Abstract
We identified a case of Alzheimer’s disease with a deletion of the lysine residue at codon 280 (ΔK280) in exon 10-encoded microtubule-binding repeat domain of the tau gene (MAPT). This mutation was originally identified in a sporadic case of frontotemporal dementia (FTD) with a family history of Parkinson’s disease. In the original report, the authors were careful in their assessment of the pathogenicity and suggested one could not be sure whether the mutation was pathogenic or not. The mutation has always presented a conundrum because it is the only known mutation, of assumed pathogenicity, which increases the proportion of 3-repeat tau mRNA in in vitro assays. Here we present the clinical and pathological features of a new case with this mutation and discuss whether the mutation is indeed pathogenic.
Introduction
While mutations in the tau gene (MAPT) are a common cause of dementia (Hutton et al., 1998; Spillantini et al., 1998; Poorkaj et al., 1999), the ΔK280 mutation in MAPT has always presented a conundrum. All the other pathogenic mutations either alter the amino acid sequence and decrease microtubule binding or alter exon 10 splicing such that more exon 10 is included in the sequence, leading to a preponderance of 4 repeat tau (4R-tau) transcripts (Pittman et al., 2006). In contrast, the ΔK280 variant alone, strongly inhibits exon 10 inclusion and leads to an excess of 3 repeat tau (3R-tau) transcripts (D’Souza et al., 1999). However, the mutant 4R-tau transcript, which would be predicted to occur in very small amounts since the mutation is in exon 10 and thus would be expected to be largely self-excluding, is extremely fibrillogenic and has frequently been used to model tau aggregation (Barghorn et al., 2000; Mukrasch et al., 2005; Vogelsberg-Ragaglia et al., 2005; von Bergen et al., 2001). Furthermore, it has been demonstrated to strongly reduce the ability of tau to promote microtubule assembly (Rizzu et al., 1999). In addition, the original report of the occurrence of this mutation, in a Dutch individual with sporadic FTD, but with a father with reported Parkinson’s disease, was very circumspect and did not claim that this mutation was pathogenic (Rizzu et al., 1999). A follow up of this original report has recently been published, showing that the tau deposition in this individual is largely in the form of Pick bodies, and that the predominant tau isoform deposited was 3R-tau but that some 4R-tau was also found (van Swieten et al. 2007).
We have been sequencing MAPT extensively to find other pathogenic MAPT mutations and polymorphisms at the locus (Malkani et al. 2006). This included the sequencing of exon 10 in several hundred individuals, during which we identified a second individual with the ΔK280 mutation; an individual with late onset Alzheimer’s disease with no documentation of a family history. Here we present the data from this case and discuss whether ΔK280 is indeed pathogenic and, if it is, what might be the mechanism of its pathogenicity.
Materials and Methods
Patient material
The autopsy program of the Kathleen Price Bryan Brain Bank has been in existence since 1985 (Hulette et al. 1997). Patient recruitment and enrolment procedures have been approved by the Institutional Review Board at Duke University Medical Center.
DNA Sequencing
We sequenced exon 10 of MAPT using the protocol we have previously described in approximately 150 frontotemporal dementia cases, 300 North American and British Controls and 200 Alzheimer’s disease cases (Malkani et al. 2006).
Immunohistochemistry
Serial sections were cut at a thickness of 6μm from formalin-fixed, wax-embedded blocks and mounted onto APES-coated slides. One set of sections was immunostained for insoluble, pathological tau proteins by a standard immunoperoxidase method using the phospho-dependent tau antibody AT8 (1:750; Innogenetics, Belgium; against phosphorylated Ser202/Thr205 tau epitopes). Other sets of sections were stained with the 3R- and 4R-tau specific monoclonal antibodies, RD3 (1:3000; Upstate/Chemicon, Dundee, UK) and RD4 (1:100; Upstate/Chemicon, Dundee, UK), respectively, as described (de Silva et al, 2003). A second set was immunostained with anti-Aβ antibody (1:100; Dako, Ely, UK) for Alzheimer’s disease β-amyloid pathology. Briefly, sections were deparaffinised in xylene and rehydrated in decreasing concentrations of alcohol. Endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol for 10 mins. For tau staining, sections were pre-treated by pressure cooking for 10 mins in 0.01M citrate buffer pH6.0. For Aβ staining, sections were first pre-treated with formic acid for 10 mins prior to pressure cooking. Sections were incubated in 10% (w/v) non-fat milk to block non-specific staining, then with the primary antibodies AT8, RD3, RD4 and anti-Aβ for 1 hour at room temperature. This was followed by several washes in PBS and treatment with biotinylated anti-mouse (Dako 1:200) for 30 mins and ABC (Dako) for 30 mins. Peroxidase activity was developed with diaminobenzidine/H2O2 solution.
RT-PCR analysis of proportion of exon 10-containing (4R-tau) mRNA and transcripts containing ΔK280
Total RNA was extracted from frontal cortex by use of RNeasy (with the RNase-free DNase set) lipid tissue mini kit (Qiagen, Crawley, UK) according to the manufacturer’s protocol. Complementary DNA was made by use of the Superscript III kit (Invitrogen, Paisley, UK). PCR was performed using the following primers spanning MAPT exons 9 to 11:
Exon9-F; 5′-ATCGCAGCGGCTACAGCAG-3′
Exon11-R; 5′-TGGTTTATGATGGATGTTGCC-3′
Amplifications were carried out in triplicate, as previously described (Hutton et al., 1998), separated on agarose gels and analysed with Kodak Molecular Imaging Software Version 4.0.
Although the AAG deletion of ΔK280 almost completely suppresses the inclusion of exon 10, any residual inclusion of exon 10 sequences containing the ΔK280 deletion will result in 4R-tau isoforms that are highly fibrillogenic. In order to obtain a measure of the production of transcripts containing the mutant exon 10 (exon 10+), we separated the above RT-PCR products on a low melting-point agarose gel (2%) in 1x TAE buffer and excised and purified the larger band representing the exon 10 containing transcripts. This product was then cloned into a pGEM-T vector (Promega, Southampton, UK) and, 91 clones containing the larger, exon 10+ insert were identified by PCR using the above primers. The PCR products were sequenced with the Exon11-R primer (Advanced Biotechnology Centre, Imperial College, London) and sequence compared with wild-type MAPT exon 10 to identify the number of clones containing the AAG (ΔK280) deletion.
Protein Chemistry
Tau protein isolation and analysis
Isolation of sarkosyl-insoluble tau was carried out as described by (Goedert et al, 1993), modified from (Greenberg & Davies, 1990). Brain tissue was homogenised in 10x volume (w/v) homogenisation buffer (10mM Tris-HCl pH 7.4, 0.8M NaCl, 1mM EGTA and 10% sucrose containing Complete protease inhibitor cocktail (Roche, Burgess Hill, UK). The suspension was then spun at 20,000×g for 20 minutes at 4°C and the supernatant set aside. The pellet was resuspended in 5x volumes of homogenisation buffer and re-centrifuged as above. The supernatants were combined and N-lauryl sarcosinate added to a concentration of 1% (w/v), and incubated at room temperature for 1 hour with shaking. The mixture was then centrifuged at 100,000×g for 1 hour at 4°C. The sarkosyl-insoluble pellet was resuspended in 50mM Tris-HCl pH7.5 at 0.2ml per gm of starting material.
Soluble tau was isolated as described (Hanger et al, 2002); brain tissue was homogenised in 4 vol. 100mM MES buffer, pH 6.5, containing 1M NaCl and Complete protease inhibitor (Roche), centrifuged at 27,000×g for 30 min at 4°C. The supernatant was centrifuged at 100,000×g for 1h at 4°C and the soluble tau in the supernatant was enriched further by heating for 10 min at 100°C and centrifuging at 15,000×g for 30 min at 4°C. The supernatant, containing heat-stable soluble proteins, was made to 45% saturation with ammonium sulphate, maintained in ice for 15 min, and centrifuged at 15,000×g for 30 min at 4°C. Precipitated proteins were resuspended in 0.1x vol. (relative to weight of starting brain material).
Dephosphorylation of tau with lambda protein phosphatase
Dephosphorylation of human tau preparations was carried out as previously described (Hanger et al, 2002). The sarkosyl-insoluble tangle tau was solubilised by addition of guanidium hydrochloride (Sigma, Poole, UK) to a final concentration of 4M and the incubation for 1 hour at room temperature. The guanidium hydrochloride was then removed by dialysis overnight at 4°C into 50mM Tris-HCL (pH 7.5). The tau samples in 50mM Tris-HCl, pH 7.5, were then dephosphorylated with 20 U/μl lambda protein phosphatase (New England Biolabs, Hitchin, UK) for 3 h at 30°C. Reactions were stopped by the addition of 2X sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and heating for 5 min at 100°C.
Western blotting
Dephosphorylated proteins together with a mixture of all six isoforms of recombinant tau (rPeptide, Bogart, GA) were separated by standard SDS-PAGE (Laemmli, 1970) using a 10% gel and then electroblotted onto a PVDF membrane. The immobilised proteins were then probed with the pan-tau antibody, HT7 (1:60,000; rPeptide) followed by a peroxidase conjugated secondary anti-rabbit antibody (1:1000; Autogen Bioclear, Calne, UK) and visualised by peroxidase-based enhanced chemiluminescence (Perbio, Cramlington, UK).
Results and Discussion
This case was an 81-year-old man with a 10-year history of dementia prior to death. He was severely demented 5 years antemortem at the time of enrolling in the Autopsy and Tissue Donation program of the Duke Alzheimer Disease Center. The subject was diagnosed with Alzheimer disease by a neurologist in private practice in 1986. A CT scan was obtained at that time which demonstrated cortical atrophy and ventricular dilation. There was a history of cerebrovascular accident and an infarct was seen in the right basal ganglia with CT scan. Cognitive testing was performed at the time of diagnosis, but detailed results are not available. He was apolipoprotein ε3ε4 heterozygote. Family history information was poor because the case had lost contact with his family, but there was no indication that his parents had had dementia. This case is the only coding variant we have identified in Alzheimer’s disease or control cases. We confirmed the ΔK280 variant in a separate sample from the same brain which was used for the protein work described below. The previous FTD case with the ΔK280 variant was Dutch, but there was no indication that this case had Dutch ancestry.
Neuropathological evaluation was limited to the fixed left hemisphere since the right hemisphere was frozen for genetic studies. There was extensive pathology with gross and microscopic evidence of Alzheimer disease, dementia with Lewy bodies and vascular dementia. In contrast to the previously described case with a ΔK280 mutation (van Swieten et al., 2007), which had severe atrophy of the frontal and temporal cortex extending into the inferior parietal lobe, the present case showed no evidence of frontotemporal degeneration.
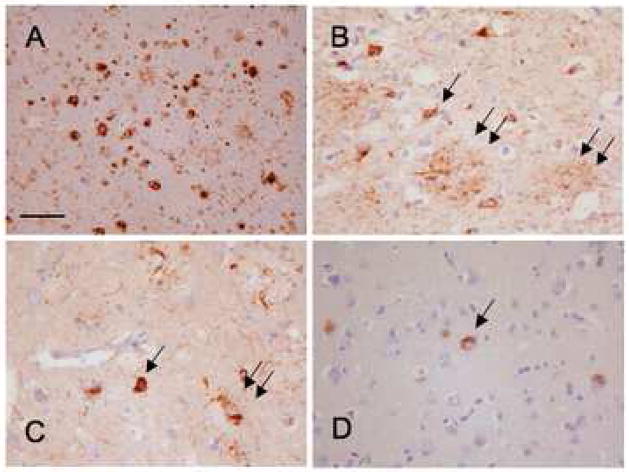
The pathology of this case was of typical Alzheimer’s disease, Braak stage IV with Aβ immunopositive plaques (Fig. 1). There were frequent mature neuritic plaques sufficient to make the diagnosis of definite Alzheimer’s disease according to CERAD criteria.
Figure 1.
A: Aβ immunohistochemistry showing numerous mature and diffuse plaques in the superior temporal cortex.
B: The AT8 anti-tau antibody shows numerous neurofibrillary tangles (arrow), neuropil threads and plaque-associated abnormal neurites (double arrow) in the same area.
C: Immunohistochemistry with the anti-4R-tau antibody, RD4, shows a similar staining pattern.
D: The anti-3R-tau antibody (RD3) mostly stained neurofibrillary tangles. Bar on A represents 100 mm on A and 50 mm on B–D.
Tau immunohistochemistry showed unremarkable staining with both 3R- and 4R- tau visualised using the antibodies RD3 and RD4, respectively (Fig 1). AT8 anti-tau antibody showed numerous neurofibrillary tangles, neuropil threads and plaque-associated abnormal neurites in the same area. Immunohistochemistry with RD4, showed a similar staining pattern, whereas RD3 staining was mostly restricted to neurofibrillary tangles (Fig 1). There were frequent neurofibrillary tangles in the frontal, parietal and temporal lobes but only a few of them in the primary visual cortex. The extent of neurofibrillary change corresponds to Braak Stage IV or intermediate likelihood of AD according to NIA/Reagan criteria (Hyman et al. 1997). No Pick bodies or ballooned Pick cells were seen in the hippocampus or in any other location. On the other hand, the other ΔK280 case described by van Swieten and co-workers (van Swieten et al., 2007) demonstrated relatively widespread Pick bodies and also, tau-positive glial cells.
There was also evidence of dementia with Lewy bodies. Lewy bodies were demonstrated by alpha synuclein immunohistochemistry in the brainstem, midbrain and limbic system (not shown). The extent of Lewy body disease was consistent with McKeith limbic category dementia with Lewy bodies (McKeith et al. 1996), or Parkinson disease related Braak Stage IV (Braak et al. 2003). There were also changes consistent with vascular dementia with moderate to severe atherosclerosis in the vessels at the base of the brain, which resulted in 50% stenosis of the left posterior cerebral artery and 75% stenosis of the left vertebral artery. There was also an old infarct in the left pons, which measured 2×2×1 cm in diameter. Mild amyloid angiopathy was also noted.
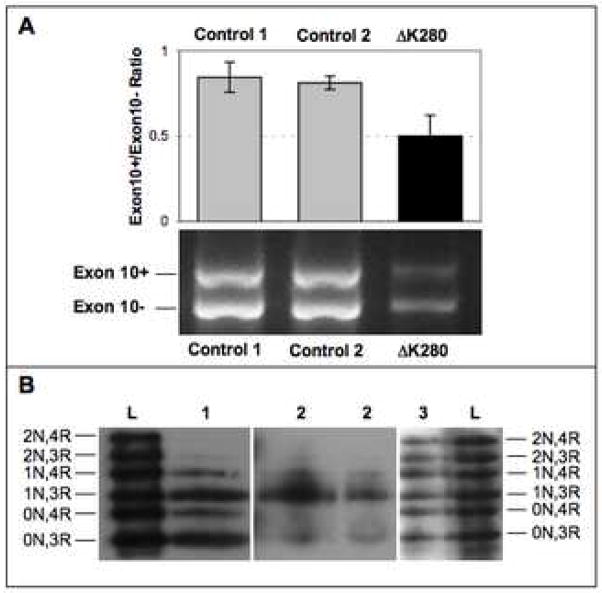
Consistent with previous findings, semi-quantitative RT-PCR analysis of ratio of the MAPT transcripts with and without exon 10 (exon10+/exon 10−) showed a preponderance of exon 10− (3R-tau) mRNA with an ~1.6-fold reduced exon10+/exon10− ratio compared to control brains (Figure 2A). The controls had mean exon 10+/exon 10− ratios of 0.851 (s. d. 0.060) and 0.850 (s. d. 0.016) compared to the case, which had the reduced mean ratio of 0.559 (s. d. 0.075). The increased 3R-tau ratio, particularly the 0N3R and 1N3R isoforms, is clearly reflected in both the soluble and sarkosyl-insoluble tau fractions from this case (Figure 2B). However, as in Fig. 1, immunohistochemical staining showed more widespread 4R-tau staining compared to 3R-tau, which was less intense and limited to the tangle structures. This could be due to masking of the 3R-tau antibody (RD3) epitope within the tau filaments.
Figure 2.
A: RT-PCR analysis of mRNA from the frontal cortex of the MAPT ΔK280 case and two healthy controls. In the top panel, the bars depict the ratios of the exon 10+ (4R-tau) and exon 10− (3R-tau) transcripts as quantified by intensities of the respective bands (see lower pane). Error bars represent standard error of the mean.
B: Western blot analysis of dephosphorylated, soluble (Lane 1) and sarkosyl-insoluble (Lanes 2) tau from the frozen frontal cortex of the ΔK280 case. Lane 3 shows sarkosyl-insoluble tau from the frontal cortex of another Alzheimer’s disease case, without MAPT mutations. Lanes L are recombinant tau isoforms, as labelled on the left and right edges.
In order to assess the degree to which the ΔK280 variant suppresses the inclusion of MAPT exon 10, we cloned the larger exon 10+ band (Figure 2A) into a pGEM-T vector and sequenced 91 individual plasmids containing the exon 10+ PCR product. Of these exon 10+ clones, 13 contained the ΔK280 deletion, constituting just over 14% of the exon 10+ transcripts as compared with the allelic ratio of 50%. It is reasonable to posit that this leads to a roughly similar ratio (14%) of the highly fibrillogenic ΔK280 4R-tau protein isoforms, compared to wild-type 4R-tau, functioning as a potent seed for tau aggregation. Barghorn and colleagues showed that the ΔK280 4R-tau protein has a 5-fold greater rate of aggregation compared to wild-type 4R-tau and, “since PHF assembly is a nucleation-dependent process, nuclei formed by mutant tau could be elongated even from the pool of normal tau and thus poison the entire tau population in a cell” (Barghorn et al., 2000). This would be compounded by the strongly reduced capacity of the ΔK280 tau variant to promote microtubule assembly (Rizzu et al., 1999).
When the ΔK280 variant was described, Rizzu and colleagues (1999) were careful not to claim it was a pathogenic variant. There are only two genetic proofs of pathogenicity: linkage and association and this variant fulfilled neither criterion since it was only found in a single case, reported three times. In many ways, the in vitro behaviour of the mutation was anomalous; it was the only mutation which increased the proportion of 3R-tau and while cDNA transfection showed abnormalities in tubulin binding, this work has uncertain relevance to the human situation because the mutation is in the potentially excluded exon 10. Despite this uncertainty, the ΔK280 protein variant has been extensively used in biochemical experiments designed to understand the effects of the “FTDP-17 mutations” on tau biophysics, tau aggregation and on tubulin binding (Barghorn et al., 2000, Mukrasch et al., 2005; Vogelsberg-Ragaglia et al,. 2005; von Bergen et al., 2001).
The data we present here adds to the ambivalence about both the pathogenic nature of the mutation and the mechanism by which it may be pathogenic. On pathogenicity: identifying the mutation in an Alzheimer’s disease case neither significantly strengthens nor weakens the argument about whether it has been pathogenic. Clearly, one should not assume that this mutation is pathogenic. However, the protein data from both this case and the original case show that the deposited protein is largely 3R-tau, albeit with consistent evidence that some 4R-tau protein is also deposited. As we show here, a significant proportion (~14%) of the 4R-tau will contain the ΔK280 deletion. These data validate the in vitro data suggesting that the mutation does indeed alter splicing in favor of the 3R-tau isoform. We are therefore left with three possibilities: first, the mutation is simply not pathogenic; second, it is pathogenic because the small amounts of the ΔK280 4R-tau protein are enough to initiate tau deposition and third, the overproduction of 3R-tau isoforms can lead to deposition and disease. All other pathogenic mutations which alter splicing increase the proportion of 4R-tau (Hutton, 2000). Therefore, this last explanation is the least likely. This is an important point, because some therapeutic strategies aim to increase the proportion of the 3R-tau isoform. We would suggest that this therapeutic approach still has validity.
Acknowledgments
This work was supported in part by the NIH, NIA intramural program, PHS P50 AG05128 and by the Reta Lila Weston Trust for Medical Research. RdS is funded by a grant from the MRC (G0501560).
Footnotes
Disclosure Statement
The RD3 and RD4 antibodies in this paper have been licensed out to Millipore/Upstate and Rohan de Silva receives a revenue share. The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barghorn S, Zheng-Fischhofer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000;39:11714–11721. doi: 10.1021/bi000850r. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- D’Souza I, Poorkaj P, Hong M, Nochlin D, Lee VM, Bird TD, Schellenberg GD. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc Natl Acad Sci USA. 1999;96:5598–5603. doi: 10.1073/pnas.96.10.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva R, Lashley T, Gibb G, Hanger D, Hope A, Reid A, Bandopadhyay R, Utton M, Strand C, Jowett T, Khan N, Anderton B, Wood N, Holton J, Revesz T, Lees A. Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol Appl Neurobiol. 2003;29:288–302. doi: 10.1046/j.1365-2990.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP, Gibb GM, de Silva R, Boutajangout A, Brion JP, Revesz T, Lees AJ, Anderton BH. The complex relationship between soluble and insoluble tau in tauopathies revealed by efficient dephosphorylation and specific antibodies. FEBS Lett. 2002;531:538–542. doi: 10.1016/s0014-5793(02)03611-6. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Crain B, Szymanski MH, Sinclaire NO, Roses AD. Rapid brain autopsy. The Joseph and Kathleen Bryan Alzheimer’s Disease Research Center experience. Arch Pathol Lab Med. 1997;121:615–618. [PubMed] [Google Scholar]
- Hutton M. Missing” tau mutation identified. Ann Neurol. 2000;47:417–418. [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malkani R, D’Souza I, Gwinn-Hardy K, Schellenberg GD, Hardy J, Momeni P. A MAPT mutation in a regulatory element upstream of exon 10 causes frontotemporal dementia. Neurobiol Dis. 2006;22:401–403. doi: 10.1016/j.nbd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen ENH, Ballard C, de Vos RAI, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB) Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Mukrasch MD, Biernat J, von Bergen M, Griesinger C, Mandelkow E, Zweckstetter M. Sites of tau important for aggregation populate β-structure and bind to microtubules and polyanions. J Biol Chem. 2005;280:24978–24986. doi: 10.1074/jbc.M501565200. [DOI] [PubMed] [Google Scholar]
- Pittman AM, Fung HC, de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet 15 Spec No. 2006;2:R188–195. doi: 10.1093/hmg/ddl190. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Rizzu P, van Swieten JC, Joosse M, Hasegawa M, Stevens M, Tibben A, Niermeijer MF, Hillebrand M, Ravid R, Oostra BA, Goedert M, van Duijn CM, Heutink P. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet. 1999;64:414–421. doi: 10.1086/302256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swieten JC, Bronner IF, Azmani A, Severijnen LA, Kamphorst W, Ravid R, Rizzu P, Willemsen R, Heutink P. The DeltaK280 mutation in MAP tau favors exon 10 skipping in vivo. J Neuropathol Exp Neurol. 2007;66:17–25. doi: 10.1097/nen.0b013e31802c39a4. [DOI] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V, Bruce J, Richter-Landsberg C, Zhang B, Hong M, Trojanowski JQ, Lee VM. Distinct FTDP-17 missense mutations in tau produce tau aggregates and other pathological phenotypes in transfected CHO cells. Mol Biol Cell. 2000;11:4093–4104. doi: 10.1091/mbc.11.12.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Li L, Marx A, Biernat J, Mandelkow EM, Mandelkow E. Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J Biol Chem. 2001;276:48165–48174. doi: 10.1074/jbc.M105196200. [DOI] [PubMed] [Google Scholar]