Abstract
Osteomyelitis often causes functional impairment due to tissue destruction. This report demonstrates a novel previously unappreciated role of osteoblasts. Samples of osteomyelitic bone and bacterially challenged osteoblasts produce increased amounts of antimicrobial peptides in order to combat bacterial bone infection. An osteomyelitis mouse model confirmed the osseous induction of the murine homologue of human β-defensin-2, suggesting a central role in the prevention of bacterial bone infection. Antimicrobial peptides are effectors of the innate defence system and play a key role in host protection at cellular surfaces. Some of them are produced constitutively, whereas others are induced during infection. Human β-defensins represent a major subclass of antimicrobial peptides and act as a first line of defence through their broad spectrum of potent antimicrobial activity. The aim of the present in-vitro and in-vivo investigations was to study the expression and regulation of human β-defensin-2 in the case of bacterial bone infection and to analyse the effects of immunosuppressive drugs on bone-derived antimicrobial peptide expression. Samples of healthy human bone, osteomyelitic bone and cultured osteoblasts (hFOB cells) were assessed for the expression of human β-defensin-2. Regulation of human β-defensin-2 was studied in hFOB cells after exposure to bacterial supernatants, proinflammatory cytokines and immunosuppressive drugs (glucocorticoids and methotrexate) and was assayed by enzyme-linked immunosorbent assay. An osteomyelitis mouse model was performed to demonstrate the regulation of the murine homologue of human β-defensin-2, named murine β-defensin-3, by real-time reverse transcription-polymerase chain reaction and immunohistochemistry. Healthy human bone and cultured osteoblasts are able to produce human β-defensin-2 under standard conditions. Samples of infected bone produce higher levels of endogenous antibiotics, such as human β-defensin-2, when compared with samples of healthy bone. A clear induction of human β-defensin-2 was observed after exposure of cultured osteoblasts to Gram-positive bacteria or proinflammatory cytokines. Additional treatment with glucocorticoids or methotrexate prevented bacteria-mediated antimicrobial peptide induction in cultured osteoblasts. The osteomyelitis mouse model demonstrated transcriptional upregulation of the murine homologue of human β-defensin-2, namely murine β-defensin-3, in bone after intraosseous contamination of the tibia. Human and murine bone have the ability to produce broad-spectrum endogenous antibiotics when challenged by micro-organisms in vitro and in vivo. Immunosuppressive drugs, such as glucocorticoids or methotrexate, may increase the susceptibility to bone infection by decreasing antimicrobial peptide expression levels in case of microbial challenge. The induction of human β-defensin-2 following bacterial contact suggests a central role of antimicrobial peptides in the prevention of bacterial bone infection.
Keywords: antimicrobial peptides, bone, human beta defensin-2, innate immunity
Introduction
Osteomyelitis is a severe infection that is characterized by a progressive inflammatory destruction of bone (Lew & Waldvogel, 1997) and the incidence of this condition appears to be increasing (Jensen et al. 1997). The advancing age of the general population, with an increased incidence of diabetes, peripheral vascular disease, musculoskeletal surgery and the intake of immunomodulating drugs such as dexamethasone or methotrexate, contributes to increased numbers of bone and joint infections. Novel approaches to management are needed, which require an understanding of the pathophysiology of disease, particularly in the era of multi-resistant bacterial strains.
Current studies have demonstrated that cultured osteoblasts produce several inflammatory cytokines (Bost et al. 1999) and chemokines (Bost et al. 2001; Gasper et al. 2002) when exposed to bacterial strains. The production of inflammatory mediators stimulates the circulation of macrophages, neutrophils and activated T-lymphocytes to infected sites and supports the anti-inflammatory host response. Alternatively, the observed high levels of cytokines, such as interleukin (IL)-1 or IL-6, produced by cultured osteoblasts in response to bacterial exposure, may perpetuate inflammation and progressive bone destruction (Marriott et al. 2004, 2005; Marriott 2004). However, the mechanisms necessary for the protective host response are not fully understood.
The host response to bacterial infection is dependent on both innate (non-antibody-mediated) and adaptive (antibody-mediated) immune systems. The adaptive immune system is primarily cellular in composition and relies on the prolonged actions of B and T cells. The innate immune response is more immediate and depends on the activity of phagocytic cells and the expression of a number of proteins and peptides, which are secreted by epithelial and mesenchymal tissues. The rapidity of the innate immune system provides effective host defence against multiple microbes in a manner that is independent of prior exposure to the invading pathogen (Zasloff, 2002).
Antimicrobial peptides (APs) are key elements in the innate immune system, providing the first line of defence in many epithelial and mesenchymal tissues against invading microbes (Harder et al. 1997, 2001; Paulsen et al. 2001, 2002; Varoga et al. 2004). Defensins are an important subfamily of APs and are able to kill microbes by destroying their cell membranes without the need for the cellular immune system. α- and β-defensins are the major subclasses of defensins and differ in the spacing and connectivity of their characteristic six-cysteine residues. To date, six human β-defensins (HBDs) (HBD-1 to HBD-6) have been identified in human tissues.
Human β-defensin-2 was originally isolated from human skin (Harder et al. 1997). Different studies revealed the expression and induction of HBD-2 in various cultured cells upon treatment with proinflammatory cytokines (Harder et al. 1997, 2000; Singh et al. 1998) or bacteria (Harder et al. 1997; Varoga et al. 2004). The constitutive or inducible expression of APs is based on appropriate stimulation (Singh et al. 1998; Harder et al. 2000; Zasloff 2002; Varoga et al. 2004) and the expression pattern differs in the examined tissues. The importance of APs in host defence becomes evident in mice with disrupted AP genes that are prone to infection in the affected organs (Salzmann et al. 2003). Taken together, the expression of APs after bacterial contact seems to be a key mechanism in the prevention of inflammatory diseases. We chose Staphylococcus aureus and Pseudomonas aeruginosa due to their huge clinical relevance in orthopaedic surgery.
Recently, our group demonstrated the induction of the endogenous antibiotics HBD-2 and HBD-3 without bacterial threat in osteoarthritic cartilage (Varoga et al. 2005a,b, 2006). HBD expression was induced after co-cultivation of healthy human cartilage or cultured chondrocytes with inflammatory cytokines such as IL-1 or tumour necrosis factor (TNF)-α. Moreover, the application of HBD-3 protein to cultured chondrocytes and cartilage discs resulted in an increased production of cartilage-degrading matrix metalloproteinases (MMPs) and downregulation of their endogenous regulators, tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 (Varoga et al. 2005a). These results may display previously unrecognized roles of HBDs but further studies will have to elucidate the role of AP in non-inflammatory diseases.
In addition to findings in human tissue, to date more than 10 different murine homologues of HBDs, named murine β-defensins (MBDs), have been isolated and characterized. The in-vivo relevance of MBDs has not yet been clearly demonstrated (Bals et al. 1999; Morrison et al. 1999; Burd et al. 2002; Yamaguchi et al. 2002) but MBD-3 is regarded as the murine homologue of HBD-2, as the amino acid sequence similarity to human counterparts is obvious (Bals et al. 1999).
The aim of the current study was to determine whether human and murine bone is protected against bacterial infection by the production of endogenous antibiotics such as HBD-2.
Materials and methods
Tissues
Healthy human bone (n = 6) was obtained during hip alloplasty surgery (age: 67–73 years). Samples of osteomyelitic bone (n = 6) were obtained from patients who underwent revision surgery at the Trauma Hospital of Hamburg (age: 58–69 years). The collection of the tissues was approved by the institutional review board (UKSH Campus Kiel). The experiments comply with the current laws of Germany.
Human cell culture
The conditionally immortalized human fetal osteoblastic cell line hFOB (Harris et al. 1995) (Promochem, Wesel, Germany, CRL-11372) was cultured in a 1 : 1 mixture of phenol-free Dulbecco's modified Eagle's medium/Ham's F12 medium (GIBCO-BRL, Gaithersburg, MD, USA) containing 10% heat-inactivated fetal calf serum supplemented with geneticin (300 mg mL−1), 2 mm L-glutamine and 10 U mL−1 penicillin/streptomycin (Sigma, Taufkirchen, Germany) at 33.5 °C, the permissive temperature for the expression of the large T antigen. Cells were trypsinized and seeded 48 h before use at an appropriate density. At 80% confluency, stimulation experiments were performed in serum-free Dulbecco's modified Eagle's medium/Ham's F12 medium in a humidified 5% CO2 atmosphere. All experiments of hFOB cells were carried out in triplicate in 25-cm2 culture flasks at the permissive temperature of 33.5 °C.
Stimulants
The hFOB osteoblasts in monolayer culture were exposed to IL-1 (10 ng mL−1), dexamethasone (100 µm), methotrexate (10 ng mL−1) or supernatants of Staph. aureus(1 : 100) or Ps. aeruginosa(1 : 200) for 24 h.
For the production of bacterial culture supernatants, clinical osteomyelitis isolates of Staph. aureus and Ps. aeruginosa were grown in tryptic soy broth (TSB) medium in shaking conditions at 37 °C until they reached an optical density of 1.0. Then 1 mL of this culture was added to 9 mL TSB and the resultant culture was incubated for 24 h in 75-cm2 flasks without shaking. Subsequently, the bacteria were heat-killed in a water bath at 65 °C for 60 min and then centrifuged at 5000 g for 15 min. The resulting supernatants were diluted 1 : 3 in EpiLife medium and used for stimulation experiments. Stimulation experiments with TSB growth medium (diluted 1 : 100 or 1 : 200) were performed to analyse unspecific effects of the medium.
RNA preparation and cDNA synthesis
Frozen tissue samples (20 mg) of healthy and infected murine bone were crushed in an achate mortar under liquid nitrogen. RNA from tissues was generated by Trizol reagent. In addition, RNA from cultured hFOB osteoblasts was extracted with the RNeasy Total RNA Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Contaminating DNA was destroyed by digestion with RNase-free DNase-I (20 min at 25 °C, Boehringer, Mannheim, Germany). After inactivation of DNase (15 min at 65 °C), cDNA was generated with 1 µL (20 pmol) of oligo (dt) primer (Amersham Pharmacia, Uppsala, Sweden) and 0.8 µL of superscript RNase H-reverse transcriptase (Gibco, Paisley, UK) for 60 min at 37 °C.
Reverse transcription-polymerase chain reaction (RT-PCR)
For polymerase chain reaction (PCR), 4 µL of cDNA were incubated with 30.5 µL water, 4 µL 25 mm MgCl2, 1 µL dNTP, 5 µL 10x PCR buffer and 0.5 µL (2.5 U) platinum Taq DNA polymerase (Gibco) and the following pairs of primers: HBD-2-for1 5′-GCCTCTTCCAG GTGTTTTTG-3′, HBD-2-ra 5′-GAGACCACAGGTGCCAATTT-3′, 60 °C, 118 bp and 18 srRNA s 5′-CGGCTACCACATCCAAGGAA-3′, 18 srRNA as 5′-GCTGGAATTACCGCGGCT-3′, 56 °C, which yielded a 186-bp amplified product, served as the internal control for equal amounts of cDNA. Thirty-five cycles were performed with each primer pair. All primers were synthesized by MWG-Biotech AG (Ebersberg, Germany). The positive control cDNA included samples from human lung and epidermis. For the negative control reaction the cDNA was replaced with water.
Real-time RT-PCR
Real-time RT-PCR was carried out using a one-step RT-PCR system (Qiagen; QuantiTect SYBR Green RT-PCR). For this purpose, 100 ng of total RNA was added. Real-time RT-PCR was used to monitor gene expression using an i-Cycler (Biorad, München, Germany) according to the standard procedure. The PCR was performed using ‘Hot StarTaq’ DNA Polymerase, which is activated by an initial heating step, whereas Omniscript Reverse Transcriptase is deactivated. The temperature profile included an initial denaturation for 15 min at 95 °C, followed by 37 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, elongation at 72 °C (the elongation time depends on the size of the fragment and the number of base pairs divided by 25 yielded the time in seconds) and fluorescence monitoring at 72 °C. I-Cycler Data Analysis Software (Biorad) was used for PCR data analysis. The specificity of the amplification reaction was determined by performing a melting curve analysis.
For SYBR-green real-time RT-PCR the following primers were used: MBD-3 sense, 5′-AAAGGAGGCAGATGCTGGAA-3′; MBD-3 antisense, 5′-AAGGAACTCCACAACTGCCAAT-3′; TNF-α sense, 3′-ATCCGCGACGTGGAACTG-5′ and TNF-α antisense, 3′-ACCGCCT GGAGTTCTGGAA-5′[primer design was performed using Primer Express 2.0 (Applied Biosystems, Darmstadt, Germany)]. Relative quantification was performed by normalizing the signals of the different genes against those of β-actin. The assessed data included three independent experiments with triplicates.
Immunohistochemistry
After the fixation of human bone in 4% paraformaldehyde, the tissue was decalcified with EDTA embedded in paraffin, sectioned and dewaxed. Sections were blocked with 3% hydrogen peroxide (endogenous peroxidases) and subsequently incubated with normal serum (1 : 5 in Tris-buffered saline) from the species in which the primary antibody was raised. Immunohistochemical staining was performed overnight at 4 °C on 6-µm paraffin sections using primary antibodies against HBD-2 (1 : 30; 900-K172, rabbit anti-human; PEPROTECH, Paris, France). This was followed by incubation with the biotinylated secondary antibody (1 : 400; E0353, swine anti-rabbit; DAKO, Glostrup, Denmark) and a peroxidase-labelled streptavidin-biotin staining technique. Diaminobenzidine was used for staining. After counterstaining with haematoxylin, the sections were finally mounted with Aquatex (Boehringer). Negative controls were carried out by absorption of the primary antibody by human recombinant proteins. Sections of human skin served as internal positive controls. The expression of MBDs was examined in a septic osteomyelitis mouse model using MBD-3 antibodies overnight at 4 °C (1 : 50, sc-30116, Santa Cruz Biotechnology, Santa Cruz, USA). This was followed by incubation with the biotinylated secondary antibody (1 : 400; E0353, swine anti-rabbit; DAKO) and a peroxidase-labelled streptavidin-biotin staining technique. Diaminobenzidine was used for staining. After the mice were killed via cervical dislocation, their tibiae were removed, fixed in 4% paraformaldehyde and demineralized with EDTA. Sections of mouse skin served as positive controls.
HBD-2 ELISA
For enzyme-linked immunosorbent assay (ELISA), 100 mg fresh weight of healthy and infected bone tissue was crushed in an achate mortar under liquid nitrogen and homogenized in 150 mm NaCl, 20 mm Tris HCl buffer, pH 7.4, using a polytron homogenizer (Kinematica, Luzern, Switzerland). A soluble fraction was obtained by centrifugation at 48 000 g for 60 min. Subsequently, 200-µL aliquots of this homogenate and aliquots of the collected cell supernatants from the stimulation experiments were examined by sandwich ELISA. Immunoplates (96-well) (MaxiSorp™, Nunc, Roskilde, Denmark) were coated at 4 °C for 24 h with 50 µL (0.5 µg mL−1) goat anti-HBD-2 antibody (PP1125P2, Acris, Hiddenhausen, Germany) diluted 1 : 500 in 0.05 m carbonate buffer, pH 9.6. Subsequently, wells were blocked with 200 µL 1% bovine serum albumin in phosphate-buffered saline (PBS) for 10 min at room temperature 20 °C. After three washes with 200 µL PBS + 0.1% Tween 20, 100 µL per well of cell culture supernatants was incubated for 30 min at room temperature. Plates were washed three times with PBS + 0.1% Tween 20 and wells were incubated for 30 min at room temperature with 50 µL of biotinylated goat anti-HBD-2 antibody (PP1125B1, Acris) diluted 1 : 2500 to 0.2 µg mL−1 in PBS + 0.1% Tween 20. Plates were again washed three times with PBS + 0.1% Tween and filled with 50 µL/well of Streptavidin-POD (1 : 10 000 in PBS + 0.1% Tween 20; Roche Diagnostics, Mannheim, Germany). The plates were then incubated for 30 min at room temperature, washed three times as described above and incubated with 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (Roche Diagnostics) as the development agent for 15–45 min at room temperature in the dark. The absorbance was measured at 405 nm with a multichannel photometer (Sunrise; Tecan, Crailsheim, Germany). Human recombinant HBD-2 (PeproTec, Rocky Hill, USA) served as the standard at the following concentrations: 0, 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50, 100, 200, 400 and 800 ng mL−1.
Osteomyelitis mouse model
BALB/c mice (8- to 12-week-old inbred males) (six for each group) were used in all osteomyelitis experiments. They were housed in the animal facility of the University of Kiel under standard conditions of light and temperature.
The intraosseous injections were performed according to the technique recently described by Lucke et al. (2003) on mice anesthetized by isofluorane. A volume of 10 µL of bacterial suspension was injected into the osseous cavity of the tibiae containing 106 CFU Staph. aureus/mL, of a clinical osteomyelitis isolate (n = 6). Animals in the control group were injected with the same volume of PBS (n = 6). At 12 h after injection of the bacteria, the mice were killed via cervical dislocation and the shinbones were removed and either fixed in 4% paraformaldehyde for immunohistochemical evaluation or prepared for real-time RT-PCR. The animal study was approved by the Ministry of Environment, Nature and Forests.
Statistical analysis
Data are expressed as the mean ± SD of tested samples. Statistical significance was evaluated by the T-test, P ≤ 0.05.
Results
Induction of HBD-2 in infected bone
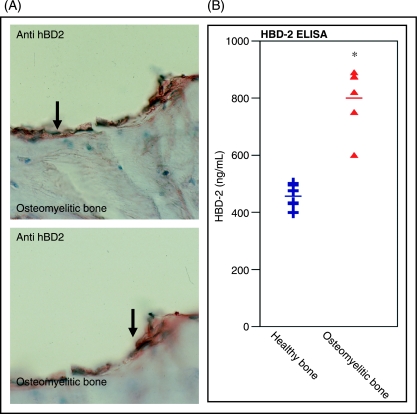
As recent reports demonstrated qualitative expression of AP transcripts and protein in healthy and inflamed human bone (Varoga et al. 2003; Warnke et al. 2006), ELISA experiments were performed to estimate quantities of HBD-2 in collected healthy and infected tissue samples. Immunhistochemistry confirmed strong HBD-2 expression in tissue samples of infected bone (Fig. 1A). In the case of Gram-positive bacterial bone infection, HBD-2 expression levels doubled to 800 ng mL−1, thus suggesting a direct influence of the bacteria in the regulation of bone-derived APs (Fig. 1B).
Fig 1.
Induction of human β-defensin (HBD)-2 in osteomyelitic bone. Immunohistochemistry revealed a strong immunoreactivity to HBD-2 antibody in tissue samples of human infected bone (A). Enzyme-linked immunosorbent assay (ELISA) experiments demonstrate basal HBD-2 expression levels in samples of healthy human bone. In the case of bacterial bone infection, increased levels of HBD-2 protein (up to 800 ng mL−1) were determined, thus suggesting a bacterial influence on the induction of bone-derived antimicrobial peptides (B). Values are the mean ± SD. *P < 0.05 vs controls.
IL-1 and Staph. aureus stimulate HBD-2 expression in cultured osteoblasts (hFOB cells)
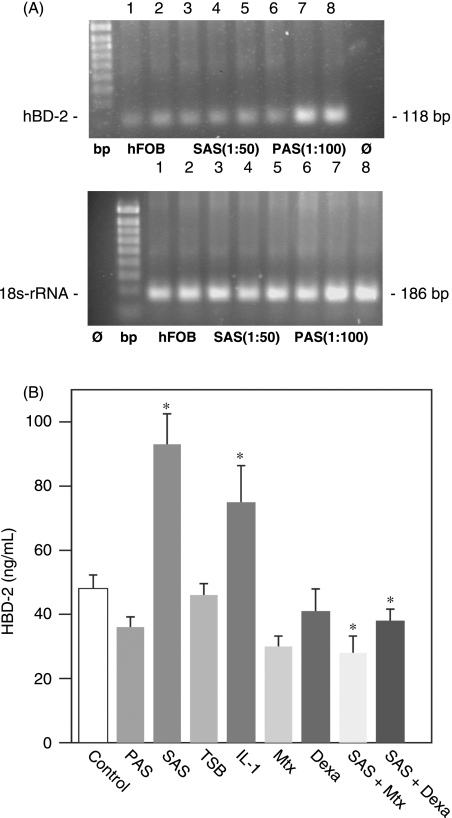
In order to establish a suitable in-vitro model, we investigated hFOB osteoblasts with regard to the production and regulation of HBD-2. Similar to recent results in human bone, cultured hFOB osteoblasts are able to produce HBD-2 transcripts under standard conditions and in the case of bacterial stimulation (Fig. 2A).
Fig 2.
Glucocorticoids and methotrexate prevent bacteria-mediated induction of human β-defensin (HBD)-2 in hFOB osteoblasts. (A) Reverse transcription-polymerase chain reaction was performed to assess the HBD-2 transcription pattern in cultured hFOB osteoblasts. As shown in A, hFOB cells are able to produce HBD-2 transcripts under standard conditions (lanes 1 and 2, unstimulated hFOB cells) and the expression pattern did not change following bacterial stimulation (lanes 3–8). Stimulation experiments with Staphylococcus supernatants (SASs) (diluted 1 : 100) demonstrated the clearest bands (lanes 6–8). PAS, Ps. aeruginosa supernatant (diluted 1 : 200). (B) Subsequently, we performed enzyme-linked immunosorbent assay experiments to analyse the secreted protein levels of cultured osteoblasts after 24 h incubation with interleukin (IL)-1 (10 ng mL−1) or supernatants of Gram-positive Staph. aureus(diluted 1 : 100) or Ps. aeruginosa (diluted 1 : 200). In contrast to unstimulated osteoblasts, the amounts of HBD-2 increased to nearly 100 ng mL−1 in the case of Gram-positive bacterial stimulation, whereas supernatants of Gram-negative Ps. aeruginosa failed to induce HBD-2 expression. Co-incubation with immunosuppressive drugs such as methotrexate (Mtx) or dexamethasone (Dexa) prevented the Staph. aureus-mediated HBD-2 induction in cultured osteoblasts. Stimulation experiments with tryptic soy broth growth medium were performed to analyse the unspecific effects of the bacterial growth medium. Values are the mean ± SD of three independent experiments. *P < 0.05 vs controls.
To assess the influence of the proinflammatory cytokine IL-1 (10 ng mL−1) or bacterial supernatants of Staph. aureus (diluted 1 : 100) or Ps. aeruginosa (diluted 1 : 200) on HBD-2 expression in hFOB cells, cell culture supernatants were collected from hFOB cells after being exposed to these inflammatory stimulators for 24 h. ELISA experiments revealed increased amounts of secreted HBD-2 protein (up to 100 ng mL−1) after stimulation with Gram-positive Staph. aureus. In contrast, Ps. aeruginosa failed to induce osteoblast-derived HBD-2 protein production. Moreover, 24 h of IL-1 stimulation resulted in an upregulation of HBD-2 protein as measured by ELISA (Fig. 2B).
Immunosuppressive drugs such as dexamethasone and methotrexate prevent Staph. aureus-mediated induction of HBD-2 in osteoblasts (hFOB cells)
As the susceptibility to bone and joint infections is increased in patients with a regular intake of immunosuppressive drugs, we analysed the effects of dexamethasone (100 µm) and methotrexate (10 ng mL−1) on the AP production of cultured osteoblasts. Incubation of hFOB cells with both drugs did not significantly alter basal HBD-2 expression. As incubation with supernatants of Staph. aureus resulted in an increased expression of HBD-2 in osteoblasts, we performed a co-incubation with dexamethasone and methotrexate for 24 h. Interestingly, the presence of both immunosuppressive drugs prevented the bacteria-mediated induction of HBD-2 in osteoblasts (Fig. 2B).
Increased expression of MBD-3 in bone after microbial contamination
To investigate the in-vivo expression pattern of MBD-3 in bone, the shinbones of BALB/c mice (age 18–40 weeks) were infected with a clinical isolate of Staph. aureus (10 µL containing 106 CFU Staph. aureus) and were examined by immunohistochemistry and real-time analysis.
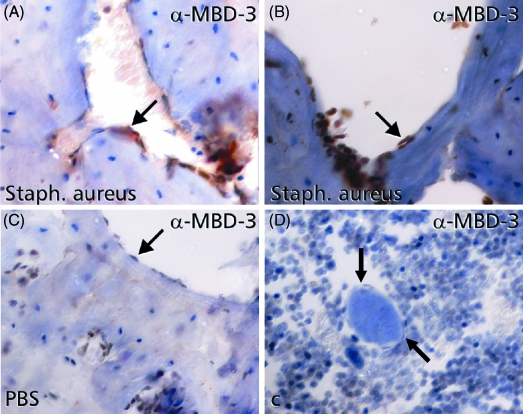
At 12 h after microbial contamination of the medullary cavity of murine shinbones, immunohistochemistry revealed increased expression of MBD-3 in osteoblasts (Fig. 3A and B). In the case of intramedullary PBS injections into murine shinbones, immunohistochemical staining revealed nearly no reactivity to MBD-3 antibody in osteoblasts of murine bone (Fig. 3C).
Fig 3.
Induction of the murine homologue of human β-defensin-2, named murine β-defensin (MBD)-3, in bone after intraosseous contamination with Staph. aureus. Cross-sections of murine shinbones, treated with intraosseous phosphate-buffered saline (PBS) injections showed nearly no immunoreactivity (arrows) to MBD-3 antibody in endostal osteoblasts (C). Application of Staph. aureus into the medullary cavity of murine shinbones resulted in an increased expression of MBD-3 in the pericellular matrix of endostal osteoblasts (A and B). Negative controls were carried out by absorption of the primary antibody by recombinant protein (D).
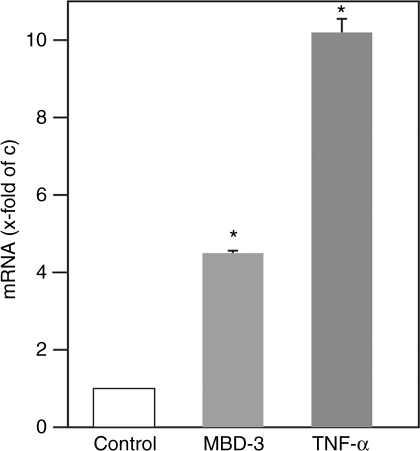
To verify these results, real-time RT-PCR of the obtained tissue samples was performed. In contrast to control animals, the relative MBD-3 expression in bone increased nearly fourfold after microbial challenge. The strong induction of the proinflammatory cytokine TNF-α in the obtained samples of infected bone confirmed the effective contamination of the medullary cavity of these mice (Fig. 4).
Fig 4.
Increased gene expression of murine β-defensin (MBD)-3 in bone after Gram-positive bacterial challenge. Real-time reverse transcription-polymerase chain reaction was performed. After bacterial challenge, MBD-3 transcripts were increased fourfold in infected animals when compared with controls. Moreover, elevated numbers of tumour necrosis factor (TNF)-α transcripts were measured in bacteria-challenged shinbones, validating an effective bone infection. *P < 0.01 vs control.
Discussion
Bone infection or osteomyelitis in general is characterized by uncontrolled inflammation and destructive bone loss, although little is known about the immunopathogenesis of infection (Wright & Friedland, 2002). The increasing incidence of Staph. aureus-caused bacterial infections and the emergence of strains of this organism that are resistant or show reduced susceptibility to antibiotics such as methicillin (Heseltine, 2000) and vancomycin (Tenover et al. 2001) have made it imperative to understand the pathogenesis of Staph. aureus-associated disease. In addition to the production of their extracellular matrix, osteoblasts are able to produce an array of immune molecules following bacterial challenge that could recruit leukocytes to sites of infection and promote inflammation during osteomyelitis. Our study demonstrates that osteoblasts are able to produce anti-inflammatory peptides such as HBD-2 in vitro and in an animal model of staphylococcal osteomyelitis. We provide evidence for a new role of osteoblasts during infection of bone tissues, i.e. the ability to produce APs and modulating immune responses in inflammatory bone diseases.
Recently, the qualitative expression of HBDs was demonstrated in tissue samples of healthy and infected mandibular bone and cultured osteoblasts by means of RT-PCR and immunohistochemistry (Varoga et al. 2003; Warnke et al. 2006) but, until now, no study has investigated the regulation and induction of APs in bone in the case of inflammatory threats.
To assess the influence of inflammatory or bacterial agents on HBD-2 expression in osteoblasts, ELISA experiments were performed to assess the osteoblast-derived HBD-2 protein levels. After 24 h of Gram-positive bacterial stimulation, HBD-2 levels increased to 100 ng mL−1. In contrast to Staph. aureus, Gram-negative bacteria such as Ps. aeruginosa failed to induce HBD-2 expression in cultured hFOB osteoblasts. Interestingly, HBD-2 demonstrates a greater microbiocidal activity against Gram-negative bacteria like Ps. aeruginosa with a lethal dose-50 near 10 µg mL−1 (Harder et al. 1997) and is therefore required at lower expression levels to defend against Gram-negative bacteria. In accordance with previous findings on epithelia and cartilage (Harder et al. 1997, 2000; Singh et al. 1998; Varoga et al. 2004), we would also have expected an HBD-2 induction in bone following Ps. aeruginosa stimulation. A plausible explanation for the absent induction of osteoblast-derived HBD-2 after Gram-negative bacterial stimulation is based on recent investigations, in which bacterial products and inactivated bacteria are far less potent in eliciting a cell-based immune response (Bost et al. 2001; Gasper et al. 2002) than live bacteria. Therefore, we propose that the observed AP expression pattern in osteoblasts following bacterial stimulation is partly related to the bacterial supernatants used and is therefore different to previous investigations (Harder et al. 1997, 2000; Singh et al. 1998; Varoga et al. 2004). We finally chose a Gram-positive strain due to its higher clinical relevance.
A very interesting observation is the missing induction of osteoblast-derived HBD-2 in the presence of immunosuppressive drugs such as dexamethasone and methotrexate. These agents were frequently used in chronic inflammatory diseases such as rheumatoid arthritis (Emery, 2006) and may be responsible for the increased susceptibility of these patients to bone and joint infection by decreasing the bone- and articular joint-derived AP expression.
Other studies have illuminated the mechanisms by which osteoblasts can perceive and respond to bacteria by demonstrating the functional expression of members of the toll-like family (toll like receptor) of cell surface pattern recognition receptors by osteoblasts (Kikuchi et al. 2001; Gasper et al. 2002). However, the expression of these toll like receptors does not preclude the involvement of other pattern recognition receptors in the initiation of osteoblast immune responses. A family of novel nucleotide-binding oligomerization domain (NOD) proteins has recently been identified in both immune and non-immune cell types, whose members include at least two that appear to serve as intracellular pattern recognition receptors (Inohara & Nunez, 2003). As such, NOD1 and NOD2 could play an important role in the detection of intracellular pathogens of osteoblasts, including Staph. aureus (Girardin et al. 2003a,b,c), but the role of NOD receptors in the induction of osteoblast-derived APs has to be evaluated in future experiments.
Human β-defensin-2 has been found to be involved in the chemotaxis of immature dendritic cells and memory T cells, thus resulting from their direct binding and activation of the chemokine C-C motif receptor 6 (Yang et al. 1999; Wu et al. 2003). By linking the innate and adaptive immune response, APs may induce the production of proinflammatory mediators, initiate antigen-specific activation of infiltrating cells and facilitate the development of cell-mediated host responses to invading pathogens. The increased expression of HBD-2 and its murine analogon MBD-3 in osteoblasts after Gram-positive bacterial challenge suggests a role in the AP-mediated host response but a chemokine function by regulating leukocyte influx into the bone and supporting the adaptive immune system is another explanation for the rapid induction.
Similarities between mammalian β-defensins may permit the use of murine models to further define the role of these peptides in innate host defence. To demonstrate the in-vivo expression of the murine analogon of HBD-2, named MBD-3 in bone, we used a mouse osteomyelitis model. MBD-3 showed the highest sequence homology to its human counterpart and was evaluated after bacterial contamination of the medullary cavity of murine shinbones. Until now, more than 10 different MBDs (Yamaguchi et al. 2002) have been characterized but the inducibility of MBDs seems to depend on the examined tissues (Bals et al. 1999; Morrison et al. 1999; Burd et al. 2002).
The expression of MBD-3 clearly increases after the inoculation of Staph. aureus into the medullary canal of murine shinbones. Compared with a basal expression in PBS-injected bones, immunohistochemistry and real-time RT-PCR revealed an increased MBD-3 expression in endost cells following bacterial contamination. The antimicrobial activity of MBD-3 against Gram-positive pathogens has not been clearly determined so far (Bals et al. 1999; Burd et al. 2002) but induction subsequent to Staph. aureus inoculation suggests antibacterial activity as well. The upregulation of TNF-α gene expression in samples of infected murine bone displayed the effective osseous contamination and served as an internal control.
In summary, based on our in-vitro and in-vivo results, we propose a novel role for osteoblasts in the case of bacterial challenge. The expression and induction of HBD-2 in osteomyelitic bone in order to combat microbial invasion in the first hours of infection may be key mechanisms in the prevention of bacterial bone disease. The biological importance of HBD-2 in bone has to be evaluated in future experiments.
Acknowledgments
We thank K. Brabänder, S. Echterhagen, I. Geurink, C. Jaeschke, P. Kozak, S. Lorenzen, U. Mundt, M. Nicolau, A. Rüben and S. Seiter for their excellent technical assistance and C. Franke for the drawing. Part of this work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 617, project A22; DFG Va 220/2-1; Pu 214/3-2; Pu 214/4-2 and Pu 214/5-2) and by a grant from the ‘Stiftung zur Förderung der Medizinischen Forschung’ of the Medical Faculty of the University of Kiel (to D.V. and T.P.).
References
- Bals R, Wang X, Meegalla RL, et al. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun. 1999;67:3542–3547. doi: 10.1128/iai.67.7.3542-3547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost KL, Bento JL, Petty CC, Schrum LW, Hudson MC, Marriott I. Monocyte chemoattractant protein-1 expression by osteoblasts following infection with Staph. aureus or Salmonella. J Interferon Cytokine Res. 2001;21:297–304. doi: 10.1089/107999001300177484. [DOI] [PubMed] [Google Scholar]
- Bost KL, Ramp WK, Nicholson N, Bento JL, Marriott I, Hudson MC. Staph. aureus infection of mouse or human osteoblasts induces high levels of IL-6 and IL-12 production. J Infect Dis. 1999;180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- Burd RS, Furrer JL, Sullivan J, Smith AL. Murine beta-defensin-3 is an inducible peptide with limited tissue expression and broad-spectrum antimicrobial activity. Shock. 2002;18:461–464. doi: 10.1097/00024382-200211000-00013. [DOI] [PubMed] [Google Scholar]
- Emery P. Treatment of rheumatoid arthritis. BMJ. 2006;332:152–155. doi: 10.1136/bmj.332.7534.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasper NA, Petty CC, Schrum LW, Marriott I, Bost KL. Bacteria induced CXCL10 secretion by osteoblasts can be mediated, in part, through TLR4. Infect Immun. 2002;70:4075–4082. doi: 10.1128/IAI.70.8.4075-4082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Carneiro LA, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003a;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003b;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Travassos LH, Herve M, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003c;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schröder JM. Isolation and characterization of human (human beta defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Harder J, Meyer-Hoffert U, Teran LM, et al. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1-beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- Harris SA, Enger RJ, Riggs BL, Spelsberg TC. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10:178–186. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- Heseltine P. Has resistance spread to the community? Clin Microbiol Infect. 2000;2:11–16. doi: 10.1046/j.1469-0691.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- Jensen AG, Espersen F, Skinhoj P, Rosdahl VT, Frimodt-Moller N. 1980–1990 Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark. J Infect. 1997;34:113–118. doi: 10.1016/s0163-4453(97)92395-1. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Matsuguchi T, Tsuboi N, et al. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J Immunol. 2001;166:3574–3579. doi: 10.4049/jimmunol.166.5.3574. [DOI] [PubMed] [Google Scholar]
- Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- Lucke M, Schmidmaier G, Sadoni S, et al. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32:521–531. doi: 10.1016/s8756-3282(03)00050-4. [DOI] [PubMed] [Google Scholar]
- Marriott I. Osteoblast responses to bacterial pathogens: a previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol Res. 2004;30:291–308. doi: 10.1385/IR:30:3:291. [DOI] [PubMed] [Google Scholar]
- Marriott I, Gray DL, Rati DM, et al. Osteoblasts produce monocyte chemoattractant protein-1 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Bone. 2005;37:504–512. doi: 10.1016/j.bone.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Marriott I, Gray DL, Tranguch SL, et al. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am J Pathol. 2004;164:1399–1406. doi: 10.1016/S0002-9440(10)63226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GM, Davidson DJ, Dorin JR. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 1999;442:112–116. doi: 10.1016/s0014-5793(98)01630-5. [DOI] [PubMed] [Google Scholar]
- Paulsen F, Pufe T, Conradi L, et al. Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes. J Pathol. 2002;198:369–377. doi: 10.1002/path.1224. [DOI] [PubMed] [Google Scholar]
- Paulsen F, Pufe T, Petersen W, Tillmann B. Expression of natural peptide antibiotics in human articular cartilage and synovial membrane. Clin Diagn Lab Immunol. 2001;8:1021–1023. doi: 10.1128/CDLI.8.5.1021-1023.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:478–479. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- Singh PK, Jia HP, Wiles K, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC, Biddle JW, Lancaster MV. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerg Infect Dis. 2001;7:327–332. doi: 10.3201/eid0702.010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoga D, Paulsen F, Kohrs S, et al. Expression and regulation of human beta-defensin-2 in osteoarthritis. J Pathol. 2006;209:166–173. doi: 10.1002/path.1974. [DOI] [PubMed] [Google Scholar]
- Varoga D, Pufe T, Harder J, et al. Production of endogenous antibiotics in articular cartilage. Arthritis Rheum. 2004;50:3526–3534. doi: 10.1002/art.20605. [DOI] [PubMed] [Google Scholar]
- Varoga D, Pufe T, Harder J, et al. Human β-defensin-3 mediates tissue remodelling processes in articular cartilage by increasing metalloproteinases and reducing their endogenous inhibitors. Arthritis Rheum. 2005a;52:1736–1745. doi: 10.1002/art.21090. [DOI] [PubMed] [Google Scholar]
- Varoga D, Pufe T, Mentlein R, et al. Expression and regulation of antimicrobial peptides in articular joints. Ann Anat. 2005b;187:499–508. doi: 10.1016/j.aanat.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Varoga D, Pufe T, Petersen W, Tillmann B, Kloppenburg O, Paulsen F. Expression of antimicrobial peptides in bone. Calcified Tissue Int. 2003;72:356. [Google Scholar]
- Warnke PH, Springer IN, Russo PA, et al. Innate immunity in human bone. Bone. 2006;38:400–408. doi: 10.1016/j.bone.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Wright KM, Friedland JS. Differential regulation of chemokine secretion in tuberculous and staphylococcal osteomyelitis. J Bone Miner Res. 2002;17:1680–1690. doi: 10.1359/jbmr.2002.17.9.1680. [DOI] [PubMed] [Google Scholar]
- Wu Z, Hoover DM, Yang D, et al. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci USA. 2003;100:8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Nagase T, Makita R, et al. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol. 2002;169:2516–2523. doi: 10.4049/jimmunol.169.5.2516. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]