Abstract
This study provides a model of the complex deltoid origin and end tendons, as a basis for further anatomical, biomechanical and clinical research. Although the deltoid is used in transpositions with upper limb paralysis, its detailed morphology and segmentation has not been object of much study. Morphologically, the deltoid faces two distinct challenges. It closely envelops a ball joint, and it reduces its width over a short distance from a very wide origin along clavicle, acromion and spina scapula, to an insertion as narrow as the humerus. These challenges necessitate specific morphological tendon adaptations. A qualitative model for these tendons is developed by the stepwise transformation of a unipennate muscle model into a functional deltoid muscle. Each step is the solution to one of the mentioned morphological challenges. The final model is of an end tendon consisting of a continuous succession of bipennate end tendon blades centrally interspaced by unipennate tendon parts. The origin tendon consists of lamellae that interdigitate with the end tendon blades, creating a natural segmentation. The model is illustrated by qualitative dissection results. In addition, in view of a proliferation of terms found in the literature to describe deltoid tendons, tendon concepts are reviewed and the systematic use of the unique and simple terminology of ‘origin and end tendons’ is proposed.
Keywords: anatomy, shoulder, deltoid, muscle, tendon, model
Introduction
The deltoid is a strong muscle superficially enveloping the shoulder joint anteriorly, laterally and posteriorly (Fig. 1). Despite its anatomic accessibility and the fact that it is used for tendon transpositions (Falconer, 1988; Herzberg et al. 1999; Friden & Lieber, 2001; Lieber et al. 2003), its complex morphology has received relatively little detailed attention. The literature provided amongst others anatomical (Kumar et al. 1997; Bailie et al. 1999; Herzberg et al. 1999; Friden & Lieber, 2001; Lorne et al. 2001; Zhao et al. 2001; Klepps et al. 2004; Cetik et al. 2006), biomechanical (Otis et al. 1994; Johnson et al. 1996; Liu et al. 1997; Chang et al. 2000, Gagey & Hue, 2000; Lee & An, 2002; Kido et al. 2003; Langenderfer et al. 2004; Scepi et al. 2004; Holzbaur et al. 2005; Bitter et al. 2007), clinical/surgical (Falconer, 1988; Wirth et al. 1993; Chen et al. 1995; Sher et al. 1997; Ko et al. 1998; Hughes et al. 1999; Chen et al. 2000; Friden & Lieber, 2001; Zhao et al. 2001; Ejeskar, 2002; Lieber et al. 2003; Gill et al. 2004; Hong et al. 2005), and functional/EMG/clinical studies (Gabriel, 1997; Roman-Liu et al. 2001; Gamulin et al. 2002; Ferreira et al. 2003; Reinold et al. 2004, Smith et al. 2004, Wise et al. 2004, Brown & Wickham, 2006).
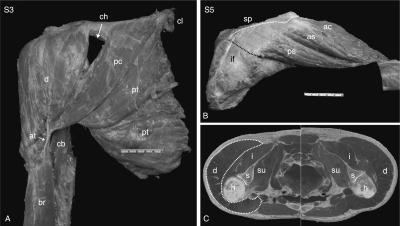
Fig. 1.
Deltoid overview. (A) (Specimen S3) Anterior deltoid (d) and pectoralis major. cl: clavicle. at: anterior deltoid ET blade. pc, pt: clavicular and thoracic parts of pectoralis major. ch: chiasma of vena cephalica. br: brachialis (biceps has been removed). cb: coracobrachialis. (B) (specimen S5) Posterior deltoid. sp: spina scapula (the supra-spinal scapula was covered by black background). ps: posterior spinal deltoid segment, mobilized from anterior spinal deltoid (as). ac: acromial deltoid. if: infraspinal fascia, covering infraspinatus and spinal deltoid OT sheet. White dotted line: origin line of spinal deltoid OT. Black dotted line: posterior deltoid edge. (C) Cross-section through humerus heads (h), left and right shoulders (from: ‘Visible Human’). Top is posterior. Deltoid (d) transected just beneath spina scapula, showing the great origin width compared to humerus width. White dotted line: outline of deltoid in right shoulder; left shoulder was left unmarked. s: scapula. i: infraspinatus. su: subscapularis.
The deltoid is commonly conceived as consisting of three parts – anterior (clavicular), lateral (middle, acromial) and posterior (spinal). However, mention is made of multiple internal tendon bands that point to greater segmentation. Fick even proposed seven functional parts (Fick, 1911). Gray's Anatomy (Williams & Warwick, 1980) mentions a triangular muscle with anterior, posterior and middle fibers, partitioned by the muscle fiber directions into multiple divisions, with four intramuscular septae descending from the acromion to interdigitate with three ascending from the deltoid tuberosity. Other authors mention: one anterior, four middle and one posterior fibrous band descending into tendinous cones (Lorne et al. 2001), three to four tendinous septa descending from the acromion (Kumar et al. 1997), four descending septa interdigitating with three ascending septa (Ko et al. 1998), three separate end tendons that unite in a trapezoid-shaped insertion (Klepps et al. 2004), or take functional EMG from seven locations (Brown & Wickham, 2006). For the purpose of posterior deltoid tendon transposition, muscle fiber lengths and pennate angles, physiological cross-sectional area of the posterior deltoid, and number of end tendon septa (3.3 ± 0.2) were quantified (Herzberg et al. 1999; Friden & Lieber, 2001). Other authors provide little detail and many consider the deltoid a three-part muscle.
The present study aims to provide a qualitative model that would explain the overarching deltoid tendon structure as a basis for improved understanding of the segmental and muscle fiber structure of the deltoid. The model may also provide a conceptual basis for further realistic biomechanical deltoid modeling in functional shoulder modeling.
Tendon model concepts, deltoid tendon nomenclature
In the literature, tendons of the deltoid were referred to by many terms: septa, raphe, tendinous bands, fibrous frame, and, in the subscaluparis, even as ligament-like bands (Klapper et al. 1992). Proliferation of terms for the same anatomical reality induces confusion. Therefore, the tendon as a musculoskeletal system element is reviewed for further model purposes and to introduce a unique terminology. The terminology ‘origin and end tendons’ is proposed and used throughout this study.
Tendon as musculo-skeletal system element
The physiological cross-sectional area of muscles generally far exceeds the skeletal surface area available for their attachment. Muscles therefore require an interface to attach to bone, which is the tendon. Tendon fibers (TF) are much stronger and thus much thinner than muscle fibers (MF) for an equal strength, and require comparatively minute surface areas for attachment (Fig. 2a). Expanding from narrow lines or small areas on bone, tendons provide the extensive surface areas required for MF attachment (Leijnse, 1997a).
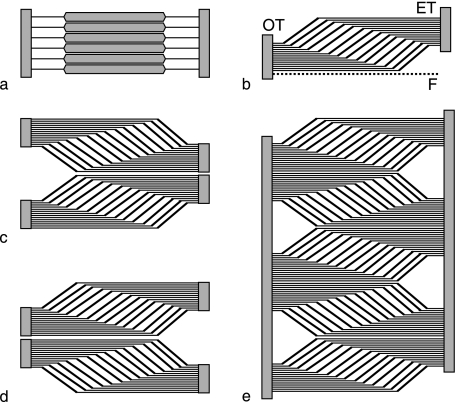
Fig. 2.
Muscle-tendon models. (a) Elementary tendon-muscle-tendon fiber (TMTF) units. (b) TMTF units in unipennate arrangement. OT, ET: origin and end tendon sheets. F: fascia. (c) Two unipennate muscles, not necessarily of the same size, stacked back-to-back, form a bipennate muscle. The unipennate ET fuse into a bipennate ET. (d) Model of (c) reversed: bipennate OT gives rise to two unipennate muscles. (e) Multipennate or multisegmented muscle, formed by stacking unipennate models back to back.
Tendon-muscle-tendon fiber (TMTF) model (Leijnse, 1997b)
As a model basis, it is further assumed that skeletal muscles are parallel assemblies of elementary units. Each unit consists of an individual TF, MF and TF in series (Fig. 2a). In large quantities, the units assume the geometry known as unipennate (Fig. 2b), which allows stacking any number of units without changing stacking geometry. The individual TF collect in TF sheets. Proximal TF sheets may form thin tendinous compartments around the muscles that originate from them. This distinct visual aspect may have inspired the often-used term aponeuroses for proximal TF sheets, as distinct from the ET sheets, which are generally called end tendons (ET). However, no principal material difference exists between origin and end TF sheets, and we will further uniquely call them origin (OT) and end tendons (ET). In their free course, ET sheets especially may remodel and change shape to satisfy, for example, functional biomechanical demands. However, being in series, the OT and ET, irrespective of shape, must be of comparable strength and no less than maximum muscle force, otherwise one would fail. As an assembly of units of equilibrated strength, the TMTF model of Fig. 2b naturally incorporates the strength balance of OT, muscle and ET along the length of the muscle. Irrespective of tendon remodeling in free course, the model seems valid within a free body diagram including the muscle-tendon junctions, as tendons lose/gain thickness with muscle fibers arising/inserting, and terminate/start with the last/first muscle fiber arising/inserting.
Bipennate tendons, intermuscular septae, multisegmented muscles
In the above model terms, a bipennate muscle can be conceived as two unipennate muscles, not necessarily of the same size, stacked back-to-back (Fig. 2c). The unipennate tendons which are back-to–back, fuse into bipennate tendons. The symmetry of the constituent unipennate model is illustrated by reflecting Fig. 2c about a vertical axis (Fig. 2d). The resulting configuration is of a bipennate OT giving rise to two unipennate muscles. Anatomically, bipennate OT sheets are commonly referred to as intermuscular septa (literally, intermuscular partitionings). However, this nomenclature does not express that these septa are actual tendons of muscle origin. Multiple unipennate muscle models can be stacked back-to-back to form multipennate or multisegmented muscles. Figure 2e shows that such muscles are basically symmetric with respect to origin and insertion, with OT and ET interdigitating, as is the case in the central deltoid (see further).
Fascias
TF sheets must be distinguished from fascias, which are here defined as connective tissue sheets enveloping muscles or muscle groups and which do not contain tendon fibers of muscle origin. An OT or ET sheet may be tightly covered by fascia tissue, but the OT or ET fibers themselves are by our definitions not fascia.
Materials and methods
Generic deltoid model
The generic deltoid model starts from the following observations. The narrow line of available bone surface at clavicle, acromion and spina scapula does not suffice for direct attachment of the very large deltoid muscle mass. Therefore, the deltoid muscle body arises from a large OT sheet, which itself originates from the available line on bone. An equally large ET sheet is required to insert the muscle body, as the humerus insertion area is also far too small for direct insertion of all muscle fibers (MF). The generic model starts from a unipennate muscle model, as wide as the deltoid, attached by its origin tendon sheet to the bone line of deltoid origin. The model consists of rectangular OT and ET sheets of equal dimensions connected by parallel MF in equal numbers to a deltoid. The modeling method consists of stepwise transforming the unipennate model into a functional deltoid. Hereby a fundamental model constraint is that the surface areas of OT and ET sheets remain invariant throughout the transformations. Each transformation is a morphological solution to a geometric constraint that must be satisfied to create a functional delta-shaped muscle enveloping a three-axial joint. By proceeding in this way, deltoid morphology emerges as a qualitative solution of a morphological optimization problem with constraints, rather than as a description. The modeling method was introduced by Leijnse (1997a,b,c).
Dissection validation
Specimens
Eight shoulders with arms severed beneath the lower third of the humerus were amputated with entire scapula, clavicle and pectoralis major, from eight lightly embalmed bodies, four male, four female, of 76 ± 12 years of age (Table 1). Light embalming does not perceptibly affect collagen stiffness but preserves a refrigerated specimen for about 3 weeks, allowing detailed observations (Anderson, 2006). The neurovascularization was dissected in detail in one preliminary specimen but was not further systematically considered.
Table 1.
Shoulder specimens
| Specimen | gender | side | Age (years) |
|---|---|---|---|
| S1 | M | L | 94 |
| S2 | M | R | 84 |
| S3 | M | R | 67 |
| S4 | M | R | 56 |
| S5 | F | R | 70 |
| S6 | F | L | 78 |
| S7 | F | L | 82 |
| S8 | F | R | 75 |
| Mean | 4M, 4F | 5R, 3L | 76 ± 12 |
Procedures
Skin and subdermal fat were removed. The scapula was clamped by its posterior edge in the anatomical position of an upright torso, the humerus hanging under gravity. Deltoid segments were identified by careful gradual dissection of MF from the ET blades and photographed with a 50-mm scale in the plane of depth (Nikon D100 6 megapixel camera on a stable tripod with bilateral fluorescent lighting, Nikon Nikkor 60- and 105-mm lenses, at a distance at least 1 m to minimize image distortion, on a black background). After photographic documentation, all MF were cleanly dissected from all tendon surfaces. All ET blades, while attached to the humerus, were photographed per adjacent pairs, spread out on a flat black background, with a 50-mm scale in plane of depth. The cleaned OT were photographed in situ, and thereafter excised in one piece from their bone origin and photographed on a flat black background, front and back, on a 50-mm scale. Normative measures were taken. Number, lengths, surface areas and other quantitative measures of OT and ET were determined.
Data presentation
From this extensive set of photographic and quantitative data, representative samples will be provided here to illustrate the main model principles. The detailed data on OT, ET, deltoid segment and muscle fiber structure will be presented in follow-up publications.
Results
Generic model of deltoid origin and end tendons
End tendons
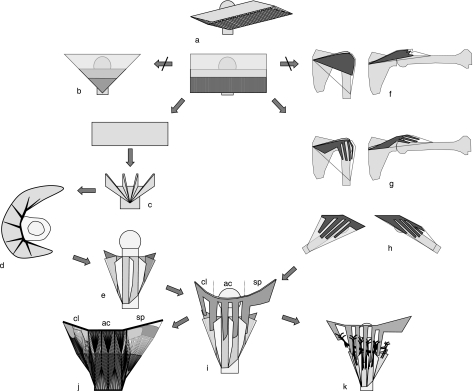
Consider a rectangular unipennate muscle model attached by its OT sheet to the long deltoid origin line at clavicle, acromion and spina scapula (Fig. 3a). The model contains the same amount of MF as a deltoid, spread evenly along its width. To obtain sufficient surface area for attaching such a large amount of MF, the OT and ET sheets need to be even longer than the MF so that these sheets partly overlap. To insert, the rectangular model must be reduced to a triangular (delta) shape. Here a morphological problem arises, as the ET sheet cannot be simply made triangular (Fig. 3b). A triangular ET has less than half the surface area of the OT sheet and is too small to insert all MF. However, ET surface area can be maintained by folding the ET sheet in a number of folds, with the MF at the outside of the folds (Fig. 3c). The innerfold tendon surfaces, at which no MF attach, are folded on each other and merge into single bipennate ET blades, as in Fig. 2c. The result is an ET consisting of a number of bipennate ET blades with interconnecting unipennate ET parts. ET blades receiving the MF from clavicular and spinal origins will concentrate at the anterior and posterior insertion area edges (Fig. 3d). Figure 3e shows a three-dimensional rendition of the resulting ET model.
Fig. 3.
Generic model of deltoid. A unipennate muscle (a) is stepwise transformed into a deltoid model (i). Left branch: generic model of ET. Right branch: OT. For further explanations, see text. The final model (i) shows bipennate OT lamellae interdigitating with bipennate ET blades. cl, ac, sp: clavicular, acromial and spinal parts, resp. j: 2D rendition of muscle fiber structure in a deltoid with seven ET blades and one clavicular, three acromial and one spinal OT lamellae. The tendon structure is detailed in Fig. 6. k: The inter-lamella spaces are natural pathways for segmental neurovascularization.
Deltoid segments
The ET blades define segmental divisions in the deltoid. However, as Fig. 3e indicates, the segments in anterior and posterior parts are not juxtaposed but in a scaled position. This and other possible irregularities, especially in the posterior part, obfuscate a regular segmental structure.
Origin tendons
The unipennate OT sheet in the model of Fig. 3a spans the line of attachment at clavicle, acromion and spina scapula and envelops the shoulder joint like a cuff (Fig. 3f). However, such an enveloping OT shoulder cuff would limit the range of motion of the shoulder as, with abduction, its inferior edge would be stretched. In addition, with abduction the OT sheet would proximally become slack and fold up, and could not functionally transfer the muscle forces to bone (Fig. 3f). This problem is resolved by splitting the OT sheet from its origin onwards along the direction of the tendon fibers into parallel lamellae (Fig. 3g). With shoulder abduction (Fig. 3g) and flexion/extension (Fig. 3h), the lamellae rotate at their bone attachment while remaining parallel, like windshield wipers. As such, they can remain taut and conduct the muscle forces in a straight line to bone throughout the shoulder range of motion. The unipennate OT lamellae can further be transformed into bipennate complements of the bipennate ET blades. This is achieved by folding the lamellae longitudinally in half, with the tendon surfaces without MF at the inside of the folds. These surfaces merge, producing the bipennate OT lamellae described variously in the literature as septa, raphe, tendinous bands or fibrous frame (see Introduction).
Combined OT and ET model
Interdigitating OT and ET blades
The bipennate OT lamellae are morphologically consistent with the ‘folded’ ET model as they naturally interdigitate with the ET blades (Fig. 3i). The ET blades then receive at their enclosed surfaces the MF of the facing surfaces of the interdigitating OT lamellae (Fig. 3j). This implies that although the OT lamellae at their origin are aligned with the bone origin line, they would realign in their distal course more parallel with the ET blades in which their MF insert.
Spinal OT sheets
The dissections revealed that the spinal OT sheets are shorter than the acromial OT and that they are only lamellated near the acromion, and this only in 50% of cases. This is consistent with the above model analysis. The spinal OT sheets are farther from the shoulder joint center of rotation than the other deltoid parts are. Therefore, the spinal OT sheets do not undergo such great deformations with shoulder abduction and flexion/extension as the acromial OT, which are directly above the shoulder joint. Although spinal OT luxation with shoulder motion may not be negligible, it would remain limited to the degree that the resulting internal strain in the spinal OT sheet would not cause its lamellation.
Final model
With the hindsight of dissection results, the final deltoid tendon model is summarized in Fig. 3i. The large continuous ET consists of a number of bipennate tendon blades, which accumulate at the anterior and posterior edges of the insertion area. Centrally, the ET blades are interspaced by and in continuity with unipennate ET sheets. From acromion and clavicle, a bipennate OT lamella descends between each successive pair of ET blades. The spinal part may have a single lamella near the acromion, but is more posteriorly not lamellated and also distinct by being unipennate and superficial to the muscle (see dissection results). Figure 3j shows a 2D rendition of the resulting multisegmented deltoid structure.
Neurovascularization
The neurovascularization (axillary nerve, posterior humeral circumflex artery) reaches the deltoid from the posterior. The interlamellar spaces are natural neurovascularization pathways, consistent with deltoid segmentation (Fig. 3k). However, especially in the posterior acromial part, where the neurovascularization runs most distal, the neurovascularization may have to pass through the posterior unipennate parts of the ET sheet to reach the deltoid muscle segments. Such neurovascular passages may proximally split the posterior ET sheets. The morphology of such neurovascular passages through tendon sheets was investigated in Leijnse (1997c).
Dissection results
General outline
The deltoid arises anteriorly from the distal third of the clavicle, lateral and adjacent to the pectoralis major (Fig. 1A). The deltoid-pectoralis separating landmark is the chiasma through which the vena cephalica passes from a distal superficial to a proximal subclavicular course. Without chiasma, the clavicular deltoid and pectoralis would likely present as a continuous muscle mass. The close relationship between clavicular deltoid and pectoralis is reflected in the narrow confluence of the pectoralis insertion with the most anterior deltoid ET blade (Fig. 1A). Posteriorly, the deltoid origin line continues from the acromion onto the spina scapula up to the posterior spinal edge (Fig. 1B). The great width of the deltoid at its origin, the degree to which it envelops the shoulder joint, and the comparative minuteness of the insertion area at the lateral humerus are illustrated by the cross-sections of Fig. 1C.
Acromial deltoid structure
In dissection of subdermal fat, the superficial fascia of the acromial and clavicular deltoid is found to be degenerate. Subdermal fat interdigitates to greater or lesser extent with superficial muscle fiber bundles, except near the bone origin, where the OT may become superficial (Fig. 4A). Removal of fat reveals in the acromial deltoid a pattern of triangular muscle parts alternatively pointing up or down (Fig. 4B). Downward-pointing triangles are of MF bipennately inserting in an ET blade, as Fig. 3j illustrates. Upward-pointing triangles are of MF diverging from an OT lamella and inserting bilaterally in the ET blades between which the lamella descends. Figure 4C,D shows the acromial OT lamellae in situ after progressive removal of muscle fibers. Figure 4E–G details a single deltoid segment formed by an OT lamella and its two ET blades. Figure 4G shows how far OT lamellae and ET blades overlap. Figure 4H presents an OT lamella and its ET blades after removal of MF, showing that OT lamella and ET blades are of approximately equal length, as in the multisegment model of Fig. 2e. The left ET blade is also of equal shape and therefore has about the same surface area as the OT lamella. This is not apparent in the right ET blade, which is spread wider than in its anatomical position.
Fig. 4.
Acromial deltoid segment structure. (A–D) Lateral deltoid (anterior = left). (A,B) (specimen S0) Deltoid after removal of subdermal fat. (B) Interdigitating muscle fiber triangles eased apart. White dotted lines: ET blades towards which muscle fibers bipennately converge. White striped lines: OT lamellae, from which muscle fibers bipennately diverge. (C,D) (specimen S5) OT lamellae in situ after gradual removal of muscle fibers. (E–G) (specimen S1) Dissection of a single acromial segment. (E) Superficial aspect. (F) Superficial muscle fibers removed from OT lamella. (G) Muscle fiber segment removed from ET blades and retracted. OT lamella and ET blades have approximately the same length and have considerable overlap (double white arrow). (H) (specimen S2) OT lamella and its ET blades, all muscle fibers removed. Shoulder in adduction to minimize OT and ET overlap for photograph. The left ET blade has the same length (white arrows) and width (black arrows) as the OT lamella. The same width relationship is not visually evident in the right ET blade, which is spread wider than in the anatomical position.
Deltoid end tendons
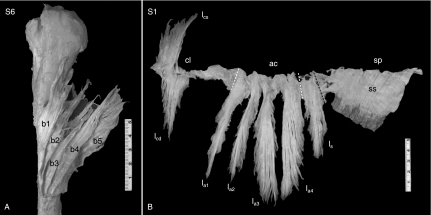
Figure 5A shows five of the seven ET blades of specimen S6 after removal of MF. The entire ET is modeled in Fig. 6. In the eight specimens S1–S8, a mean of 7.8 ± 1.2 blades were found. From an anterior ET blade trunk, inserting anteriorly at the lateral humerus surface, 3.3 ± 0.7 ET blades divided. From a posterior ET blade trunk, inserting posterior at the lateral humerus, 2.9 ± 0.8 ET blades divided. In between, one, two or three single independent ET blades were found, in 4/8, 3/8 and 1/8 cases, respectively, separated by unipennate ET parts. Figure 6 models an ET with two central ET blades, as were found in specimen 6. The anterior ET trunk and the central ET blades inserted in remarkably straight parallel lines (Fig. 6). The posterior ET trunk insertion line was distally more convex.
Fig. 5.
Overview of deltoid origin and end tendons. (A) (specimen S6) ET blades, superimposed, anterior view. This specimen had seven ET blades. The anterior five ET blades are visible (b1–b5); the posterior two blades are hidden beneath the others (see Fig. 6). (B) (specimen S1) Origin tendons, front view, amputated in one piece at origin line after removal of muscle fibers. cl, ac, sp: clavicular, acromial and spinal parts, respectively, marked by white dashed lines. This specimen had two superimposed clavicular OT lamellae (superficial lcs, deep lcd); four acromial lamellae (la1–la4); and one spinal lamella (ls). The posterior spinal OT sheet (ss) is not lamellated. It is superficial to the muscle, with muscle fibers arising unipennately from the backside, as shown by the posterior deltoid muscle mass left in situ. The black dashed line marks the posterior border of the bipennate part of the spinal lamella, which is posterior continuous with the superficial spinal OT sheet.
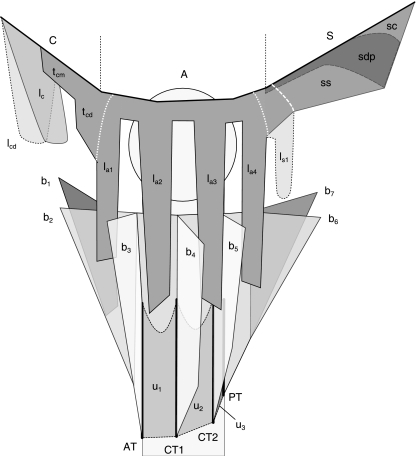
Fig. 6.
Summary model of dissection results. C, A, S: clavicle, acromion, spina. Origin tendons. Proximal at the clavicle, one (lc) or two superimposed (lc, lcd) lamellae are present. More distally, a short, often flimsy superficial unipennate OT sheet arises (tcm) that variably expands (tcd) near the clavicular–acromial joint and is continuous with the first acromial lamella la1. tcd, white dotted line: transition from unipennate OT to bipennate acromial OT. lai: bipennate acromial lamellae, counting three or four. ls1 (dotted outline): single spinal lamella, shorter than the acromial lamellae, found in 50% of cases. ss: superficial spinal OT sheet, continuous with and shorter than acromial OT. la4, white dotted line: transition from bipennate acromial to unipennate spinal OT, if no spinal OT lamella is present. ls1, white striped line: bi- to unipennate transition when a spinal lamella is present. sdp (black striped line): consistently present deep posterior OT sheet, degenerating into a flimsy structure near the acromion. sc: common OT sheet, dividing in superficial and deep posterior OT sheets at some distance from the spinal line of origin S. End tendons. ET model of specimen 6 (Fig. 5A). Blades b1–b3 split from the anterior ET trunk, inserting at line AT (thick black line). Two central single blades b4 and b5, inserting at lines CT1 and CT2, were continuous with unipennate ET sheets u1, u2 and u3, inserting in the humerus surface between the ET blade insertion lines (gray areas within black dotted lines). The posterior ET trunk, inserting in line PT, splits at some distance from its insertion line in two ET blades b6 and b7.
Deltoid origin tendons
Figure 5B presents the entire OT of specimen S1, amputated at the line of bone origin after removal of MF fibers, to illustrate the general findings in specimens S1–S8. Clavicular and spinal OT were in all cases shorter than the acromial OT. Clavicular OT were consistently lamellated, consisting of one (50% of cases) or two (50%) superimposed lamellae of variable size (Fig. 6). Figure 5B is a case with two large clavicular lamellae. In all specimens, clavicular lamellae originated proximally at the clavicular deltoid origin. Deltoid muscle fibers also arose from the clavicular bone surface, especially distal to the lamellae. At mid-clavicular deltoid origin, a short, often flimsy unipennate superficial OT sheet was found (tcm, Fig. 6). At the distal clavicle this OT sheet variably expanded (tcd, Fig. 6) to continue into the anterior acromial lamella, where the OT became internal and bipennate (Figs 5B and 6). At the acromion, all OTs were internal and bipennate up to the spina, where the OT became superficial and unipennate again. In all specimens, the acromial OTs were lamellated, with three (3/8, or 38%) or four (5/8, or 62%) lamellae. Lamella were generally flat and sword-shaped, as in Fig. 5B. However, incidental expansions were found in the form of longitudinal OT flaps or rims. At the posterior side of the posterior acromial lamella, an additional OT expansion of highly variable size was systematically present, in one specimen almost as large as the posterior acromial lamella itself. Spinal OT were continuous with the posterior OT lamella, generally connected by a neck of shorter tendon fibers. Posteriorly at the acromion, a single spinal lamella, in all cases smaller than the acromial lamellae, was present in 50% of specimens (Fig. 5B). In all specimens, posterior to the posterior OT lamella (spinal or acromial), the spinal OT sheet was unlamellated, unipennate and superficial to the muscle (Figs 5B and 6). The transition from internal bipennate OT lamella to superficial unipennate spinal OT sheet is indicated in Figs 5B and 6. Posteriorly at the spina, another OT sheet, deep to the deltoid, was also consistently found, of equal or lesser length than the superficial OT sheet. Anteriorly, near the acromion, this deep OT sheet generally degenerated into a flimsy structure. Together, the superficial and deep posterior spinal OT sheets formed a posterior deltoid OT compartment. Posteriorly, the superficial and deep spinal OT sheets divided from a common tendon sheet at some distance from its line of origin at the posterior spina (Fig. 6).
Discussion
Geometric relationships between deltoid origin and end tendons
A qualitative model was developed to clarify deltoid tendon morphology. The model generated the basic OT and ET shapes, validated by detailed dissections of eight specimens. The model implies quantitative relationships between OT and ET. Indeed, the transformation steps of longitudinal tendon sheet folding or splicing changed neither OT and ET lengths nor surface areas, which were equal in the starting unipennate muscle model. Therefore, in the final model, OT and ET lengths and surface areas would still be equal. This model prediction can be experimentally validated by measuring OT and ET lengths and surface areas. This would allow validation of the model assumption that deltoid OT and ET morphology is fundamentally determined by the requirement for surface area, apart from the further geometric determinants elaborated in the model.
On proliferation and ambiguity in nomenclature for tendons
In the literature, a proliferation of terms for deltoid OT and ET was encountered (septa, raphe, tendinous or even ligament-like bands, fibrous frame). More generally, fascia or ‘investing’ fascia is also occasionally used to indicate what are essentially or mainly OT or ET sheets (e.g. lumbar fascia, fascia lata). Having multiple names for a structure introduces confusion and inadequate names may obfuscate its vital purpose. Specifically, the terms ‘septa’, ‘raphe’ and ‘(investing) fascia’ do not reflect that the named structures are functional tendons, which, when cut or dissected from the muscle, leave the muscle fibers without attachment and consequently dysfunctional. We therefore propose to consistently and uniquely name all tendon fiber collectives to which muscle fibers attach as ‘tendons’– whatever their shape.
Clinical applications
Surgically, the deltoid is involved in two important applications. The proximal part covers the shoulder joint and must be split or detached for open shoulder joint access in rotator cuff surgery and related procedures such as acromionectomy (Neer and Marberry, 1981; Bosley, 1991; Sher et al. 1997; Jeon et al. 2005, McCallister et al. 2005). Improved understanding of the OT structure may help to optimize procedures and minimize functional deltoid comorbidity. Distally, posterior deltoid transposition to the triceps tendon by an interposed tendon graft is a common procedure for restoration of elbow extension in the paralyzed upper limb. Detailed knowledge of deltoid ET morphology and deltoid segment structure is a prerequisite for optimizing surgical aspects, providing information such as how much of the deltoid is actually harvested, the morphologically optimal fixation of the posterior deltoid ET to the tendon graft, anatomic variations that can be expected and the practical problems they may cause, and the achievable excursion. Morphological aspects of these questions will be discussed in follow-up studies.
Acknowledgments
The authors thank G. Prater, PhD, S. Galandiuk, MD, A. Gupta, MD, S. McCabe, MD, R. Shatford, MD, and R. Acland, MD, for their support.
References
- Anderson SD. Practical light embalming technique for use in the surgical fresh tissue dissection laboratory. Clin Anat. 2006;19:8–11. doi: 10.1002/ca.20216. [DOI] [PubMed] [Google Scholar]
- Bailie DS, Moseley B, Lowe WR. Surgical anatomy of the posterior shoulder: effects of arm position and anterior-inferior capsular shift. J Shoulder Elbow Surg. 1999;8:307–313. doi: 10.1016/s1058-2746(99)90151-9. [DOI] [PubMed] [Google Scholar]
- Bitter NL, Clisby EF, Jones MA, Magarey ME, Jaberzadeh S, Sandow MJ. Relative contributions of infraspinatus and deltoid during external rotation in healthy shoulders. J Shoulder Elbow Surg. 2007;16:563–568. doi: 10.1016/j.jse.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bosley RC. Total acromionectomy. A twenty-year review. [See comment] J Bone Joint Surg Am. 1991;73:961–968. [PubMed] [Google Scholar]
- Brown JM, Wickham J. Neuromotor coordination of multisegmental muscle during a change in movement direction. J Musculoskeletal Res. 2006;10:63–74. [Google Scholar]
- Cetik O, Uslu M, Acar HI, Comert A, Tekdemir I, Cift H. Is there a safe area for the axillary nerve in the deltoid muscle? A cadaveric study. [See comment] J Bone Joint Surg [Am] 2006;88:2395–2399. doi: 10.2106/JBJS.E.01375. [DOI] [PubMed] [Google Scholar]
- Chang YW, Hughes RE, Su FC, Itoi E, An KN. Prediction of muscle force involved in shoulder internal rotation. J Shoulder Elbow Surg. 2000;9:188–195. [PubMed] [Google Scholar]
- Chen WJ, Wu CC, Shih CH. Surgical treatment for deltoid contracture in adults. Am J Orthop. 1995;24:488–491. [PubMed] [Google Scholar]
- Chen WJ, Wu CC, Lin YH, Shih CH. Treatment of deltoid contracture in adults by distal release of the deltoid. Clin Orthop. 2000:136–142. doi: 10.1097/00003086-200009000-00022. [DOI] [PubMed] [Google Scholar]
- Ejeskar A. Elbow extension. Hand Clin. 2002;18:449–459. doi: 10.1016/s0749-0712(02)00083-5. [DOI] [PubMed] [Google Scholar]
- Falconer DP. Tendon transfers about the shoulder and elbow in the spinal cord injured patient. Hand Clin. 1988;4:211–221. [PubMed] [Google Scholar]
- Ferreira MI, Bull ML, Vitti M. Participation of the deltoid (anterior portion) and pectoralis major (clavicular portion) muscles in different modalities of supine and frontal elevation exercises with different grips. Electromyogr Clin Neurophysiol. 2003;43:131–140. [PubMed] [Google Scholar]
- Fick R. Handbuch der Anatomie und Mekanik der Gelenke. Jena: Gustav Fischer; 1911. [Google Scholar]
- Friden J, Lieber RL. Quantitative evaluation of the posterior deltoid to triceps tendon transfer based on muscle architectural properties. J Hand Surg [Am] 2001;26:147–155. doi: 10.1053/jhsu.2001.20161. [DOI] [PubMed] [Google Scholar]
- Gabriel DA. Shoulder and elbow muscle activity in goal-directed arm movements. Exp Brain Res. 1997;116:359–366. doi: 10.1007/pl00005763. [DOI] [PubMed] [Google Scholar]
- Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop. 2000;375:250–257. doi: 10.1097/00003086-200006000-00030. [DOI] [PubMed] [Google Scholar]
- Gamulin A, Pizzolato G, Stern R, Hoffmeyer P. Anterior shoulder instability: histomorphometric study of the subscapularis and deltoid muscles. Clin Orthop Relat Res. 2002;398:121–126. [PubMed] [Google Scholar]
- Gill DR, Cofield RH, Rowland C. The anteromedial approach for shoulder arthroplasty: the importance of the anterior deltoid. J Shoulder Elbow Surg. 2004;13:532–537. doi: 10.1016/j.jse.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Herzberg G, Urien JP, Dimnet J. Potential excursion and relative tension of muscles in the shoulder girdle: relevance to tendon transfers. J Shoulder Elbow Surg. 1999;8:430–437. doi: 10.1016/s1058-2746(99)90072-1. [DOI] [PubMed] [Google Scholar]
- Holzbaur KR, Murray WM, Delp SL. A model of the upper extremity for simulating musculoskeletal surgery and analyzing neuromuscular control. Ann Biomed Eng. 2005;33:829–840. doi: 10.1007/s10439-005-3320-7. [DOI] [PubMed] [Google Scholar]
- Hong TC, Kumar VP, Nather A. The posterior neuromuscular compartment of the deltoid. Plastic Reconstr Surg. 2005;115:1660–1664. doi: 10.1097/01.prs.0000161460.95026.15. [DOI] [PubMed] [Google Scholar]
- Hughes RE, Johnson ME, O'Driscoll SW, An KN. Normative values of agonist-antagonist shoulder strength ratios of adults aged 20 to 78 years. Arch Phys Med Rehabil. 1999;80:1324–1326. doi: 10.1016/s0003-9993(99)90037-0. [DOI] [PubMed] [Google Scholar]
- Jeon I-H, Koorevaar R, Neumann L, Wallace WA. Reconstruction of the deltoid and acromion after failed acromionectomy. Clin Orthop Relat Res. 2005;430:100–107. doi: 10.1097/01.blo.0000146535.77776.5f. [DOI] [PubMed] [Google Scholar]
- Johnson GR, Spalding D, Nowitzke A, Bogduk N. Modelling the muscles of the scapula morphometric and coordinate data and functional implications. J Biomech. 1996;29:1039–1051. doi: 10.1016/0021-9290(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Kido T, Itoi E, Lee SB, Neale PG, An KN. Dynamic stabilizing function of the deltoid muscle in shoulders with anterior instability. Am J Sports Med. 2003;31:399–403. doi: 10.1177/03635465030310031201. [DOI] [PubMed] [Google Scholar]
- Klapper RC, Jobe FW, Matsuura P. The subscapularis muscle and its glenohumeral ligament-like bands. A histomorphologic study. Am J Sports Med. 1992;20:307–310. doi: 10.1177/036354659202000312. [DOI] [PubMed] [Google Scholar]
- Klepps S, Auerbach J, Calhon O, Lin J, Cleeman E, Flatow E. A cadaveric study on the anatomy of the deltoid insertion and its relationship to the deltopectoral approach to the proximal humerus. J Shoulder Elbow Surg. 2004;13:322–327. doi: 10.1016/j.jse.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Ko JY, An KN, Yamamoto R. Contracture of the deltoid muscle. Results of distal release. J Bone Joint Surg Am. 1998;80:229–238. doi: 10.2106/00004623-199802000-00010. [DOI] [PubMed] [Google Scholar]
- Kumar VP, Satku K, Liu J, Shen Y. The anatomy of the anterior origin of the deltoid. J Bone Joint Surg Br. 1997;79:680–683. doi: 10.1302/0301-620x.79b4.7353. [DOI] [PubMed] [Google Scholar]
- Langenderfer J, Jerabek S, Thangamani VB, Kuhn JE, Hughes RE. Musculoskeletal parameters of muscles crossing the shoulder and elbow and the effect of sarcomere length sample size on estimation of optimal muscle length. Clin Biomech. 2004;19:664–670. doi: 10.1016/j.clinbiomech.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Lee SB, An KN. Dynamic glenohumeral stability provided by three heads of the deltoid muscle. Clin Orthop Relat Res. 2002;400:40–47. doi: 10.1097/00003086-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Leijnse JN. A generic morphological model of a muscle group – application to the muscles of the forearm. Acta Anat. 1997a;160:100–111. doi: 10.1159/000148002. [DOI] [PubMed] [Google Scholar]
- Leijnse JN. A generic morphological model of the anatomic variability in the m. flexor digitorum profundus, m. flexor pollicis longus and mm. lumbricales complex. Acta Anat. 1997b;160:62–74. doi: 10.1159/000147997. [DOI] [PubMed] [Google Scholar]
- Leijnse JN. Morphology of holes in aponeuroses as caused by perforating nerves or vessels at the medial epicondyle of the elbow. Acta Anat. 1997c;160:42–50. doi: 10.1159/000147995. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J, Hobbs T, Rothwell G. Analysis of posterior deltoid function one year after surgical restoration of elbow extension. J Hand Surg [Am] 2003;28:288–293. doi: 10.1053/jhsu.2003.50057. [DOI] [PubMed] [Google Scholar]
- Liu J, Hughes RE, Smutz WP, Niebur G, Nan-An K. Roles of deltoid and rotator cuff muscles in shoulder elevation. Clin Biomech. 1997;12:32–38. doi: 10.1016/s0268-0033(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Lorne E, Gagey O, Quillard J, Hue E, Gagey N. The fibrous frame of the deltoid muscle. Its functional and surgical relevance. Clin Orthop. 2001;386:222–225. doi: 10.1097/00003086-200105000-00029. [DOI] [PubMed] [Google Scholar]
- McCallister WV, Parsons IM, Titelman RM, Matsen FA., 3rd Open rotator cuff repair without acromioplasty. J Bone Joint Surg Am. 2005;87:1278–1283. doi: 10.2106/JBJS.D.02432. [DOI] [PubMed] [Google Scholar]
- Neer CS, Marberry TA. On the disadvantages of radical acromionectomy. J Bone Joint Surg Am. 1981;63:416–419. [PubMed] [Google Scholar]
- Otis JC, Jiang CC, Wickiewicz TL, Peterson MG, Warren RF, Santner TJ. Changes in the moment arms of the rotator cuff and deltoid muscles with abduction and rotation. J Bone Joint Surg Am. 1994;76:667–676. doi: 10.2106/00004623-199405000-00007. [DOI] [PubMed] [Google Scholar]
- Reinold MM, Wilk KE, Fleisig GS, et al. Electromyographic analysis of the rotator cuff and deltoid musculature during common shoulder external rotation exercises. J Orthop Sports Phys Ther. 2004;34:385–394. doi: 10.2519/jospt.2004.34.7.385. [DOI] [PubMed] [Google Scholar]
- Roman-Liu D, Tokarski T, Kaminska J. Assessment of the musculoskeletal load of the trapezius and deltoid muscles during hand activity. Int J Occup Saf Ergon. 2001;7:179–193. doi: 10.1080/10803548.2001.11076485. [DOI] [PubMed] [Google Scholar]
- Scepi M, Faure JP, Ridoux N, Kamina P, Richer JP. A three-dimensional model of the shoulder girdle. Forces developed in deltoid and supraspinatus muscles during abduction. Surg Radiol Anat. 2004;26:290–296. doi: 10.1007/s00276-004-0225-3. [DOI] [PubMed] [Google Scholar]
- Sher JS, Iannotti JP, Warner JJ, Groff Y, Williams GR. Surgical treatment of postoperative deltoid origin disruption. Clin Orthop Relat Res. 1997;343:93–98. [PubMed] [Google Scholar]
- Smith J, Padgett DJ, Dahm DL, et al. Electromyographic activity in the immobilized shoulder girdle musculature during contralateral upper limb movements. J Shoulder Elbow Surg. 2004;13:583–588. doi: 10.1016/j.jse.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Williams P, Warwick L. Gray's Anatomy. Edinburgh: Churchill Livingstone; 1980. [Google Scholar]
- Wirth MA, Butters KP, Rockwood CA., Jr The posterior deltoid-splitting approach to the shoulder. Clin Orthop Relat Res. 1993;296:92–98. [PubMed] [Google Scholar]
- Wise MB, Uhl TL, Mattacola CG, Nitz AJ, Kibler WB. The effect of limb support on muscle activation during shoulder exercises. J Shoulder Elbow Surg. 2004;13:614–620. doi: 10.1016/j.jse.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Zhao X, Hung LK, Zhang GM, Lao J. Applied anatomy of the axillary nerve for selective neurotization of the deltoid muscle. Clin Orthop Relat Res. 2001;390:244–251. doi: 10.1097/00003086-200109000-00028. [DOI] [PubMed] [Google Scholar]