Abstract
The intervertebral disc is important in maintaining flexibility and dissipating loads applied to the spine. The disc comprises a heterogeneous population of cells, including those of the nucleus pulposus and annulus fibrosus, which are diverse in phenotype, partly due to the different mechanical loads they experience. Several studies have implicated the cytoskeleton in mechanotransduction, but little characterization of the three major cytoskeletal elements – actin, tubulin and vimentin – in the intervertebral disc has been undertaken. In this study we show that there are differences in both the organization and the amounts of these cytoskeletal proteins across the regions of immature bovine intervertebral disc (nucleus pulposus and outer annulus fibrosus), which differs with skeletal maturity. These differences are likely to reflect the diverse mechanical characteristics of the disc regions, and the loads that they experience, i.e. tension in the annulus fibrosus and compression in the nucleus pulposus. Alterations to the organization and amount of cytoskeletal element proteins may change the ability of the cells to respond to mechanical signals, with a loss of tissue homeostasis, suggesting that the cytoskeleton has a potential role in intervertebral disc degeneration.
Keywords: confocal microscopy, cytoskeleton, intervertebral disc, mRNA expression
Introduction
The intervertebral disc (IVD) is avascular and alymphatic, and comprises three morphologically distinct zones, namely the nucleus pulposus (NP), the annulus fibrosus [inner (IAF) and outer (OAF)] and the cartilaginous end plate. The IVD has important mechanical functions in maintaining flexibility and dissipating loads applied to the spine. IVD cells exposed to physiological mechanical loads experience complex physical stimuli including compressive, tensile and shear stresses and strains, fluid flows, hydrostatic pressure and osmotic pressure (Weidenbaum et al. 1992; Urban et al. 1993; Iatridis et al. 1996).These physical stimuli are believed to be important regulators of IVD cell metabolism contributing to matrix turnover, both synthesis and degradation, in normal and degenerative discs.
However, it has been reported that the distinct cell populations of the IVD respond differently to compressive loads (Chen et al. 2004; Setton & Chen, 2006; Wang et al. 2007), suggesting that there may be intrinsic differences in the cell types. The IVD comprises a sparse population of heterogeneous cells; the NP cells resident at the centre of the disc closely resemble chondrocytes, whereas the OAF cells at the periphery of the disc are characteristic of fibroblasts (Errington et al. 1998). In bovine IVDs, the OAF cells have been shown to contain elongated processes containing filamentous F-actin (Errington et al. 1998; Bruehlmann et al. 2002; Li et al. 2007b) and vimentin (Bruehlmann et al. 2002). In the study by Errington et al. (1998), specific cytoskeletal components in the cell processes were hypothesized to play a role in ‘sensing’ mechanical loads. More recently, it has been demonstrated that the cytoskeleton may play a crucial role in mechanotransduction between the IVD cells and their surrounding extracellular matrix (Hayes et al. 1999; Chen et al. 2004; Li et al. 2007a).
The cytoskeleton, comprising actin microfilaments, tubulin microtubules and vimentin intermediate filaments in the IVD, is fundamental to the dynamic functions of the cell. Collectively, the three major cytoskeletal elements play important roles in cell division, motility, protein trafficking and secretion (Benjamin et al. 1994; Thyberg & Moskalewski, 1999).
Therefore, the activities of the NP and OAF cells of the IVD are likely to be dependent upon their morphology and hence cytoskeletal composition. With the exception of a few studies which have investigated F-actin and vimentin organization in bovine IVD (Errington et al. 1998) and in normal vs. pathological human discs (Johnson & Roberts, 2003) very little is known about the content and organization of the three major cytoskeletal elements in the two distinct IVD cell populations, and whether there are differences with skeletal maturity.
The aim of this study was to characterize fully the abundance of the three major cytoskeletal elements – F-actin, β-tubulin and vimentin – in the NP and OAF of immature (7-day-old) and mature (18-month-old) bovine IVDs using a combination of scanning laser confocal microscopy, quantitative PCR and Western blotting to examine, in detail, network organization, mRNA and protein levels. Our study illustrates that there are both zonal (NP vs. OAF) and age-related (7 days vs. 18 months) differences in the cytoskeletal elements of the IVD, which may reflect the diverse phenotypic characteristics of the disc regions.
Materials and methods
All chemicals were obtained from Sigma (Poole, UK) unless otherwise stated and were of analytical grade or above.
Source of bovine intervertebral disc
Bovine tails from immature (7-day-old) and skeletally mature (18-month-old) steers were obtained from the abattoir within 6 h of slaughter. The NP and OAF were dissected out of the IVDs (cc1–cc5), snap frozen in liquid nitrogen and stored at −80 °C until required. Dissected NP and OAF from cc1–cc5 discs were pooled per animal and between four and six individual animals were utilized in this study. Accurate dissection of NP from OAF was verified by immunoblotting for type II and type I collagen, respectively (data not shown).
Analysis of cytoskeletal element organization using confocal microscopy
Frozen cryosections (20 µm thickness) of NP and OAF tissue were taken and fixed in 4% paraformaldehyde. After repeated washes in modified Hanks’ balanced salt solution (mHBSS), tissue sections were permeabilized in 5% Triton X-100 and blocked with 10% goat serum prior to overnight incubation with anti-vimentin (V9 clone, 1 : 40) or anti-β-tubulin (E7 clone, 1 : 100; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA). Sections were washed extensively and incubated in goat anti-mouse fluorescein isothiocyanate FITC-conjugated secondary antibody (1 : 64) for 1 h. F-actin was stained with Alexa 488™ conjugated phalloidin (1 : 200; Molecular Probes, Invitrogen). Sections were mounted in Vectorshield™ containing propidium iodide (10 µg mL−1) (Vectashield, USA). Sections were examined using a Leica DM6000B upright digital microscope (Leica, Wetzlar, Germany) and controlled by the Leica Control software, set up for dual-channel fluorescence recordings as described previously (Blain et al. 2006). Representative cells were scanned using a 63× oil immersion objective with appropriate excitation and emission settings for fluorescein isothiocyanate and propidium iodide. Negative controls, omitting the primary antibody, conducted in parallel were devoid of fluorescent signal (data not shown).
Analysis of cytoskeletal element gene expression using quantitative PCR
Total RNA was extracted from both NP and OAF of bovine IVDs, and the absolute amounts of cytoskeletal genes expressed by the two cell populations were assessed using quantitative PCR. Tissue (50 mg) was powdered in a liquid-nitrogen cooled Mikro-Dismembrator (Braun Biotech) and 1 mL Trizol™ reagent (Invitrogen, UK) was added directly to the powdered tissue and warmed to room temperature. Following chloroform extraction, samples were precipitated with 70% ethanol and the RNA purified using Qiagen RNeasy™ mini kits according to the manufacturer's protocols (Qiagen, West Sussex, UK). First strand cDNA was synthesized (2 µL RNA per 20 µL reaction volume) using Superscript™ II reverse transcriptase (Invitrogen, Paisley, UK) according to the manufacturer's instructions and as previously described (Blain et al. 2003). Quantitative real-time PCR was performed using the Mx3000P® QPCR System (Stratagene, Edinburgh, UK) with primers designed to the open reading frame of β-actin, β-tubulin and vimentin (Table 1). Calculation of starting concentration was based on standard curves for each target DNA run in parallel. GAPDH was used as an internal reference of housekeeping gene transcription for normalization between different cDNA samples as described previously (Blain et al. 2006).
Table 1.
Primer sequences for real-time quantification of cytoskeletal genes
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| β-actin | Forward: 5′-TTCGAGACCTTCAACACCCC-3′ | 68* |
| Reverse: 5′-GGCCAGAGGCATACAGGGA-3′ | ||
| β-tubulin | Forward: 5′-GAGGAGGAGGTGGCCTAGAG-3′ | 175 |
| Reverse: 5′-GCTTTAATGGTGGTGGCTGT-3′ | ||
| Vimentin | Forward: 5′-AAGGAAGAGATGGCTCGTCA-3′ | 164 |
| Reverse: 5′-TTGGTTTCCCTCAGGTTCAG-3′ |
Analysis of cytoskeletal element protein expression by Western blotting
Cytoskeletal proteins –β-actin, β-tubulin and vimentin – expressed by the NP and OAF were assessed using Western blotting. Due to the inability to extract sufficient amounts of intracellular proteins direct from the NP tissue, the NP and OAF tissue were subject to a short period of enzymatic digestion to release the cells prior to analysis. Tissue was finely minced and cells extracted from IVD tissue by rapid digestion, over 2 h, using 2.5 mg mL−1 type I collagenase for OAF tissue (Wuertz et al. 2007) and 1 mg mL−1 type II collagenase for NP tissue (Chelberg et al. 1995). Preparations were centrifuged (3000 r.p.m., 10 min), filtered through a cell strainer to remove debris, cell viability confirmed (trypan blue exclusion) and preparations resuspended to yield 3 × 106 cells mL−1. Total protein concentration was determined using the BCA kit (Pierce, UK) according to manufacturer's instructions, and a direct correlation of cell number and protein concentration was observed for the NP (R2 = 0.968) and OAF cells (R2 = 0.988). Cells were subsequently lysed in 2× sample buffer (1 × 106 cells 100 µL−1[0.06 m Tris pH 6.8, 2% (w/v) SDS, 10% (v/v) glycerol, 0.2% (w/v) bromophenol blue]) (Blain et al. 2001). Protein extracts were reduced with 2.5% (v/v) β-mercaptoethanol and denatured for 30 min at 60 °C. Protein, loaded on an equal cell number basis (5 × 105 cells), was separated on 10% polyacrylamide gels and transferred to PVDF membrane (Millipore, Dundee, UK) at room temperature for 60 min. Membranes were blocked with 3% milk, prior to immunoblotting with specific primary antibodies that recognize β-actin (AC15, 1:100, Abcam, Cambridge, UK), vimentin (V9, 1 : 400), and β-tubulin (E7, 1 : 500). Membranes were then incubated with HRP-conjugated sheep anti-mouse secondary antibody (1 : 10 000) and the HRP signal detected using Hyperfilm® and ECL® detection system (GE Healthcare, Bucks, UK), as described previously (Blain et al. 2006). All Western blots were scanned and differences in the chemiluminescence intensity of digitized bands were assessed using NIH Image software.
Statistical analysis
Data are presented as mean ± SE, with tissue derived from between four and six individual animals. Data was tested for normality (Anderson–Darling test) and equal variance. One-way analysis of variance (anova) or paired t-test was carried out using SPSS 12.0 software. Differences were considered significant at P-values of less than 0.05.
Results
Differential F-actin organization and increased β-actin mRNA and protein expression in cells of the outer annulus fibrosus
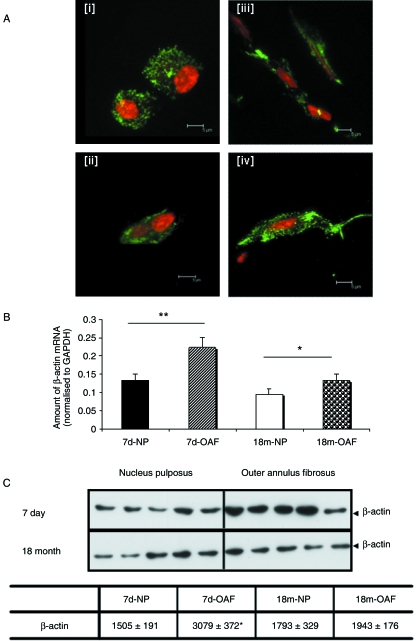
F-actin organization, mRNA and protein expression were compared in the OAF and NP of young (7-day-old) and skeletally mature (18-month-old) IVDs (Fig. 1). The F-actin network, investigated using Alexa 488™-conjugated phalloidin in conjunction with confocal microscopy, was observed in dense punctate regions throughout the cytoplasm of both young (Fig. 1Ai) and mature (Fig. 1Aii) NP cells. In contrast, F-actin was distributed throughout the cytoplasm extending into the cell processes of young (Fig. 1Aiii) and mature (Fig. 1Aiv) OAF cells. There was no discernible difference in actin architecture between young (Fig. 1Ai,iii) and mature (Fig. 1Aii,iv) populations of IVD cells. To determine whether the zonal variations observed in F-actin distribution were purely organizational or were a consequence of altered gene or protein expression, β-actin mRNA and protein levels were assessed by quantitative PCR (Fig. 1B) and Western blotting (Fig. 1C), respectively. After normalization to the housekeeping gene GAPDH, significantly more β-actin mRNA was observed in the OAF compared with NP in both young (P = 0.008) and mature disc (P = 0.025) (Fig. 1B). However, there was no age-related difference in β-actin mRNA levels between young and mature NP (P = 0.283) or OAF (P = 0.304). As there were zonal variations in β-actin mRNA levels, potential differences in protein expression were also investigated by Western blotting (Fig. 1C). Greater β-actin expression was found in young OAF cells compared with NP cells (P = 0.0159) (Fig. 1C). However, there was no zonal variation in β-actin content in mature disc (P = 1.00). Additionally, there were no age-related differences in the amount of β-actin protein detected in either NP or OAF cells (P = 0.6905 and 0.0537, respectively) (Fig. 1C).
Fig. 1.
The actin cytoskeleton of intervertebral disc. (A) F-actin organization in (i) immature and (ii) mature nucleus pulposus and (iii) immature and (iv) mature outer annulus fibrosus discs. F-actin is predominantly punctate in nucleus pulposus cells, but extends into the processes of the outer annulus fibrosus cells; however, there were no striking differences with skeletal maturity. Cells were visualized using Alexa-488™-phalloidin (counterstained with propidium iodide) in conjunction with scanning laser confocal microscopy. (B) β-actin mRNA expression was assessed using quantitative ‘real-time’ PCR. There was a significant increase in β-actin mRNA levels in both the immature (P < 0.01) and mature (P < 0.05) outer annulus fibrosus. Expression was normalized to the housekeeping gene GAPDH, and normalized data are presented as mean ± SE (n = 6). (C) β-actin protein expression was detected by Western blotting (equivalent number of cells loaded) using a monoclonal antibody AC15, and densitometric analyses performed. Significantly more β-actin was observed in immature outer annulus fibrosus cells (P < 0.05), but no differences were observed in the mature disc cell populations. Normalized data are presented as mean densitometric units ± SE (n = 5). *P < 0.05; **P < 0.01.
Increased β-tubulin mRNA and protein expression in cells of the nucleus pulposus
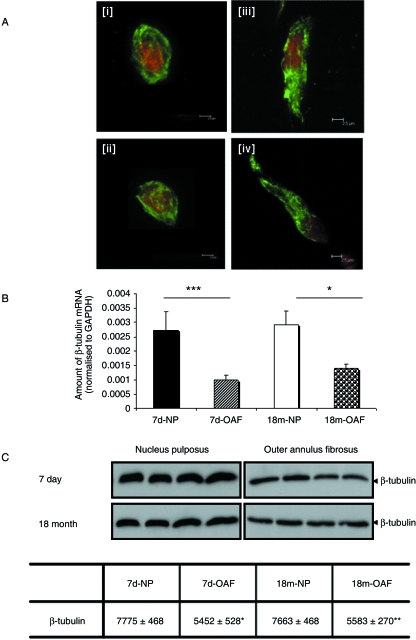
The architecture of the β-tubulin networks, as well as mRNA and protein expression, were compared in the OAF and NP of young (7-day-old) and skeletally mature IVDs (18-month-old). β-tubulin was observed throughout the cytoplasm in cells from NP and OAF tissue (Fig. 2A). Analysis of the confocal images indicated that there were no obvious differences in β-tubulin organization across the tissue zones or with skeletal maturity (Fig. 2Ai–iv). For completeness, we also investigated the relative amounts of β-tubulin mRNA (Fig. 2B) and protein (Fig. 2C) in the NP and OAF. Significantly more β-tubulin mRNA was detected in NP than in the OAF in both young (P = 0.023) and mature IVD (P = 0.026; Fig. 2B). However, there was no age-related difference in the expression of β-tubulin mRNA in the OAF (P = 0.071) or in the NP (P = 0.829). The changes observed at the mRNA level were confirmed at the protein level using Western blotting (Fig. 2C); significantly more β-tubulin protein was detected in NP cells than in OAF cells in both young (P = 0.0165) and mature IVD (P = 0.0084; Fig. 2C). As with mRNA levels, no age-related differences in β-tubulin protein expression were detected in NP (P = 0.114) and OAF cells (P = 0.677; Fig. 2C).
Fig. 2.
The β-tubulin cytoskeleton of intervertebral disc. (A) Organization in (i) immature and (ii) mature nucleus pulposus and (iii) immature and (iv) mature outer annulus fibrosus discs. β-tubulin is observed as a dense meshwork spanning throughout the cytoplasm of both nucleus pulposus and outer annulus fibrosus cells. No obvious differences were observed across the tissue zones or with skeletal maturity. Cells were visualized using FITC-conjugated antibodies (counterstained with propidium iodide) in conjunction with scanning laser confocal microscopy. (B) β-tubulin mRNA expression was assessed using quantitative ‘real-time’ PCR. There was a significant increase in β-tubulin mRNA expression in both the immature (P < 0.001) and mature (P < 0.05) nucleus pulposus. Expression was normalized to the housekeeping gene GAPDH, and normalized data are presented as mean ± SE (n = 6). (C) β-tubulin protein expression was detected by Western blotting (equivalent number of cells loaded) using a monoclonal antibody E7, and densitometric analyses performed. Significantly less β-tubulin was observed in both immature (P < 0.05) and mature (P < 0.01) outer annulus fibrosus cells, but no age-related differences were observed. Normalized data are presented as mean densitometric units ± SE (n = 4). *P < 0.05; **P < 0.01.
Differential vimentin organization associated with age-related decreases in vimentin mRNA and protein expression in cells of the nucleus pulposus
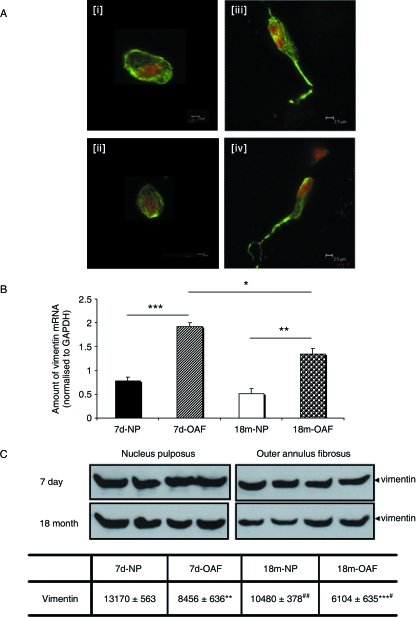
The architecture of the vimentin intermediate filaments, as well as corresponding mRNA and protein levels were analysed in the NP and OAF of young and skeletally mature bovine IVDs (Fig. 3). Vimentin filaments were observed traversing the cytoplasm from the plasma to the nuclear membrane (Fig. 3A). Vimentin filaments were also observed extending into the cellular processes, especially in OAF cells (Fig. 3Aiii,iv). There was no discernible difference in vimentin architecture between young (Fig. 3Ai,iii) and mature IVD (Fig. 3Aii,iv). However, quantitative mRNA analysis demonstrated appreciably more vimentin mRNA in both young (P < 0.001) and mature OAF (P = 0.002; Fig. 3B), although vimentin gene expression in the OAF decreased with skeletal maturity (P = 0.018; Fig. 3B). There was no difference in vimentin mRNA levels in the NP with age (P = 0.154). In contrast, Western blotting revealed less vimentin protein in the OAF than in the NP in both young (P = 0.0015) and mature IVD (P = 0.001; Fig. 3C). An age-related reduction in vimentin protein expression was also evident in both the NP (P = 0.007) and the OAF cells (P = 0.04; Fig. 3C).
Fig. 3.
The vimentin cytoskeleton of intervertebral disc. (A) Organization in (i) immature and (ii) mature nucleus pulposus, and in (iii) immature and (iv) mature outer annulus fibrosus discs. Vimentin displays an extensive network traversing the cytoplasm of both nucleus pulposus and outer annulus fibrosus cells; vimentin filaments also extend into the processes of the outer annulus fibrosus cells. Cells were visualized using FITC-conjugated antibodies (counterstained with propidium iodide) in conjunction with scanning laser confocal microscopy. (B) Expression levels of vimentin mRNA as assessed using quantitative ‘real-time’ PCR. There was a significant increase in vimentin mRNA levels in both the immature (P < 0.001) and mature (P < 0.01) outer annulus fibrosus. An age-related decrease in vimentin gene expression was also observed in the outer annulus fibrosus cells (P < 0.05). Expression was normalized to the housekeeping gene GAPDH, and normalized data are presented as mean ± SE (n = 6). (C) Vimentin protein expression was detected by Western blotting (equivalent number of cells loaded) using a monoclonal antibody V9, and densitometric analyses performed. Significantly more vimentin was observed in the immature (*P < 0.002) and mature nucleus pulposus cells (*P < 0.001). An age-related reduction in vimentin protein was observed in both the nucleus pulposus (#P = 0.01) and the outer annulus fibrosus cells (#P < 0.05). Normalized data are presented as mean densitometric units ± SE (N = 5). *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Intervertebral discs, which are important for dissipating loads and providing flexibility to the spine, comprise at least two distinct cell populations – cells of the NP are ‘chondrocyte-like’ in morphology, whereas those of the OAF appear ‘fibroblast-like’. This likely reflects both the different matrix organization and the different mechanical characteristics of the disc regions (Urban & Roberts, 2003). The NP resists compressive loading, whereas the OAF mainly counteracts tensional forces in vivo (Klein et al. 1983). It has previously been hypothesized that the cytoskeleton, which in IVD cells comprises actin microfilaments, tubulin microtubules and vimentin intermediate filaments, can act as a transducer of mechanical signals in tissues including IVD (Chen et al. 2004; Chiquet et al. 2007), bone (Myers et al. 2007) and articular cartilage (Durrant et al. 1999; Knight et al. 2006; Campbell et al. 2007). Furthermore, the presence/organization of specific cytoskeletal elements may reflect the diverse mechanical functions of these cells.
To date, there has been little characterization of the composition of the three major cytoskeletal elements in the IVD (Errington et al. 1998; Guilak et al. 1999; Bruehlmann et al. 2002; Johnson & Roberts, 2003; Li et al. 2007b), and none directly comparing the cytoskeletal composition of NP and OAF. Therefore, in this present study we have utilized a combination of scanning laser confocal microscopy, quantitative PCR and Western blotting to analyse the organization of and quantify F-actin, β-tubulin and vimentin of NP and OAF cells in situ IVDs. Interestingly, our data demonstrate that the organization, mRNA and protein content of the cytoskeletal elements alter across the IVD, coincident with the two distinct cell populations.
Of the three major cytoskeletal elements, the composition of the actin cytoskeleton is most altered between the NP and OAF of bovine IVDs. F-actin is cytoplasmically punctate in its localization in NP cells. In the OAF, actin filaments are observed extending out into the cell processes, reflecting the fibroblast-like morphology (Dunlevy & Couchman, 1993; Baschong et al. 1997; Pritchard et al. 2002). The distinct localization of F-actin in the NP and OAF is evident in both young and mature IVD. As scanning laser confocal microscopy can only indicate cell morphology and is not quantitative, both mRNA and protein levels were also analysed to determine whether there were inherent differences in expression levels. Interestingly, β-actin mRNA levels were significantly higher in OAF than in NP cells, irrespective of age. This trend was also reflected in enhanced β-actin protein amounts in the OAF cells; however, this was only true for young IVD and not mature IVD.
To date, the microtubular networks have not been characterized in IVD cells; our study demonstrates that β-tubulin is expressed in cells of the NP and OAF. In both cell populations, β-tubulin forms an extensive meshwork throughout the cytoplasm correlating with its organization in chondrocytes (Blain et al. 2006). In contrast to β-actin, there is significantly more β-tubulin mRNA in NP than in OAF cells, irrespective of age; this increase in β-tubulin mRNA is also reflected in the increased protein expression.
Vimentin is a highly expressed cytoskeletal protein providing the cell with rigidity; the dense meshwork of filaments supports the cell shape (Brown et al. 2001) and acts to absorb mechanical loads (Kreplak et al. 2005). The vimentin intermediate filaments of the NP were displayed as a dense meshwork that traversed the cytoplasm from the plasma to the nuclear membrane. The organization of vimentin in cells of the NP is characteristic of articular chondrocytes (Postacchini et al. 1982; Durrant et al. 1999; Langelier et al. 2000; Blain et al. 2006) and cells of the meniscus (Hellio Le Graverand et al. 2001). In the OAF, vimentin filaments were observed throughout the cytoplasm as well as extending into the cell processes. This organization has previously been reported in cells of the OAF, with cell processes parallel to the collagen fibre direction (Bruehlmann et al. 2002).
Significantly higher vimentin mRNA levels were observed in the OAF than in the NP cells, although there was an age-related decrease in the OAF. This age-related reduction in vimentin expression was also evident at the protein level in OAF cells, suggestive of a transcriptional shutdown in expression of this cytoskeletal element. However, we consistently observed more vimentin protein in the NP tissue, although mRNA levels were shown to decrease. This may be due to the dynamic nature of the intermediate filaments, as polymerized vimentin is associated with the insoluble fraction, whereas the depolymerized monomers form the soluble component (Chen et al. 2004). If the cytoskeletal element turnover is more dynamic in OAF cells, the amount of polymerized vimentin may not represent the total amount, thereby yielding lower levels of expression than the NP tissue when using Western blotting; we are currently trying to determine whether this is the case; this may offer some explanation for the contrasting vimentin mRNA and protein data.
Concluding remarks
In summary, using a combination of scanning laser confocal microscopy, quantitative PCR and Western blotting, we have demonstrated that β-actin, β-tubulin and vimentin have altered architectures as well as differences in gene and protein levels in NP and OAF cells from young and skeletally mature IVD. It is hypothesized that these differences arise due to the distinct mechanical features of these two regions, suggesting that β-tubulin and vimentin are distributed so as to resist the effects of compressive loads on the NP cells, and β-actin to endure the tensional forces exerted on the cells of the OAF.
Acknowledgments
We would like to acknowledge funding from the EPSRC (Dorothy Hodgkin Postgraduate Award – S.L.) and Arthritis Research Campaign (Grant No. 14874 and 18221: E.J.B.). The E7 monoclonal antibody developed by Michael Klymkowsky was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA.
References
- Baschong W, Sutterlin R, Aebi U. Punch-wounded, fibroblast populated collagen matrices: a novel approach for studying cytoskeletal changes in three dimensions by confocal laser scanning microscopy. Eur J Cell Biol. 1997;72:189–201. [PubMed] [Google Scholar]
- Benjamin M, Archer CW, Ralphs JR. Cytoskeleton of cartilage cells. Microsc Res Tech. 1994;28:372–377. doi: 10.1002/jemt.1070280503. [DOI] [PubMed] [Google Scholar]
- Blain EJ, Gilbert SJ, Wardale RJ, Capper SJ, Mason DJ, Duance VC. Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch Biochem Biophys. 2001;396:49–55. doi: 10.1006/abbi.2001.2575. [DOI] [PubMed] [Google Scholar]
- Blain EJ, Mason DJ, Duance VC. The effect of cyclical compressive loading on gene expression in articular cartilage. Biorheology. 2003;40:111–117. [PubMed] [Google Scholar]
- Blain EJ, Gilbert SJ, Hayes AJ, Duance VC. Disassembly of the vimentin cytoskeleton disrupts articular cartilage chondrocyte homeostasis. Matrix Biol. 2006;25:398–408. doi: 10.1016/j.matbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Hallam JA, Colucci-Guyon E, Shaw S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J Immunol. 2001;166:6640–6646. doi: 10.4049/jimmunol.166.11.6640. [DOI] [PubMed] [Google Scholar]
- Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat. 2002;201:159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JJ, Blain EJ, Chowdhury TT, Knight MM. Loading alters actin dynamics and up-regulates cofilin gene expression in chondrocytes. Biochem Biophys Res Commun. 2007;361:329–334. doi: 10.1016/j.bbrc.2007.06.185. [DOI] [PubMed] [Google Scholar]
- Chelberg MK, Banks GM, Geiger DF, Oegema TR., JR Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat. 1995;186:43–53. Pt 1. [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yan W, Setton LA. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573–583. doi: 10.1016/j.matbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Tunc-Civelek V, Sarasa-Renedo A. Gene regulation by mechanotransduction in fibroblasts. Appl Physiol Nutr Metab. 2007;32:967–973. doi: 10.1139/H07-053. [DOI] [PubMed] [Google Scholar]
- Dunlevy JR, Couchman JR. Controlled induction of focal adhesion disassembly and migration in primary fibroblasts. J Cell Sci. 1993;105(Pt 2):489–500. doi: 10.1242/jcs.105.2.489. [DOI] [PubMed] [Google Scholar]
- Durrant LA, Archer CW, Benjamin M, Ralphs JR. Organisation of the chondrocyte cytoskeleton and its response to changing mechanical conditions in organ culture. J Anat. 1999;194(Pt 3):343–353. doi: 10.1046/j.1469-7580.1999.19430343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington RJ, Puustjarvi K, White IR, Roberts S, Urban JP. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J Anat. 1998;192(Pt 3):369–378. doi: 10.1046/j.1469-7580.1998.19230369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Ting-Beall HP, Baer AE, Trickey WR, Erickson GR, Setton LA. Viscoelastic properties of intervertebral disc cells. Identification of two biomechanically distinct cell populations. Spine. 1999;24:2475–2483. doi: 10.1097/00007632-199912010-00009. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev Dyn. 1999;215:179–189. doi: 10.1002/(SICI)1097-0177(199907)215:3<179::AID-AJA1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hellio Le Graverand MP, Ou Y, Schield-Yee T, et al. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. J Anat. 2001;198:525–535. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatridis JC, Weidenbaum M, Setton LA, Mow VC. Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine. 1996;21:1174–1184. doi: 10.1097/00007632-199605150-00009. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Roberts S. Human intervertebral disc cell morphology and cytoskeletal composition: a preliminary study of regional variations in health and disease. J Anat. 2003;203:605–612. doi: 10.1046/j.1469-7580.2003.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JA, Hickey DS, Hukins DW. Radial bulging of the annulus fibrosus during compression of the intervertebral disc. J Biomech. 1983;16:211–217. doi: 10.1016/0021-9290(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Knight MM, Toyoda T, Lee DA, Bader DL. Mechanical compression and hydrostatic pressure induce reversible changes in actin cytoskeletal organisation in chondrocytes in agarose. J Biomech. 2006;39:1547–1551. doi: 10.1016/j.jbiomech.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kreplak L, Bar H, Leterrier JF, Herrmann H, Aebi U. Exploring the mechanical behavior of single intermediate filaments. J Mol Biol. 2005;354:569–577. doi: 10.1016/j.jmb.2005.09.092. [DOI] [PubMed] [Google Scholar]
- Langelier E, Suetterlin R, Hoemann CD, Aebi U, Buschmann MD. The chondrocyte cytoskeleton in mature articular cartilage: structure and distribution of actin, tubulin, and vimentin filaments. J Histochem Cytochem. 2000;48:1307–1320. doi: 10.1177/002215540004801002. [DOI] [PubMed] [Google Scholar]
- Li H, Li S, Wu TJ, Xu Y, Chen YX. Early effects of the cyclic uniaxial compressive stress on actin and vimentin of the rat condylar chondrocytes [in Chinese] Hua Xi Kou Qiang Yi Xue Za Zhi. 2007a;25:422–425. [PubMed] [Google Scholar]
- Li S, Duance VC, Blain EJ. F-actin cytoskeletal organization in intervertebral disc health and disease. Biochem Soc Trans. 2007b;35:683–685. doi: 10.1042/BST0350683. [DOI] [PubMed] [Google Scholar]
- Myers KA, Rattner JB, Shrive NG, Hart DA. Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem Biophys Res Commun. 2007;364:214–219. doi: 10.1016/j.bbrc.2007.09.109. [DOI] [PubMed] [Google Scholar]
- Postacchini F, Bellocci M, Ricciardi-Pollini PT, Modesti A. An ultrastructural study of recurrent disc herniation: a preliminary report. Spine. 1982;7:492–497. doi: 10.1097/00007632-198209000-00014. [DOI] [PubMed] [Google Scholar]
- Pritchard S, Erickson GR, Guilak F. Hyperosmotically induced volume change and calcium signaling in intervertebral disk cells: the role of the actin cytoskeleton. Biophys J. 2002;83:2502–2510. doi: 10.1016/S0006-3495(02)75261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl. 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- Wang DL, Jiang SD, Dai LY. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine. 2007;32:2521–2528. doi: 10.1097/BRS.0b013e318158cb61. [DOI] [PubMed] [Google Scholar]
- Weidenbaum M, Foster RJ, Best BA, et al. Correlating magnetic resonance imaging with the biochemical content of the normal human intervertebral disc. J Orthop Res. 1992;10:552–561. doi: 10.1002/jor.1100100410. [DOI] [PubMed] [Google Scholar]
- Wuertz K, Urban JP, Klasen J, et al. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res. 2007;25:1513–1522. doi: 10.1002/jor.20436. [DOI] [PubMed] [Google Scholar]