Abstract
HMG-CoA reductase inhibitors have been reported to increase circulating bone marrow progenitor cells (BMPCs) and variably improve global function in heart failure. The potential role of improved perfusion vs. direct effects of statins on cardiac myocytes has not been established. We chronically instrumented swine with an LAD stenosis to produce chronic hibernating myocardium with regional contractile dysfunction in the absence of heart failure. Hemodynamics, function, perfusion and histopathology were assessed in pigs treated for five-weeks with pravastatin (n=12) vs. untreated controls (n=10). Regional LAD wall thickening was depressed under baseline conditions (LAD 3.7±0.3 vs. 6.6 ±0.3 in remote regions, p<0.01). It remained unchanged in untreated animals but increased from 3.8±0.6 to 5.2±0.5 mm after pravastatin (p<0.01). There was no increase in myocardial perfusion at rest or during vasodilation. Pravastatin mobilized circulating CD133+/cKit+ BMPCs and increased myocardial tissue levels (LAD CD133+ cells from 140±33 to 884±167 cells/106myocyte nuclei and cKit+ cells from 223±49 to 953±123 cells/106myocyte nuclei). Pravastatin increased myocytes in mitosis (phospho-histone-H3; 9±5 to 43±7 nuclei/106myocyte nuclei, p<0.05) and the growth phase of the cell cycle (Ki67; 410±82 to 1261±235 nuclei/106myocyte nuclei, p<0.05) in diseased but not normal hearts. As a result, pravastatin increased LAD myocyte nuclear density from 830±41 to 1027±55 nuclei/mm2 (p<0.05). These data indicate that, in the absence of impaired endothelial function and heart failure, dysfunctional hibernating myocardium improves after pravastatin. This effect is independent of myocardial perfusion and related to mobilization of CD133+/cKit+ BMPCs which stimulate myocyte proliferation resulting in quantitative increases in myocyte nuclear density.
Keywords: statins, hibernating myocardium, cardiac repair, bone marrow progenitor cells
Introduction
Although HMG-CoA reductase inhibitors were originally developed as lipid-lowering drugs and demonstrated to retard the progression of atherosclerosis and improve endothelial function, pleotrophic actions of statins may be of equal importance in reducing death and disability from cardiovascular disease. Statins have been shown to have anti-inflammatory, anti-hypertrophic and pro-angiogenic actions that may favorably impact ventricular function independently of their actions on stabilizing and/or regressing atherosclerotic lesions1, 2. In addition, statins have been demonstrated to mobilize endothelial progenitor cells (EPCs) into the circulation in humans and animals3, 4. Preclinical studies have demonstrated that EPCs can arise from bone marrow and incorporate into the vascular wall5. Some studies have also demonstrated the ability of bone marrow progenitor cell (BMPC) populations to differentiate into cardiac myocytes6, 7. Several small randomized clinical studies have demonstrated objective increases in myocardial function after statins8, 9 but this may not be a class effect10. While angiogenesis could play a role in some of these actions, improvements in myocardial function have also been demonstrated in nonischemic cardiomyopathy.
We performed the present study to identify the effects of pravastatin on myocardial perfusion and ischemic myocardial dysfunction that are independent of cholesterol lowering. We used a well characterized porcine model of collateral-dependent myocardium resulting in hibernating myocardium from a chronic LAD stenosis. Since regional rather than global LV dysfunction is present, the confounding role of statins on inflammation, cytokines and neurohormonal activation in heart failure are absent11. In addition, since hypercholesterolemia and atherosclerosis are absent, the effects of pravastatin on angiogenesis were studied independently of inhibiting plaque progression and improving endothelium-dependent vasodilation. Our results demonstrate that pravastatin mobilizes BMPCs in hibernating hearts and promotes myocytes to reenter the growth and mitotic phase of the cell cycle. As a result, myocyte nuclear density is increased and myocardial function improves without a change in myocardial perfusion.
Materials and Methods
Procedures and protocols conformed to institutional guidelines for the care and use of animals in research. Detailed experimental and histological protocols are described in the online data supplement.
Mobilization of Bone Marrow Progenitor Cells with Pravastatin
We initially assessed the dose-dependency of pravastatin to mobilize bone marrow progenitor cells in normal swine using flow-cytometry (FACS, n=11). Baseline measurements were compared to those 2-weeks after daily treatment with low-dose (20 mg/day) or high-dose (160 mg/day) pravastatin. We assessed changes in peripheral blood (30ml) as well as bone marrow (30ml). Data from FACS analysis are expressed as CD133+ or cKit+ cells per million mononuclear cells.
Effects of Pravastatin on Flow and Function in Hibernating Myocardium
Pigs were chronically instrumented with a 1.5 mm Delrin stenosis on the proximal LAD as previously described (online supplement)12. We performed baseline physiological studies 4-months after instrumentation (n=22) when hibernating myocardium with reductions in LAD wall thickening and resting LAD perfusion was present. Under propofol sedation, a Millar-catheter was inserted into the left ventricle (LV) for microsphere injection. Regional wall-thickening was assessed with transthoracic echocardiography. Microsphere flow was assessed at rest and following adenosine vasodilation. Animals were subsequently treated with pravastatin (160 mg/day, n=12) for 5-weeks and compared to untreated controls (n=10). At the end of the study, physiological studies were repeated and the heart was excised for TTC, flow and histological analysis.
Myocyte Nuclear Density and Morphometry
Samples from hibernating LAD and normal remote regions were fixed and paraffin-embedded. Trichrome-stained sections were used to quantify connective tissue13. PAS stained sections were used to quantify myocyte diameter, nuclear length and regional myocyte nuclear density. Histological results in hibernating animals were compared to normal sham animals (pravastatin n=10, untreated n=5).
Quantitative Analysis of Myocyte Proliferation and CD133+/cKit+ Cells
To identify myocyte proliferation, anti-Ki67 and anti-phospho-Histone-H3 staining were performed14. CD133+/cKit+ BMPCs in myocardial tissue were quantified using confocal immunofluoresence (online supplement). Preliminary studies demonstrated that tissue CD133+/cKit+ BMPCs were CD45 negative. To evaluate whether CD133+/cKit+ BMPCs differentiated into a cardiac lineage we co-stained BMPCs with GATA-4 and cTnI antibodies14.
Statistics
Data are expressed as mean±standard error. Differences between groups were assessed by two-way ANOVA and the post-hoc Holm-Sidak test. Temporal physiological changes between initial and final studies were assessed by paired t-tests. P<0.05 was considered significant.
Results
Pigs were in good health at the time of study and TTC staining showed no significant infarction. Initial physiological studies were performed 130±3days after instrumentation and repeated 5-weeks after pravastatin (166±3 days).
Pravastatin Dose-dependently Mobilizes CD133+ and cKit+ BMPCs
Figure 1 summarizes flow-cytometry data before and after low- and high-dose pravastatin for 2-weeks. There were no differences in circulating mononuclear cells (n=11, 11.6 × 106±1.8 × 106 cells/ml at baseline vs. 16.6 × 106±4.7 × 106 cells/ml after pravastatin, p-ns). In contrast, pravastatin dose-dependently increased both CD133+ and cKit+ cells in blood and bone marrow aspirates. Circulating and bone marrow CD133+ and cKit+ cells were much higher after a 160 mg dose of pravastatin. For example, circulating CD133+ cells averaged 18±3 cells/106 mononuclear cells at baseline, 32±5 cells/106 mononuclear cells after 20 mg pravastatin (p<0.05, vs. baseline) and 272±54 cells/106 mononuclear cells after 160 mg pravastatin (p<0.05 vs. baseline and 20mg pravastatin).
Figure 1. Pravastatin Dose-dependently Increases Circulating and Bone Marrow CD133+ and cKit+ Cells.
A.) Flow cytometry for cKit (CD117) and CD133 before and after pravastatin.
B.)Quantitative impact of statins on CD133+/cKit+ BMPCs levels normalized to mononuclear cell numbers. There was a dose-dependent increase in cKit+ and CD133+ cells. Increases in blood were paralleled by similar increases in bone marrow aspirates and greatest after 160 mg of pravastatin.
Effects of Pravastatin on Function and Flow in Hibernating Myocardium
Hemodynamics (online Table 1) and echocardiographic measurements of function (online Table 2) are summarized in the supplement. Hemodynamics were similar between treatment groups with the exception of LVEDP which tended to be lower in animals receiving pravastatin. Pravastatin increased LAD systolic wall thickening (percent WT 28±2.8% vs. 48±5.1% after pravastatin, p<0.05, online Table 2) with systolic excursion (ESWT-EDWT) summarized in Figure 2A. There were no differences in remote regions. Serial analysis of function within the same animal confirmed a similar improvement with no change in untreated controls. Likewise, global function was normal and did not change after pravastatin (Ejection Fraction 64±5% vs. 69±2% after pravastatin, p-ns). Although pravastatin improved regional function in hibernating myocardium, there was no effect on resting flow (Figure 2B). When analyzed in terms of relative full-thickness perfusion, relative reductions in resting flow (LAD/Remote 0.79±0.07 in pravastatin vs.0.70±0.05 in untreated) were similar with no serial changes between studies in the same animal. Likewise relative adenosine flow (LAD/Remote 0.27±0.04 in pravastatin vs. 0.23±0.02 in untreated) was not significantly different nor were there serial changes over time. Thus, while pravastatin increased LAD wall thickening, there was no functionally significant angiogenesis.
Figure 2. Pravastatin Improves Regional Function in Hibernating Myocardium.
A.) In hibernating myocardium, systolic LAD wall thickening (ESWT-EDWT) was depressed and significantly increased after pravastatin (160 mg/day) in comparison to untreated animals. There was no significant change in remote zone wall thickening. Similar changes were seen for percent wall thickening. B.)There was no effect of pravastatin on myocardial perfusion in hibernating myocardium.
Increase in Myocardial CD133+ and cKit+ Cells after Pravastatin
We analyzed myocardial tissue to determine whether the increase in circulating CD133+/cKit+ BMPCs produced corresponding increases in myocardial tissue levels (Figure 3A and 3B). Pravastatin increased CD133+ cells in hibernating myocardium (LAD: 884±167 vs. 140±33 cells/106myocyte nuclei in untreated animals, p<0.01). While CD133+ cells were rare in sham hearts (17±5 cells/106myocyte nuclei), myocardial levels also increased after pravastatin (673±122 cells/106myocyte nuclei, p<0.001). Increases in CD133+ cells were global and occurred in nonischemic remote regions in hibernating and sham hearts (944±183 in hibernating Remote and 607±79 cells/106myocyte nuclei in sham Remote).
Figure 3. Pravastatin increases myocardial CD133+ and cKit+ BMPCs in hibernating and sham animals.
A) Both cKit+ (a) and CD133+ (b) cells (stained green) localized in interstitial regions and were frequently clustered. Myocytes were stained with troponin-I (red) and nuclei with TO-PRO-3 (blue). B) There was a global increase in myocardial CD133+ and cKit+ cells in LAD and remote regions after 5-weeks of pravastatin. Levels were similar in hearts from hibernating and sham animals.
Like CD133+ cells, myocardial cKit+ cells were rare in normal hearts (27±3 cells/106myocyte nuclei) and increased after pravastatin (hibernating LAD: 953±123 and normal LAD: 1031±244 cells/106myocyte nuclei) as well as their corresponding remote regions. Dual staining in six animals demonstrated that 33±4% of cKit+ cells also co-stained for CD133. Thus, pravastatin produced global increases in myocardial CD133+ and cKit+ cell levels that were independent of ischemia or LV dysfunction.
GATA4 Expression in CD133+ and cKit+ Cells
To identify whether CD133+ and cKit+ BMPCs could differentiate into a cardiac lineage, they were co-stained for the cardiac transcription factor GATA4 (Figure 4A–a). These were a small fraction of CD133+/GATA4+ cells. They rarely co-expressed troponin I (Figure 4A–b). While CD133+/GATA4+ cells increased after pravastatin (43±9 cells/106 vs. 8±5 cells/106 myocytes in untreated LAD, Figure 4B) the proportion of CD133+ BMPCs expressing GATA4 was unchanged (4.4±2.9% in untreated vs. 6.2±1.6% in pravastatin, p-ns). This indicates that increases in GATA4+ BMPCs were secondary to mobilizing CD133+ BMPCs to the heart and not from pravastatin enhancing the differentiation of CD133+ BMPCs to myocytes. Similar results were found for cKit+ cells.
Figure 4. Co-expression of GATA4 in CD133+ and cKit+ BMPCs.
A) Example of a CD133+ cell co-staining for GATA4 (a) and troponin I (b). Similar changes were seen in cKit+ cells. B) Pravastatin increased myocardial CD133+ and cKit+ BMPCs co-expressing GATA4 in normal and hibernating hearts. Since the percentage of CD133+/cKit+ BMPCs co-expressing GATA4 remained fairly constant (4.4±2.9% vs. 6.2±1.6% after pravastatin), the increase reflected a greater total number of CD133+/cKit+ cells in the tissue (Figure 3B).
Increased Myocytes in the Growth Phase of the Cell Cycle after Pravastatin
We quantified the frequency of myocyte nuclei expressing Ki67, a marker of the growth phase of the cell cycle and phospho-histone-H3, a marker of mitosis (Figure 5). Untreated hibernating animals had low values of Ki-67 positivity (LAD 410±82 and Remote 285±88/106 myocyte nuclei). After pravastatin, Ki-67 positivity increased (LAD 1261±235 and remote 969±132/106 nuclei/106 myocyte nuclei, p<0.05 vs. untreated). Surprisingly, although CD133+ and cKit+ cells increased in sham hearts, there was no increase in Ki-67 positivity after pravastatin (LAD 274±82 vs. remote 284±69 nuclei/106 myocyte nuclei, p-ns vs. untreated). Likewise, myocyte nuclear phospho-histone-H3 positivity was selectively increased in pravastatin treated hibernating animals vs. sham treated animals (LAD 43±7 vs. 9±5 and Remote 34±9 per 106 vs. 6±3 per 106 myocyte nuclei, p<0.05 respectively). Thus, pravastatin only increased myocytes in the growth phase of the cell cycle in diseased hearts.
Figure 5. Pravastatin Increases Ki-67 and Phospho-histone-H3 Positive Myocytes in Hibernating Hearts.
A)Pravastatin increased myocyte Ki-67 (green) (a,b) and phospho-histone H3 (pHH3) (c) indicating an increased number of myocytes in the growth and mitotic phase of the cell cycle. Ki-67 and pHH3 positive nuclei were confirmed as myocytes by co-staining with troponin I (red). B) Quantitative data demonstrated significant increases in Ki-67 and pHH3 in the LAD and remote regions in treated pigs with hibernating myocardium. In contrast, these were not altered in sham animals despite increased myocardial CD133+ and cKit+ BMPCs after pravastatin.
Effects of Pravastatin on Myocyte Nuclear Density in Hibernating Myocardium
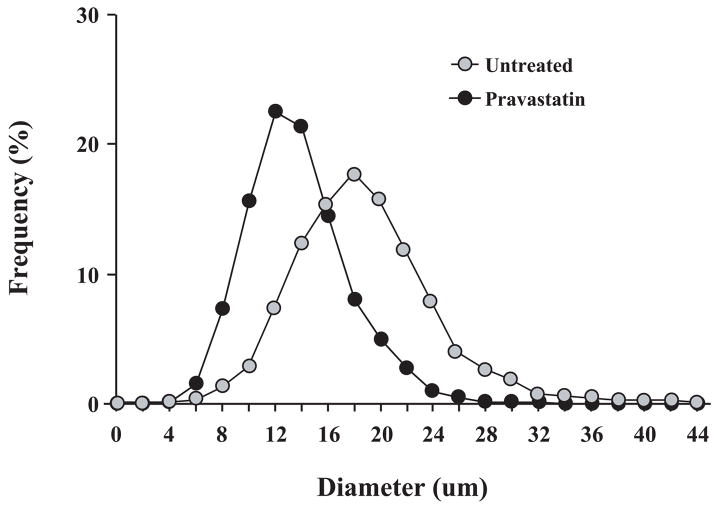
To determine whether increases in cell cycle markers led to myocyte regeneration, we assessed the effects of pravastatin on myocyte nuclear density (Figure 6). While LAD connective tissue was mildly increased in hibernating animals receiving pravastatin (LAD 8.3±1.9% vs. Remote 4.1±0.7%, p<0.05), it was similar to untreated animals (LAD 6.9±0.7% vs. Remote 4.5±0.2% p<0.05, pravastatin vs. untreated p-ns, respectively). In untreated animals, LAD myocyte nuclear density was reduced (LAD 830±41 vs. Remote 1027±55 nuclei/mm2, p<0.05) as we have previously demonstrated13. After pravastatin, LAD myocyte nuclear density increased from 830±41 to 1054±19 nuclei/mm2 (p<0.01) but values remained significantly lower than normal myocardium (p<0.05). Measurements of myocyte diameter (Figure 7) demonstrated that increases in myocyte nuclear density after pravastatin were accompanied by a reduction in myocyte size (mean diameter 13.2±0.6 μm vs. 15.7±0.4 μm, p<0.05). The diameter of Ki67 positive myocytes was even smaller (11.9±0.2 μm, p<0.05). Thus, the increase in myocyte nuclear density coupled with a reduction in myocyte size is consistent with myocyte proliferation after pravastatin in hibernating myocardium.
Figure 6. Pravastatin Increases Myocyte Nuclear Density in Hibernating Myocardium.
There was a regional reduction in LAD myocyte nuclear density in untreated hibernating animals that was greatest in the subendocardium. After pravastatin, LAD myocyte nuclear density increased but remained significantly lower than values found in normal sham animals. This demonstrates a cumulative increase in myocytes after 5-weeks of pravastatin.
Figure 7. Redistribution of LAD Myocyte Diameters in Hibernating Myocardium.
After pravastatin, LAD myocyte diameters were significantly smaller (mean 13.2±0.6 μm) than untreated animals (15.7±0.4 μm, p<0.05). Ki67 positive myocytes were even smaller (11.9±0.2, p<0.01). The shift in size supports the notion that proliferating myocytes arose from a stem cell population.
Discussion
There are several important new findings from our study. First, pravastatin dose-dependently increases CD133+ and cKit+ cells in bone marrow and blood. This leads to corresponding increases in myocardial tissue from normal and diseased hearts. Second, in hibernating myocardium, pravastatin improves regional wall thickening with no demonstrable effect on myocardial perfusion indicating that functional improvement is independent of angiogenesis. Third, immunohistochemical analysis of myocardial tissue demonstrates that improved function is accompanied by an increase in myocytes in the proliferative phase of the cell cycle. This leads to corresponding increases in myocyte nuclear density and reductions in myocyte diameter in hibernating myocardium. While statins mobilized CD133+ and cKit+ BMPCs to normal hearts, evidence of cardiac myocyte proliferation was absent. Collectively, the observations are consistent with the notion that chronic mobilization of CD133+/cKit+ BMPCs with pravastatin leads to significant myocyte regeneration in diseased hearts.
Mobilization of BMPCs and Localization in the Heart
There is now substantial support for the notion that BMPCs can facilitate myocyte regeneration under selected conditions. Orlic and colleagues demonstrated the ability of cKit+ bone marrow cells injected into murine infarcts to regenerate myocardium15. In a subsequent study, myocyte regeneration was effected by mobilizing BMPCs with stem cell factor and granulocyte colony stimulating factor16. While controversial, the ability of BMPCs to repair the heart in humans is supported by myocyte chimerism in transplant recipients17, 18. This as well as other preclinical work stimulated rapid translation of these findings to early clinical trials using a variety of largely unfractionated intracoronary and intramyocardial adult bone marrow preparations in patients with myocardial infarction. While the therapy appears safe and functional effects are generally favorable, the magnitude of improvement is modest (reviewed in a recent meta analysis19). Importantly, BMPC mobilization strategies using granulocyte colony stimulating factor in humans have not demonstrated significant functional improvement20. Thus, myocardial homing as well as mobilization is required for a physiological effect.
Our results demonstrate that pravastatin affords an alternative approach to effect bone marrow-mediated cardiac repair. Since statins have been demonstrated to mobilize EPCs, previous basic and clinical studies have focused upon evaluating their effect on angiogenesis and vasculogenesis rather than myocyte regeneration. A variety of HMG-CoA-reductase inhibitors can mobilize bone marrow derived CD34+ and CD133+ EPCs. These can differentiate into endothelial cells and have been demonstrated to improve perfusion in the severely ischemic murine hind limb model21, 22. Dimmeler et al. demonstrated that simvastatin can also increase bone marrow cKit+ cells22. Previous studies have established that EPC mobilization involves activation of the PI3-kinase/Akt pathway by statins22, 23. Our results extend these studies in swine by showing a parallel dose-dependent increase in cKit+ and CD133+ cells in bone marrow and blood. This supports the notion that the primary action of statins is to increase the proliferation of CD133+ and cKit+ cells in bone marrow. It is also possible that CD133+/cKit+ BMPCs arise from other tissue reservoirs which will require further study. The corresponding global increase in myocardial CD133+ and cKit+ cell levels in normal as well as hibernating hearts is probably a function of their circulating concentration. While the number of CD133+/GATA-4+ and cKit+/GATA-4+ cells increased, pravastatin did not enhance differentiation into a cardiac lineage since the percentage of GATA-4+ cells was unchanged.
Lack of Effect of Pravastatin on Perfusion in Hibernating Myocardium
Mobilization of EPCs can salvage tissue in rodent hind limb ischemia models via increases capillary density suggesting small vessel proliferation. The ability of statins to increase myocardial perfusion is less clear. Indeed, this question provided the initial rationale for our study where pravastatin was anticipated to improve perfusion to collateral-dependent hibernating myocardium. Surprisingly, there was no difference in resting or vasodilated flow in LAD or remote myocardium after pravastatin (on-line supplement). Likewise, relative perfusion differences between LAD and remote regions were unchanged. While we did not assess anatomic variables such as capillary density, the failure of coronary flow to increase in ischemic myocardium supports the notion that statins do not have any meaningful effect on coronary collateral resistance vessels. Dissociations between flow and capillary density can occur since the latter vessels contribute negligibly to coronary vascular resistance24. Nevertheless, since there were small increases in end-end-diastolic wall thickness after pravastatin (<15%), we cannot exclude the possibility that angiogenesis matched to changes in regional mass occurred.
A potential explanation for the failure of pravastatin to increase perfusion may relate to a biphasic dose-dependent effect on angiogenesis. Weis et al. found that low dose cerivastatin or atorvastatin potentiated endothelial cell proliferation whereas higher doses inhibited it25. In vivo, Boodhwani demonstrated that high dose atorvastatin (1.5 mg/kg/day) impairs collateral development in normocholesterolemic swine26. Our findings are similar using a less potent HMG-CoA reductase inhibitor. In disease states where endothelium-dependent vasodilation of coronary resistance vessels is impaired such as atherosclerosis, statins may have important beneficial effects in improving endothelium-dependent vasodilation of resistance arteries including collateral resistance vessels24. These functional effects rather than de novo arteriogenesis or vasculogenesis likely contribute to the well known improvement in symptomatic myocardial ischemia after administering statins to patients27.
Effect of Statins on Myocardial Function
Previous animal studies have demonstrated beneficial effects of statins on global myocardial function in a variety of models of heart disease. In general, all have used high doses of statins that greatly exceed the pharmacological range used in humans (e.g. 20 mg/kg simvastatin/day). These are also even higher than doses demonstrated to impair angiogenesis in vivo and in vitro. In rodent models of infarction, statins improved global LV function by preventing deleterious LV remodeling without reducing infarct size28–31. Statins have also been demonstrated to prevent the progression of hypertrophy following angiotensin infusion and aortic banding32 and prevent heart failure in genetic models of cardiomyopathy31. Studies evaluating the effect of statins on heart failure in large animals are limited. In dogs with heart failure statins attenuate the rate of decline in LV function in pacing-induced cardiomyopathy33 and cardiomyopathy due to coronary microembolization34. In general, mechanistic insight has largely focused on the ability of statins to improve NO signaling and reduce circulating markers of inflammation. None of the previous studies have elucidated a quantitative contribution of statins to myocyte regeneration.
Our results demonstrate that pravastatin increases regional function in hibernating myocardium in the absence of infarction or heart failure. While functional improvement was not related to improved perfusion, it was accompanied by an increase in regional end-diastolic wall thickness and an increase in myocyte nuclear density. Resolution of myocyte cellular hypertrophy in LAD myocardium could also contribute improved wall-thickening. Thus, the mechanism of improvement appears to be related to myocyte regeneration which restores regional myocyte loss from apoptosis during the development of hibernating myocardium13. While clinical trials in hibernating myocardium are lacking, Gheorghiade reported a dramatic serial functional improvement in a patient with hibernating myocardium from a chronic LAD occlusion after simvastatin35.
Statins Increase Myocytes in the Growth Phase of the Cell Cycle in Diseased Hearts
We found that statins increased myocytes in the proliferative phase of the cell cycle in a fashion similar to that which we have reported after intracoronary administration of AdvFGF-514. While this was accompanied by a global increase in myocardial tissue CD133+ and cKit+ BMPCs, increases in myocyte Ki67 and phospho-Histone-H3 staining were restricted to hibernating hearts. The possibility that increased circulating CD133+/cKit+ BMPCs leads to increased myocyte regeneration is unlikely since pravastatin increased CD133+/cKit+ BMPCs but did not cause myocyte proliferation in normal hearts. Thus, additional myocardial factors are probably required to stimulate myocyte proliferation. Since Ki67 increased in the remote myocardial region of hibernating hearts as well as the dysfunctional LAD region, the stimulus is global. Circulating factors related to neurohormonal activation like elevated catecholamines, angiotensin, endothelin and cytokines are plausible but seem unlikely in this model since global function is normal and hemodynamic evidence of neurohormonal activation is absent. A more likely possibility is that a factor released following transient myocardial ischemia recirculates to normal as well as ischemic myocardium. Alternatively, it is possible that myocardial stretch related to increased preload stimulates the expression of preload-induced myocardial growth factors and genes which facilitate myocyte proliferation in conjunction with the increase in tissue CD133+ and cKit+ cells36. Additional studies will be required to address these possibilities directly. Finally, while statins could have increased myocyte number by inhibiting apoptosis, we have previously demonstrated that apoptosis has returned to baseline 4-months after instrumentation in this model which is the time-frame over which the present studies were conducted14.
The actual origin of cycling myocytes cannot be unambiguously determined from our study. One possibility is that the CD133+ and cKit+ cells differentiate directly into cardiac myocytes37. This is supported by the observation that a portion of them express GATA4 and Troponin I. Another possibility is that CD133+ and cKit+ cells stimulate resident cardiac stem cells (CSCs) to proliferate with Ki67+ myocytes arising from CSCs as has been suggested after administration of mesenchymal stem cells38. It is also plausible that CD133+ and cKit+ cells fuse with cardiac myocytes and facilitate their reentry into the growth phase of the cell cycle39. Finally, paracrine factors released from CD133+ and cKit+ cells could stimulate CSC proliferation or facilitate myocytes to reenter the cell cycle40. Additional studies using models employing genetic fate mapping and stem cell tracking will be required to address these possibilities36, 41. While the precise signaling is unknown, the increase in myocyte nuclear density, smaller myocyte size and improvement in regional function demonstrated in vivo all suggest an effect of pravastatin on myocyte regeneration.
Clinical Implications
Our results support the hypothesis that the improvement in ejection fraction in patients with heart failure previously demonstrated with simvastatin8 and atorvastatin9 may result from stimulation of endogenous cardiac repair mechanisms. Nevertheless, this may not be a class effect as evidenced by the failure of the extremely potent drug rosuvastatin to improve ejection fraction10. In concert with the latter study, the recently completed large placebo controlled clinical trials of rosuvastatin in patients who have predominantly Class II and III heart failure with depressed ejection fraction (CORONA42 AND GISSI-HF43) have failed to show any impact of this drug on cardiovascular mortality. Results of rosuvastatin on serial function in these studies have not yet been reported. Individual statins likely vary in their ability to affect myocardial function through differential potency (or dose range) as well as their ability to affect relevant pleotrophic pathways (e.g. upregulation of Akt or inhibiting myocardial proliferative pathways such as Rho, Rac and Ras). Finally, age related impairment in cardiac stem cell repair mechanisms may be important11 and the clinical trials have focused upon elderly patients with heart failure (mean ages of 68–73 years) vs. younger patients in small positive studies using myocardial function as an end-point.
In summary, our study demonstrates functional improvement in hibernating myocardium after pravastatin that is independent of angiogenesis or atherosclerotic plaque regression that occurs in the absence of a proinflammatory state or neurohormonal activation. Beneficial effects may be absent when infarction rather than hibernating myocardium predominates since residual perfusion to scar is low. Nevertheless, since patients with ischemic cardiomyopathy usually have patchy fibrosis averaging ~20% of LV mass, our observations may be very relevant to understanding statin-mediated functional improvement in nonischemic cardiomyopathies as well as settings where dysfunction from reversible ischemia and LV remodeling play major roles. Finally, the ability of statins to mobilize circulating BMPCs may be an important variable to consider in understanding the independent effects of exogenous cell based therapies administered in clinical trials examining cardiac repair.
Supplementary Material
Acknowledgments
We thank Anne Coe, Deana Gretka, Elaine Granica and Amy Johnson for their technical assistance.
Supported by the VA, AHA, Buswell Fellowship NHLBI, Albert and Elizabeth Rekate Fund and the Empire State Stem Cell Board.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript accepted for publication in Circulation Research, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circres.ahajournals.org/. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Lipinski MJ, Abbate A, Fuster V, Vetrovec GW. Drug insight: statins for nonischemic heart failure--evidence and potential mechanisms. Nat Clin Pract Cardiovasc Med. 2007;4:196–205. doi: 10.1038/ncpcardio0855. [DOI] [PubMed] [Google Scholar]
- 2.Ramasubbu K, Estep J, White DL, Deswal A, Mann DL. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:415–426. doi: 10.1016/j.jacc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 4.Deschaseaux F, Selmani Z, Falcoz PE, Mersin N, Meneveau N, Penfornis A, Kleinclauss C, Chocron S, Etievent JP, Tiberghien P, Kantelip JP, Davani S. Two types of circulating endothelial progenitor cells in patients receiving long term therapy by HMG-CoA reductase inhibitors. Eur J Pharmacol. 2007;562:111–118. doi: 10.1016/j.ejphar.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 6.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 8.Node K, Fujita M, Kitakaze M, Hori M, Liao JK. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation. 2003;108:839–843. doi: 10.1161/01.CIR.0000084539.58092.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sola S, Mir MQ, Lerakis S, Tandon N, Khan BV. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol. 2006;47:332–337. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 10.Krum H, Ashton E, Reid C, Kalff V, Rogers J, Amarena J, Singh B, Tonkin A. Double-blind, randomized, placebo-controlled study of high-dose HMG CoA reductase inhibitor therapy on ventricular remodeling, pro-inflammatory cytokines and neurohormonal parameters in patients with chronic systolic heart failure. J Card Fail. 2007;13:1–7. doi: 10.1016/j.cardfail.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallavollita JA, Perry BJ, Canty JM., Jr 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium: Evidence for transmural variations in chronic hibernating myocardium. Circulation. 1997;95:1900–1909. doi: 10.1161/01.cir.95.7.1900. [DOI] [PubMed] [Google Scholar]
- 13.Lim H, Fallavollita JA, Hard R, Kerr CW, Canty JM., Jr Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation. 1999;100:2380–2386. doi: 10.1161/01.cir.100.23.2380. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki G, Lee TC, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res. 2005;96:767–775. doi: 10.1161/01.RES.0000162099.01268.d1. [DOI] [PubMed] [Google Scholar]
- 15.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 16.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 18.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 20.Zohlnhofer D, Dibra A, Koppara T, de Waha A, Ripa RS, Kastrup J, Valgimigli M, Schomig A, Kastrati A. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51:1429–1437. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 21.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow--derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canty JM., Jr . Coronary blood flow and myocardial ischemia. In: Libby P, Bonow RO, Mann DL, Zipes DP, editors. Braunwald’s Heart Disease. 8. Philadelphia: Elsevier; 2007. pp. 1167–1194. [Google Scholar]
- 25.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 26.Boodhwani M, Mieno S, Voisine P, Feng J, Sodha N, Li J, Sellke FW. High-dose atorvastatin is associated with impaired myocardial angiogenesis in response to vascular endothelial growth factor in hypercholesterolemic swine. J Thorac Cardiovasc Surg. 2006;132:1299–1306. doi: 10.1016/j.jtcvs.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 27.Stone PH, Lloyd-Jones DM, Kinlay S, Frei B, Carlson W, Rubenstein J, Andrews TC, Johnstone M, Sopko G, Cole H, Orav J, Selwyn AP, Creager MA. Effect of intensive lipid lowering, with or without antioxidant vitamins, compared with moderate lipid lowering on myocardial ischemia in patients with stable coronary artery disease: the Vascular Basis for the Treatment of Myocardial Ischemia Study. Circulation. 2005;111:1747–1755. doi: 10.1161/01.CIR.0000160866.90148.76. [DOI] [PubMed] [Google Scholar]
- 28.Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation. 2001;104:982–985. doi: 10.1161/hc3401.095946. [DOI] [PubMed] [Google Scholar]
- 29.Hayashidani S, Tsutsui H, Shiomi T, Suematsu N, Kinugawa S, Ide T, Wen J, Takeshita A. Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;105:868–873. doi: 10.1161/hc0702.104164. [DOI] [PubMed] [Google Scholar]
- 30.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, Mueller M, Fuchs M, Hornig B, Haller H, Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 31.Abraham SS, Osorio JC, Homma S, Wang J, Thaker HM, Liao JK, Mital S. Simvastatin preserves cardiac function in genetically determined cardiomyopathy. J Cardiovasc Pharmacol. 2004;43:454–461. doi: 10.1097/00005344-200403000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108:1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trochu JN, Mital S, Zhang X, Xu X, Ochoa M, Liao JK, Recchia FA, Hintze TH. Preservation of NO production by statins in the treatment of heart failure. Cardiovasc Res. 2003;60:250–258. doi: 10.1016/j.cardiores.2003.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaca V, Rastogi S, Imai M, Wang M, Sharov VG, Jiang A, Goldstein S, Sabbah HN. Chronic monotherapy with rosuvastatin prevents progressive left ventricular dysfunction and remodeling in dogs with heart failure. J Am Coll Cardiol. 2007;50:551–557. doi: 10.1016/j.jacc.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 35.Gheorghiade M, Klein L, Stone NJ, Bonow RO, Kim RJ. Improvement in the function of hibernating myocardium in a patient with heart failure due to coronary artery disease receiving high-dose simvastatin. Ital Heart J. 2004;5:160–162. [PubMed] [Google Scholar]
- 36.Muller P, Kazakov A, Semenov A, Bohm M, Laufs U. Pressure-induced cardiac overload induces upregulation of endothelial and myocardial progenitor cells. Cardiovasc Res. 2008;77:151–159. doi: 10.1093/cvr/cvm037. [DOI] [PubMed] [Google Scholar]
- 37.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4 (Suppl 1):S21–26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 39.Matsuura K, Wada H, Nagai T, Iijima Y, Minamino T, Sano M, Akazawa H, Molkentin JD, Kasanuki H, Komuro I. Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. J Cell Biol. 2004;167:351–363. doi: 10.1083/jcb.200312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Janosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 43.GISSI-HF I. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008 doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.