Abstract
The attachment of the Achilles tendon is part of an ‘enthesis organ’ that reduces stress concentration at the hard–soft tissue interface. The organ also includes opposing sesamoid and periosteal fibrocartilages, a bursa and Kager's fat pad. In addition, the deep crural and plantar fasciae contribute to Achilles stress dissipation and could also be regarded as components. Here we describe the sequence in which these various tissues differentiate. Serial sections of feet from spontaneously aborted foetuses (crown rump lengths 22–322 mm) were examined. All slides formed part of an existing collection of histologically sectioned embryological material, obtained under Spanish law and housed in the Universidad Complutense, Madrid. From the earliest stages, it was evident that the Achilles tendon and plantar fascia had a mutual attachment to the calcaneal perichondrium. The first components of the enthesis organ to appear (in the 45-mm foetus) were the retrocalcaneal bursa and the crural fascia. The former developed by cavitation within the mesenchyme that later gave rise to Kager's fat pad. The tip of the putative fat pad protruded into the developing bursa in the 110-mm foetus and fully differentiated adipocytes were apparent in the 17-mm foetus. All three fibrocartilages were first recognisable in the 332-mm foetus – at which time adipogenesis had commenced in the heel fat pad. The sequence in which the various elements became apparent suggests that bursal formation and the appearance of the crural fascia may be necessary to facilitate the foot movements that subsequently lead to fibrocartilage differentiation. The later commencement of adipogenesis in the heel than in Kager's pad probably reflects the non-weight environment in utero. The direct continuity between plantar fascia and Achilles tendon that is characteristic of the adult reflects the initial attachment of both structures to the calcaneal perichondrium rather than to the skeletal anlagen itself.
Keywords: calcaneal tendon, fat pads, fibrocartilage, osteotendinous junction, plantar fascia
Introduction
The site at which a tendon, ligament or joint capsule attaches to bone is known as an enthesis. It is often part of a collection of tissues jointly making up an ‘enthesis organ’, which reduces stress concentration at the hard–soft tissue interface (Benjamin & McGonagle, 2001; Benjamin et al. 2004). The archetypal enthesis organ in man is that of the Achilles tendon. Here, in addition to the enthesis itself, there is a ‘sesamoid fibrocartilage’ in the deep surface of the tendon, immediately adjacent to the attachment site, and a complementary ‘periosteal fibrocartilage’ on the posterior aspect of the calcaneus (Benjamin & McGonagle, 2001; Benjamin et al. 2004). These may be regarded as ancillary fibrocartilages that are adaptations allowing the tendon to be compressed against the calcaneus, as the superior tuberosity serves as a pulley during foot movements. The sesamoid and periosteal fibrocartilages form the walls of the distal part of the retrocalcaneal bursa. Protruding into this bursa is a synovial-lined wedge of adipose tissue – the tip of Kager's (retromalleolar) fat pad. This has a wide variety of functions that have been considered previously (Theobald et al. 2006; Shaw et al. 2007). Briefly, the fat pad is thought to facilitate the movement of tendon relative to bone, prevent tendon–bone adhesion, aid in the spread of bursal fluid and act as a space filler and immune organ (Theobald et al. 2006; Shaw et al. 2007). Although the enthesis itself, the periosteal and sesamoid fibrocartilages, the retrocalcaneal bursa and Kager's fat pad constitute the components of the Achilles enthesis organ as it was originally defined, the deep crural fascia in the lower part of the leg has subsequently been added to the list, because of its role as a retinaculum, limiting bowstringing of the tendon as the foot is plantarflexed (Benjamin et al. 2007).
Although it is known that the periosteal and sesamoid fibrocartilages develop postnatally in the rat (Rufai et al. 1992), we do not know the sequence in which the different components of the human enthesis organ differentiate. Neither do we know the relationship between the development of the Achilles tendon enthesis organ and that of the plantar fascia. As several authors have commented on the continuity of these two structures (Wood Jones, 1949; Snow et al. 1995; Milz et al. 2002), there is an argument for including part of the plantar fascia as a component of the Achilles enthesis organ, as it may aid in stress dissipation in the Achilles region. The purpose of the present paper is to address these issues by examining a rare collection of histological sections of embryonic feet that are housed in the Department of Human Anatomy and Embryology at the Universidad Complutense in Madrid.
Materials and methods
Routine histology
The material examined in this study is part of a long-established collection of histologically sectioned, human embryonic material housed in the Department of Human Anatomy and Embryology at the Universidad Complutense, Madrid. All tissue was donated to the University with parental consent following spontaneous miscarriage and in accordance with Spanish law. A complete set of serial sections from foetuses of 17 different crown rump lengths were available for study. The crown rump lengths (with corresponding gestational ages given in brackets – based on Patten (1968)) were 21 mm (51 days), 22 mm (52 days), 24 mm (54 days); 32 mm and 34 mm (8 weeks), 37 mm, 41 mm and 42 mm (9 weeks), 45 mm (9.5 weeks), 53 mm and 57 mm (10.5 weeks), 88 mm and 90 mm (13 weeks), 110 mm (14.5 weeks), 116 mm and 117 mm (19.5 weeks) and 332 mm (32.5 weeks). All the sections had been stained with Hansen's hematoxylin, VOF stain (light green – orange G – acid fuchsin), Azan, and Bielchowsky's silver stain. All sections had been cut in the sagittal plane, except for the 322-mm fetus, where the sections had been cut coronally.
MRI
With Institutional Review Board approval, magnetic resonance imaging (MRI) was performed at 3T in six normal adult volunteers (two females, four males; ages 22–63 years) with a circular receiver coil. Non fat suppressed T1-weighted spin echo sequences were obtained in the sagittal and axial planes, with a slice thickness of 3 mm, field of view 12 cm and 512 × 512 reconstruction matrix.
Results
The adult enthesis organ
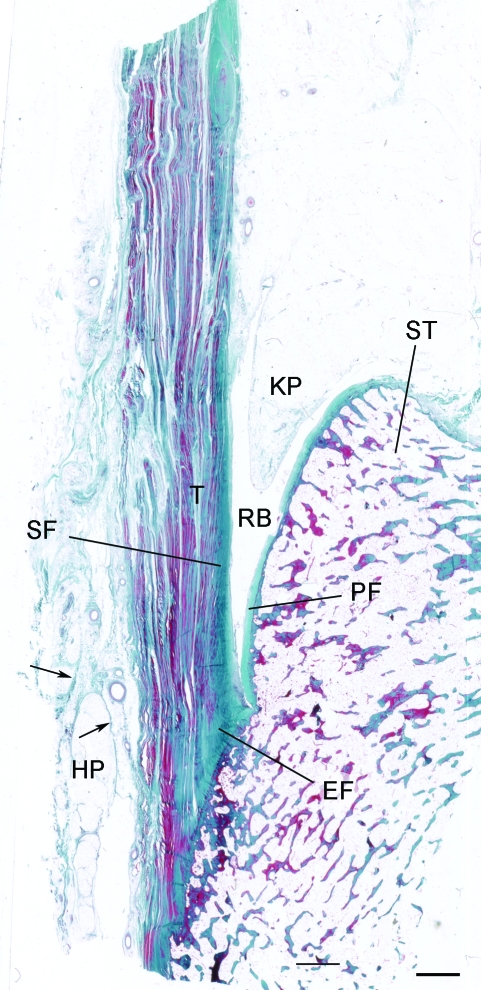
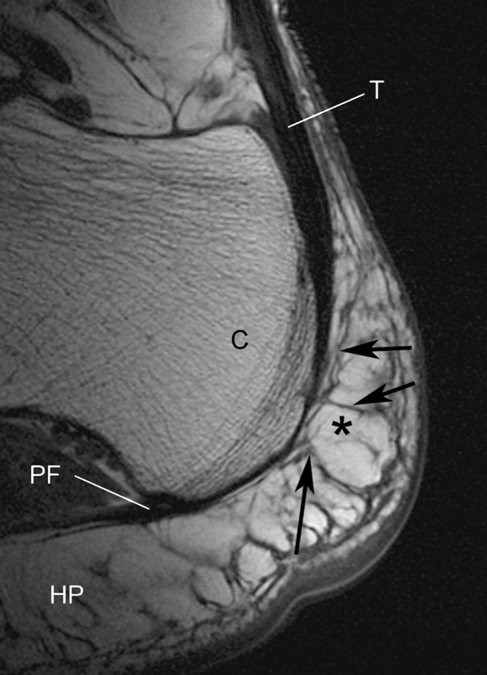
Although the structure of the adult enthesis organ has been described in detail previously (Benjamin & McGonagle, 2001; Benjamin et al. 2004), it is illustrated here in Fig. 1 for comparison with the foetal material. MR images show that the distal part of the tendon is often directly continuous with the plantar fascia via a thickened periosteum (Fig. 2). Additionally, a network of fibrous septa within the heel fat pad is evident that is functionally important for compression tolerance of the pad. This network is anchored to the posterior aspect of the distal Achilles tendon attachment, indicating a close functional integration between the heel pad and the Achilles enthesis organ in the adult (Fig. 2).
Fig. 1.
The enthesis organ of the adult Achilles tendon. The enthesis itself is characterised by an enthesis fibrocartilage (EF) which is most prominent in the superior part of the attachment. In addition, there is a sesamoid fibrocartilage (SF) in the deep surface of the tendon, immediately adjacent to the enthesis, and a corresponding periosteal fibrocartilage (PF) on the opposing surface of the superior tuberosity (ST) of the calcaneus. The tendon (T) and bone are separated by the retrocalcaneal bursa (RB) into which protrudes the tip of Kager's fat pad (KP). Note that the heel fat pad (HP) extends upwards from the sole of the foot to the posterior part of the Achilles tendon. Arrows, fibrous septa of the fat pad. Masson's trichrome. Scale bar: 2 mm.
Fig. 2.
A non-fat suppressed sagittal MR image of the normal heel of a young female volunteer (age 42 years) showing how the Achilles tendon (T) is directly continuous with the plantar fascia (PF) around the posterior aspect of the calcaneus (C). Fat (asterisk) is high signal (bright). Tendons, fascia and septa are low signal (black). Note how the heel fat pad (HP) extends upwards behind the tendon and that its fibrous septa are connected to it (arrows).
The developing enthesis organ
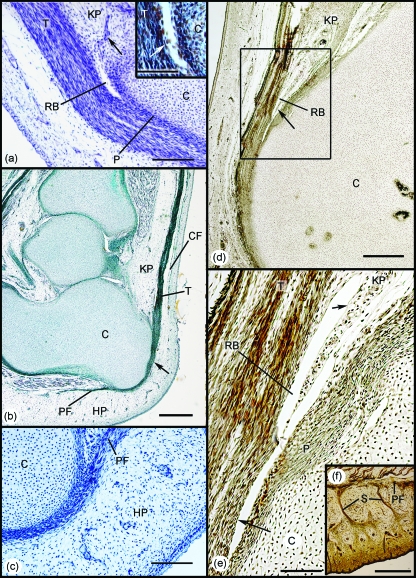
During early development (45–57-mm foetuses), the Achilles tendon was highly cellular and showed little sign of fibrillogenesis (Fig. 3a). It attached indirectly to the cartilaginous anlagen of the calcaneus via a thick perichondrium (Fig. 3a,b). Its continuity with the adjacent plantar fascia, via the perichondrium, was particularly striking (Fig. 3b). At these early stages of development, there were no signs of any fat cells in either Kager's triangle (in relation to the Achilles tendon) or the heel pad (in relation to the plantar fascia).
Fig. 3.
Development of the enthesis organ in 45–110-mm foetuses. (a) Initially the Achilles tendon (T) attaches to the perichondrium (P) of the calcaneus (C). The earliest sign of the differentiation of the enthesis organ beyond the establishment of the enthesis itself, was the development of the retrocalcaneal bursa (RB) by cavitation within the vascular mesenchymal tissue occupying the position of the future Kager's fat pad (KP). Arrow – blood vessels. 45-mm foetus. Hansen's hematoxylin. Scale bar: 100 µm. Inset: Higher power view of the developing bursa to show the appearance of a layer of flattened mesenchymal cells (putative synovium – arrow). (b) The continuity of the Achilles tendon (T) and the plantar fascia (PF) is strikingly evident in this 57-mm foetus (arrow). A condensation of mesenchyme marks the first evidence of differentiation of the crural fascia (CF), but there is no adipogenesis in the region of the future Kager's (KP) or putative heel (HP) pads. Azan. Scale bar: 500 µm. (c) Higher power view of the region of the developing heel pad in a 45-mm foetus. Note that as in (b), the heel pad (HP) is purely mesenchymal and there is no evidence of the characteristic fibrous septa of the adult. (d) In the 110-mm foetus, the tip (arrow) of the future Kager's fat pad (KP) can now be seen to protrude into the retrocalcaneal bursa (RB). C, calcaneus. Bielchowsky. Scale bar: 500 µm. (e) Enlargement of the region in the rectangle in (d). The entire bursa (RB) is lined with a single layer of synovial cells (arrows). C, calcaneus; KP, Kager's fat pad; P, perichondrium; T, tendon. (f) The heel region of a 110-mm foetus. Prominent fibrous septa (S) are obvious, but adipogenesis has not commenced.
The retrocalcaneal bursa had begun to form in the 45-mm foetus and appeared as a small cavity lined with putative synovial cells, adjacent to the deep surface of the Achilles tendon, immediately proximal to its enthesis (Fig. 3a). The cavity appeared at the tip of an extensive mesenchymal condensation within Kager's triangle that was the precursor of the fat pad characterising this region in the adult (Fig. 3a). Small blood vessels were conspicuous in the mesenchyme, but there was no evidence of adipogenesis (Fig. 3a). The presumptive heel pad on the plantar surface of the calcaneus was also entirely mesenchymal, and as in Kager's triangle there were no signs of fat cell differentiation (Fig. 3c). Neither had the fibrous septa, typical of the adult fat pad, differentiated (Fig. 3c). However, the crural fascia had begun to develop in the distal part of the leg (Fig. 3b).
At a slightly later stage of development (110-mm foetus), the synovial bursa had enlarged and the tip of the putative fat pad projected conspicuously into it (Fig. 3d,e). The fat pad region was still entirely mesenchymal and was covered with synovium that reflected back on itself, to line the whole of the retrocalcaneal bursa at this stage (Fig. 3e). The perichondrium on the posterior surface of the calcaneus had thickened considerably, but neither a periosteal nor a sesamoid fibrocartilage was yet evident (Fig. 3e). Adipogenesis had still not commenced in the heel pad, but prominent septa were now obvious (Fig. 3f).
Signs of fat cell development within Kager's triangle were first detected in the 177-mm foetus (Fig. 4a). Curiously, adipocytes had yet to appear within the calcaneal heel pad, although mesenchymal condensations marked the position of the thick fibrous septa characteristic of the adult (Fig. 4b) and Pacinian corpuscles were present. The retrocalcaneal bursa was considerably larger and more clearly defined than in earlier stages, and a superior tuberosity was evident on the calcaneus (Fig. 4c). The perichondrium covering the tuberosity had thickened, but fibrocartilage differentiation had still not commenced, either here or in the adjacent deep surface of the tendon (Fig. 4d). Furthermore, a distinct enthesis fibrocartilage could not yet be identified. However, there were signs that the collagen fibres of the tendon curved gently as they approached the calcaneus – suggesting that the extracellular matrix at the enthesis had begun to stiffen prior to histological signs of chondrogenesis.
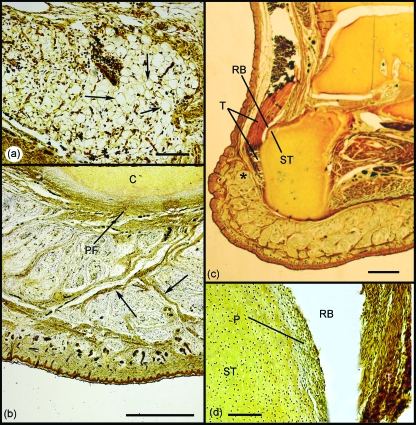
Fig. 4.
Development of the enthesis organ in the 177-mm foetus. (a) Adipocytes were first identifiable within Kager's fat pad at this stage (arrows). Scale bar:100 µm. (b) Although fat cells had not yet differentiated in the heel pad, fibrous septa were conspicuous (arrows). C, calcaneus; PF, plantar fascia. Scale bar: 500 µm. (c) A superior tuberosity (ST) had now developed on the posterior aspect of the calcaneus adjacent to the retrocalcaneal bursa (RB). Note also how the heel pad extends upwards behind the Achilles tendon (T) in the foetus as well as the adult (compare with Fig. 2). Scale bar: 2 mm. (d) Although the perichondrium (P) covering the superior tuberosity (ST) was greatly thickened, periosteal fibrocartilage had not yet differentiated. RB, retrocalcaneal bursa; T, tendon. Scale bar: 100 µm. All sections were stained with Bielchowsky's silver stain.
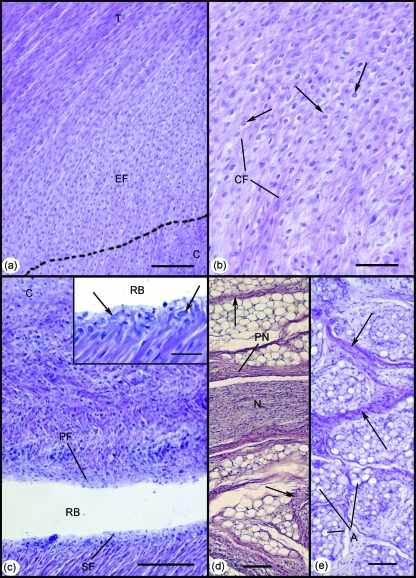
The enthesis, sesamoid and periosteal fibrocartilages were all first recognisable in the 332-mm foetus (Fig. 5a–c), and Kager's fat pad had developed further, as evident from the fibrous strands and nerves that had become conspicuous between groups of fat cells within it (Fig. 5d). Adipogenesis had now commenced in the neighbouring heel pad (Fig. 5d inset).
Fig. 5.
Development of the enthesis organ in the 332-mm foetus. (a) The enthesis fibrocartilage (EF) was first identifiable at this stage. Note the gradual bending of collagen fibres, as the Achilles tendon (T) inserts into the cartilaginous calcaneus (C). Scale bar: 100 µm. (b) Higher power view of the enthesis fibrocartilage in (a). Note the characteristic rounded fibrocartilage cells (arrows) interspersed between small bundles of collagen fibres (CF). Scale bar: 50 µm. (c) Both the sesamoid (SF) and periosteal (PF) fibrocartilages were also evident in the 332-mm foetus – forming the walls of the distal part of the retrocalcaneal bursa (RB). C, calcaneus. Scale bar: 100 µm. Inset: Higher power view of the sesamoid fibrocartilage in (c). Note the characteristic rounded cells (arrows). Scale bar: 100 µm. (d) Large myelinated nerve fascicles (N) surrounded by a prominent perineurium (PN) were evident within Kager's fat pad at this stage. Collections of well-differentiated adipocytes were separated by thick fibrous septa (arrows). Scale bar: 100 µm. (e) At this stage of development, adipogenesis was evident for the first time in the heel pad. Note the organisation of adipocytes (A) into locules, separated by thick fibrous septa (arrows). Scale bar: 100 µm. All slides were stained with Hansen's hematoxylin.
Discussion
The first sign of differentiation of the Achilles enthesis organ was the appearance of a cavity heralding the formation of the retrocalcaneal bursa. This preceded the development of the ancillary fibrocartilages in the bursal walls (i.e. the sesamoid and periosteal fibrocartilages) that were typical of the adult. There are thus clear parallels with the cavitation process that occurs in the mesenchymal interzone of a developing diathrodial joint, signalling the development of the joint cavity (Archer et al. 2003). Here, too, the appearance of a synovial cavity occurs prior to the development of the opposing articular cartilages. This supports the statement of Canoso (1998), which he credits to Bywaters, that the insertion site of the Achilles tendon has much in common with a synovial joint, i.e. the ancillary fibrocartilages ‘articulate’ with each other in a dorsiflexed foot and are thus the equivalent of articular cartilages in a synovial joint. In contrast to the adult enthesis organ, where the distal part of the bursa lacks a synovial membrane (Rufai et al. 1995), the developing bursa was initially lined throughout by synovium. It is likely that the synoviocytes differentiated from mesenchyme, filling the space between the putative periosteal and sesamoid fibrocartilages, i.e. the equivalent of the interzone tissue in a developing synovial joint. Such a mechanism has previously been observed during the development of synovial membrane in diarthrodial joints (Andersen & Bro-Rasmussen, 1961; Andersen, 1963). The early development of the bursa facilitates movement of the Achilles tendon and this in turn could lead to the differentiation of the enthesis organ fibrocartilages. Alternatively, interzone development per se could be the primary driver for fibrocartilage differentiation. With continued development, the bursa gradually increases in size, thus allowing the tip of the putative fat pad to protrude into it. By the time the periosteal and sesamoid fibrocartilages could be recognised in the 332-mm foetus, the synovium lining the distal part of the bursa had disappeared. This was probably a consequence of the increasing levels of mutual compression between tendon and bone. However, synovial membrane continued to cover the tip of the presumptive fat pad protruding into the cavity, in accordance with earlier findings in the rat (Rufai et al. 1992). Indeed, this remains a feature of the adult Achilles enthesis organ, where the synovium at the tip of Kager's fat pad may even give rise to synovial villi (Benjamin & McGonagle, 2007). The retention of synovium over the tip of the presumptive fat pad is likely to facilitate the movement of the fat pad in and out of the bursa (Canoso et al. 1988; Theobald et al. 2006).
It has been proposed that the increased mechanical loading that occurs as development progresses, induces metaplasia of tendon fibroblasts into fibrocartilage cells (Vogel & Koob, 1989; Gao et al. 1996). The importance of mechanical stimuli is endorsed by the recent experimental studies of Thomopoulos et al. (2007) on mouse supraspinatus tendons. They showed that enthesis fibrocartilage development could be delayed if the supraspinatus muscle was paralysed. The arrangement of enthesis fibrocartilage cells in longitudinal rows at the tendon attachment site probably reflects the earlier organisation of the tendon fibroblasts (Rufai et al. 1992; Gao et al. 1996).
Initially, the developing Achilles tendon attached to the perichondrium of the calcaneus – as did the plantar fascia. Consequently, the continuity between these two structures that is typical of the adult reflects their early development and represents a functional adaptation for dissipating stress over a wide area (Benjamin et al. 2006). Such a direct mechanical link is mirrored by many other tendons throughout the limbs and highlights the importance of recognising the fascial continuity between adjacent tendons/ligaments that is a general feature of the musculoskeletal system (Wood Jones, 1949; Benjamin et al. 2008). It should further be noted that because the heel fat pad extends upwards behind the calcaneus at the Achilles insertion, its fibrous septa can also be connected to the tendon at its enthesis. This connection develops relatively late in the differentiation of the enthesis organ, but is a striking feature of MRIs in young and middle-aged adults. It is thus logical to add both the fat pad septa and the posterior part of the plantar fascia to the list of components of the Achilles tendon enthesis organ. It is also possible that this close integration between the Achilles tendon and plantar fascia could be important in the pathogenesis of paediatric pathology – especially Severs’ disease, where the bone growth plate has not fused.
The results showed that adipogenesis preceded fibrocartilage differentiation in the development of the Achilles enthesis organ. This suggests that a fully functional bursa with a protruding fat pad is necessary before pressure of tendon against tuberosity in a dorsiflexed foot can be tolerated. That the perichondrium covering the calcaneal tuberosity thickens substantially prior to any sign of fibrocartilage within the tendon suggests that the periosteal fibrocartilage is likely to develop shortly before the sesamoid fibrocartilage – as in the rat (Rufai et al. 1992). The early appearance of nerve fibres within Kager's fat pad may indicate that the proprioceptive role of the fat discussed by Shaw et al. (2007) is established in utero.
Adipogenesis occurred in Kager's fat pad before it commenced in the heel pad. As the latter functions as a weight-bearing cushion for the foot, this developmental sequence is logical, as limb movements commence in utero in what is obviously a non-weight-bearing environment. The development of the fibrous strands of the adult heel pad precedes the first appearance of fat cells and suggests that the characteristic fibrous compartments within the heel pad are a key feature for pressure tolerance, in line with earlier views (Jahss et al. 1992).
In summary, this study defines the developmental stages of the normal Achilles tendon enthesis organ and draws attention to the similarities with synovial joint formation. The findings also confirm the close integration between the Achilles tendon attachment sites and that of the neighbouring plantar fascia and show that the relationship between the structures is underpinned by a developmental association of both with the perichondrium covering the calcaneus, rather than with the skeletal anlagen itself.
Acknowledgments
Hannah Shaw was in receipt of a Postgraduate Studentship from the Anatomical Society of Great Britain and Ireland.
References
- Andersen H. Histochemistry and development of the human shoulder and acromioclavicular joints with particular reference to the early development of the clavicle. Acta Anat (Basel) 1963;55:124–165. doi: 10.1159/000142467. [DOI] [PubMed] [Google Scholar]
- Andersen H, Bro-Rasmussen F. Histochemical studies on the histogenesis of the joints in human fetuses with special reference to the development of the joint cavities in the hand and foot. Am J Anat. 1961;108:111–122. [Google Scholar]
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, McGonagle D. Histopathologic changes at ‘synovio–entheseal complexes’ suggesting a novel mechanism for synovitis in osteoarthritis and spondylarthritis. Arthritis Rheum. 2007;56:3601–3609. doi: 10.1002/art.23078. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The ‘enthesis organ’ concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50:3306–3313. doi: 10.1002/art.20566. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Theobald P, Suzuki D, Toumi H. The Anatomy of the Achilles tendon. In: Maffulli N, Almekinders L, editors. The Achilles Tendon. London: Springer-Verlag; 2007. pp. 5–16. [Google Scholar]
- Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoso JJ. The premiere enthesis. J Rheumatol. 1998;25:1254–1256. [PubMed] [Google Scholar]
- Canoso JJ, Liu N, Traill MR, Runge VM. Physiology of the retrocalcaneal bursa. Ann Rheum Dis. 1988;47:910–912. doi: 10.1136/ard.47.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Messner K, Ralphs JR, Benjamin M. An immunohistochemical study of enthesis development in the medial collateral ligament of the rat knee joint. Anat Embryol. 1996;194:399–406. doi: 10.1007/BF00198542. [DOI] [PubMed] [Google Scholar]
- Jahss MH, Michelson JD, Desai P, et al. Investigations into the fat pads of the sole of the foot: anatomy and histology. Foot Ankle. 1992;13:233–242. doi: 10.1177/107110079201300502. [DOI] [PubMed] [Google Scholar]
- Milz S, Rufai A, Buettner A, Putz R, Ralphs JR, Benjamin M. Three-dimensional reconstructions of the Achilles tendon insertion in man. J Anat. 2002;200:145–152. doi: 10.1046/j.0021-8782.2001.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten B. Human Embryology. 3rd edn. New York: McGraw-Hill; 1968. [Google Scholar]
- Rufai A, Benjamin M, Ralphs JR. Development and ageing of phenotypically distinct fibrocartilages associated with the rat Achilles tendon. Anat Embryol (Berl) 1992;186:611–618. doi: 10.1007/BF00186984. [DOI] [PubMed] [Google Scholar]
- Rufai A, Ralphs JR, Benjamin M. Structure and histopathology of the insertional region of the human Achilles tendon. J Orthop Res. 1995;13:585–93. doi: 10.1002/jor.1100130414. [DOI] [PubMed] [Google Scholar]
- Shaw HM, Santer RM, Watson AH, Benjamin M. Adipose tissue at entheses: the innervation and cell composition of the retromalleolar fat pad associated with the rat Achilles tendon. J Anat. 2007;211:436–43. doi: 10.1111/j.1469-7580.2007.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow SW, Bohne WH, DiCarlo E, Chang VK. Anatomy of the Achilles tendon and plantar fascia in relation to the calcaneus in various age groups. Foot Ankle Int. 1995;16:418–421. doi: 10.1177/107110079501600707. [DOI] [PubMed] [Google Scholar]
- Theobald P, Bydder G, Dent C, Nokes L, Pugh N, Benjamin M. The functional anatomy of Kager's fat pad in relation to retrocalcaneal problems and other hindfoot disorders. J Anat. 2006;208:91–97. doi: 10.1111/j.1469-7580.2006.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007;25:1154–1163. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Wood Jones F. Structure and Function as Seen in the Foot. London: Balliere, Tindall and Cox; 1949. [DOI] [PubMed] [Google Scholar]