiana: Zool. 1970;51:169–205. [Google Scholar]
- Simonetta A. Anatomia e significato morfologico e sistematico dell’orecchio medio e delle strutture ad esso connesse in alcuni insettivori (Suncus, Talpa, Chrysochloris) Arch Ital Anat Embriol. 1957;62:55–94. [PubMed] [Google Scholar]
- Smith KK. Development of the craniofacial musculature in Monodelphis domestica(Marsupialia, Didelphidae) J Morphol. 1994;222:149–173. doi: 10.1002/jmor.1052220204. [DOI] [PubMed] [Google Scholar]
- Werner CF. Das Gehörorgan der Wirbeltiere und des Menschen. Leipzig: Thieme; 1960. [Google Scholar]
- West CD. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am. 1985;77:1091–1101. doi: 10.1121/1.392227. [DOI] [PubMed] [Google Scholar]
- Wible JR. Transformations in the extracranial course of the internal carotid artery in mammalian phylogeny. J Vertebr Paleontol. 1986;6:313–325. [Google Scholar]
- Wible JR. Petrosals of Late Cretaceous marsupials from North America, and a cladistic analysis of the petrosal in therian mammals. J Vertebr Paleontol. 1990;10:183–205. [Google Scholar]
- Wible JR. On the cranial osteology of the short-tailed opossum Monodelphis brevicaudata(Didelphidae, Marsupialia) Ann Carnegie Mus. 2003;72:137–202. [Google Scholar]
- Wible JR, Hopson JA. Basicranial evidence for early mammal phylogeny. In: Szalay FS, Novacek MJ, McKenna MC, editors. Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials. New York: Springer-Verlag; 1993. pp. 45–62. [Google Scholar]
- Wible JR, Hopson JA. Homologies of the prootic canal in mammals and non-mammalian cynodonts. J Vertebr Paleontol. 1995;15:331–356. [Google Scholar]
- Wible JR, Rougier GW, Novacek MJ, McKenna MC. Earliest eutherian ear region: a petrosal referred to Prokennalestes from the Early Cretaceous of Mongolia. Am Mus Novit. 2001;3322:1–44. [Google Scholar]
- Wroe S. The geologically oldest dasyurid, from the Miocene of Riversleigh, north-west Queensland. Palaeontology. 1999;42:501–527. [Google Scholar]
- Zeller U. Die Ontogenese und Morphologie der Fenestra rotunda und des Aquaeductus cochleae von Tupaia und anderen Säugern. Gegenbaurs Morphol Jahrb. 1985;131:179–204. [PubMed] [Google Scholar]
- Zeller U. Ontogeny and cranial morphology of the tympanic region of the Tupaiidae, with special reference to Ptilocercus. Folia Primatol(Basel) 1986;47:61–80. doi: 10.1159/000156266. [DOI] [PubMed] [Google Scholar]