Abstract
Lipoprotein(a) [Lp(a)] is assembled by the binding of apolipoprotein B (apoB) lysine residues on LDL to lysine binding sites in apolipoprotein(a) [apo(a)] and the subsequent formation of a disulphide bond between apoB and apo(a). In this study, we induced changes in apoB conformation by adding phospholipids to LDL and tested the effect of the altered apoB conformation on Lp(a) assembly. The addition of dimyristoylphosphatidylcholine (DMPC) to isolated LDL induced a decrease in the α-helical content of apoB and increased the immunoreactivity of the apoB C terminus toward monoclonal antibodies in the region. These conformational changes were associated with a reduction in the ability of the DMPC-modified LDL to form Lp(a) in in vitro assays. Furthermore, administration of DMPC to Lp(a) transgenic mice lead to a significant but transient decrease in Lp(a) levels (18.6% decrease at 2 h, P < 0.001) which coincided with the association of DMPC with LDL in plasma. Our study shows that changes in apoB conformation in the C-terminal region alter the exposure of sequences required for Lp(a) assembly and reduce the formation of Lp(a) both in vitro and in vivo. We conclude that manipulation of LDL surface phospholipids alters Lp(a) levels.
Keywords: apolipoprotein B, apolipoprotein(a), phospholipids, transgenic mice
Lipoprotein(a) [Lp(a)] consists of an LDL covalently attached via its surface protein, apolipoprotein B (apoB), to the unique glycoprotein, apolipoprotein(a) [apo(a)] (1). Many large clinical trials have identified high plasma Lp(a) levels as a risk factor for the development of cardiovascular disease (2–4). Plasma Lp(a) levels are largely determined by the rate of production (5), which is dependent upon the level of apo(a) synthesis and the efficiency of assembly into Lp(a) particles. The majority of evidence suggests that Lp(a) is assembled extracellularly after secretion of apo(a) by the liver and production of LDL from VLDL in circulation (6). Factors affecting Lp(a) assembly are of intense interest since the assembly process is a potential target for intervention to reduce Lp(a) levels.
Lp(a) assembly is well recognized as a two-step process involving an initial noncovalent interaction between apo(a) and apoB that is followed by a disulphide linkage between the two proteins (7, 8). The protein sequences in both apo(a) and apoB that are required for Lp(a) formation have been reasonably well defined. They consist of the KIV type 7 and 8 lysine binding domains in apo(a) (9, 10) that interact with specific apoB lysine residues (11, 12) in the initial step and the two cysteine residues, apo(a) 4057 (7, 13) and apoB4326 (14, 15), required for the disulphide bond. The correct conformation of both apo(a) and apoB is clearly important to enable these sequences to be presented for efficient Lp(a) assembly. In support of this are studies of apo(a) that have shown a marked reduction in Lp(a) assembly rate with a “closed” versus “open” conformation of the apo(a) protein (16).
There is a lack of information regarding the effect of apoB conformation on Lp(a) assembly. It is well established that the conformation of apoB on the lipoprotein surface changes as VLDL particles are processed to LDL in vivo and that the changes are particularly apparent in specific domains of the apoB C-terminal region (17). Interestingly, VLDL forms Lp(a) poorly compared with LDL, indicating that changes in apoB conformation do alter its ability to interact with apo(a) (18). It is likely that even within the LDL density range, changes in lipid composition, particularly surface lipids, may induce changes in apoB conformation that could alter its ability to form Lp(a). Support for this hypothesis comes from a study of LDL isolated from LCAT-deficient patients that is enriched in surface-free cholesterol that was unable to form Lp(a) with recombinant apo(a) (19). There is evidence that conformational changes in apoB also occur with modification of LDL phospholipids. Phospholipase A2-treated LDL exhibits less immunoreactivity in the apoB C terminus compared with untreated LDL (20). The effect of conformational changes in apoB induced by the modification of LDL phospholipids on Lp(a) assembly is unknown.
In this study, we investigated the effect of dimyristoylphosphatidylcholine (DMPC) on the conformation of apoB on LDL and the efficiency of Lp(a) assembly both in vitro and in vivo.
MATERIALS AND METHODS
Isolation of human LDL
Human LDL was isolated from the plasma of two healthy, normolipidemic donors with low Lp(a) levels (<10 nmol/l). The lipid levels of the two donors were 3.46 mmol/l cholesterol, 0.96 mmol/l HDL cholesterol, and 0.55 mmol/l triglyceride for donor 1 and 4.18 mmol/l cholesterol, 1.23 mmol/l HDL cholesterol, and 1.17 mmol/l triglyceride for donor 2. The LDL was isolated by ultracentrifugation using a single-step discontinuous gradient as previously described (21). The isolated LDL [designated LDL1 (from donor 1) and LDL2 (from donor 2)] was stored under argon gas in the dark at 4°C for a maximum of 2 weeks and desalted into PBS just before use. The purity of the LDL samples was analyzed by agarose gel electrophoresis, and a Western blot was performed to detect any Lp(a). A chemical composition analysis was performed on both LDL samples. Cholesterol and triglyceride content were measured using the Roche CHOD-PAP and GPO-PAP reagents, respectively (Roche Diagnostics, Mannheim, Germany), and phospholipids were measured using the Wako phospholipid assay kit (Wako Pure Chemical Industries, Osaka, Japan). The LDL protein concentration was determined by the modified Lowry method (22). The LDL1 sample was used for the sizing, electrophoretic mobility, immunoreactivity, and Lp(a) assembly studies. Both samples were used for the circular dichroism studies.
Preparation of phospholipid vesicles
Small unilamellar vesicles of DMPC (Avanti Polar Lipid, Alabaster, AL) were prepared using the method described by New (23) with slight modification. The DMPC was suspended in PBS, pH 7.4, and sonicated using a microtip 3 mm probe (Sonics VibraCell, Newtown, CT) at an amplitude of 20 MHz for 20 × 30 s with 30-s breaks in between. The temperature was maintained at 30°C, above the phase transition temperature of DMPC (∼24°C). The vesicles were centrifuged at 2,200 rpm for 5 min to pellet titanium originating from the sonicator probe. The DMPC vesicles were extruded through a polycarbonate membrane with a pore size of 30 nm using the Avanti Mini extruder (Avanti Polar Lipid). The concentration of phospholipid was determined using the Wako phospholipid assay kit.
Lipoprotein sizing and gel electrophoresis
The effect of DMPC on LDL particle size was determined by dynamic light scattering analysis using a Malvern Zetasizer (Nano ZS; Malvern Instruments, Worcestershire, UK) after incubation of LDL with 1 mM DMPC vesicles at 25°C. The effect of DMPC on LDL electrophoretic mobility was investigated by incubating LDL (275 μg/ml protein) with 0.5, 1, or 2 mM DMPC vesicles at 37°C for 2 h and subsequent electrophoresis of the samples using the Helena TITAN™ Gel Lipoprotein electrophoresis system (Helena Laboratories, Beaumont, TX). Following electrophoresis, gels were dried and stained with Fat Red 7B stain.
Circular dichroism spectroscopy
The circular dichroism (CD) spectra of both LDL samples in the presence or absence of DMPC were recorded at 37°C on an Olis DSM 10 spectrophotometer (Olis, Bogart, GA) using a 1 mm path length quartz cuvette. Spectra were recorded between the wavelengths of 260 and 190 nm with a 1.0 nm step size and slit bandwidth of 1.5 nm. Signal averaging time was 1 s and ellipticites reported as mean residue ellipticity (θ) in degree cm2/dmol. Spectra are presented as an average of four separate scans with baseline correction. The LDL samples at a protein concentration of 30 μg/ml were incubated with DMPC (220 μM) in 10 mM sodium phosphate buffer, pH 7.4, at 37°C for 1 h before CD analysis. The percentage of α-helix in apoB-100 was calculated according to Equation 1 of Aggerbeck et al. (24) using the mean residue ellipticity at 222 nm and a mean residue weight of 113.
apoB immunoreactivity
The immunoreactivity of apoB on LDL treated with 1 mM DMPC to multiple human apoB-specific monoclonal antibodies was tested using a modified form of the double sandwich ELISA method described by Sharp et al. (25).
In this case, the capture antibody was a polyclonal sheep anti-human apoB antisera (1 in 1,000 dilution) from Roche Diagnostics. The immunoreactivity of the following five mouse monoclonal antibodies to increasing amounts of DMPC-treated LDL was tested: 1D1, which binds between apoB amino acids 474 and 539 (26); MB47, which binds near amino acid 3500 (27); 4H11 and 605, which both bind between amino acids 4342 and 4536 (17); and Bsol7, which binds between amino acids 4521 and 4536 (26). All mouse monoclonal antibodies were in the form of ascites and were used at a 1 in 4,000 dilution. The binding of the mouse monoclonal antibodies was detected by incubation with a goat-anti mouse IgG-hrp (Pierce Biochemicals, Rockford, IL) for 1 h and the plates developed. Average absorbances were calculated from triplicate data points. All data were normalized to the average absorbance of the untreated (control) LDL at the highest concentration (275 μg/ml protein) to give a relative average absorbance for the DMPC-treated LDL at the various LDL concentrations. A comparison of the relative absorbances of the DMPC treated versus control LDL at 275 μg/ml was made for each antibody.
Mice
Lp(a) transgenic mice were generated by cross-breeding of human apo(a) transgenic mice (18) with human apoB-100 transgenic mice (28). All mice were housed in a specific pathogen free animal facility with a 12 h light/dark cycle. The plasma Lp(a) levels of the mice used for these studies ranged between 35 and 65 nmol/l as determined by an Lp(a) ELISA (29).
In vitro Lp(a) formation assays
The effect of DMPC on Lp(a) assembly was investigated using the Lp(a) formation assay described by Sharp et al. (25). Plasma (1 μl) from a human apo(a) transgenic mouse was incubated with isolated LDL (275 μg/ml) or plasma (2 μl) from a human apoB transgenic mouse for 3 h at 37°C in the presence of increasing amounts (0–2 mM) of DMPC vesicles. The amount of Lp(a) formed in each incubation was assessed after aliquots were size-fractionated on 4% SDS-PAGE gels under nonreducing conditions, and the separated proteins were transferred to nitrocellulose membrane for Western blotting with the MAb-a-5-hp antibody (29). Bands were visualized using the ECL detection system (Amersham Pharmacia Biotech, Uppsala, Sweden). The amount of Lp(a) formed in the incubations was quantified by an Lp(a)-specific ELISA, as detailed by Sharp et al. (25). The percentage of Lp(a) formed in each incubation was calculated relative to the incubation containing no DMPC. The incubations (4 μl) were also subject to agarose gel electrophoresis to investigate the effect of DMPC on plasma lipoproteins.
Administration of DMPC to Lp(a) transgenic mice
Six male Lp(a) transgenic mice (25 g) were injected intravenously with 0.2 mg/g body weight of DMPC vesicles (to give an estimated initial plasma concentration of 4 mM DMPC) in 200 μl PBS via the tail vein. Six control mice were injected with 200 μl PBS only. Blood (50 μl) was collected from the tail vein at 0, 1, 2, 4, and 8 h following injection, and plasma Lp(a) levels were quantified by the Lp(a)-specific ELISA (25). Plasma (3 μl) from the treated animals was also subject to agarose gel electrophoresis to visualize plasma lipoproteins.
Statistics
For the dynamic light scattering analysis, results are presented as the mean particle size ± SEM as determined from triplicate runs. In vitro data were analyzed with a two tailed Student's t-test using the statistical program within the Sigmaplot software (version 7.0) to test for a significant difference in mean values between the untreated and DMPC-treated LDL samples. For the in vivo studies, a comparison was made between the mean percentage change values of the DMPC-treated versus untreated animals at the same time point using a two-tailed Student's t-test. Individual P values have only been reported if there was a significant difference.
RESULTS
Characterization of isolated LDL
The two LDL samples used for this study (LDL1 and LDL2) were pure as judged by agarose gel electrophoresis and subsequent Western blot analysis of the isolated LDL showed that the samples were free of Lp(a) (Fig. 4A). The chemical composition of the LDL samples was 40% cholesterol, 17% triglyceride, 28% phospholipid, and 15% protein for LDL1 and 43% cholesterol, 21% triglyceride, 21% phospholipid, and 15% protein for LDL2.
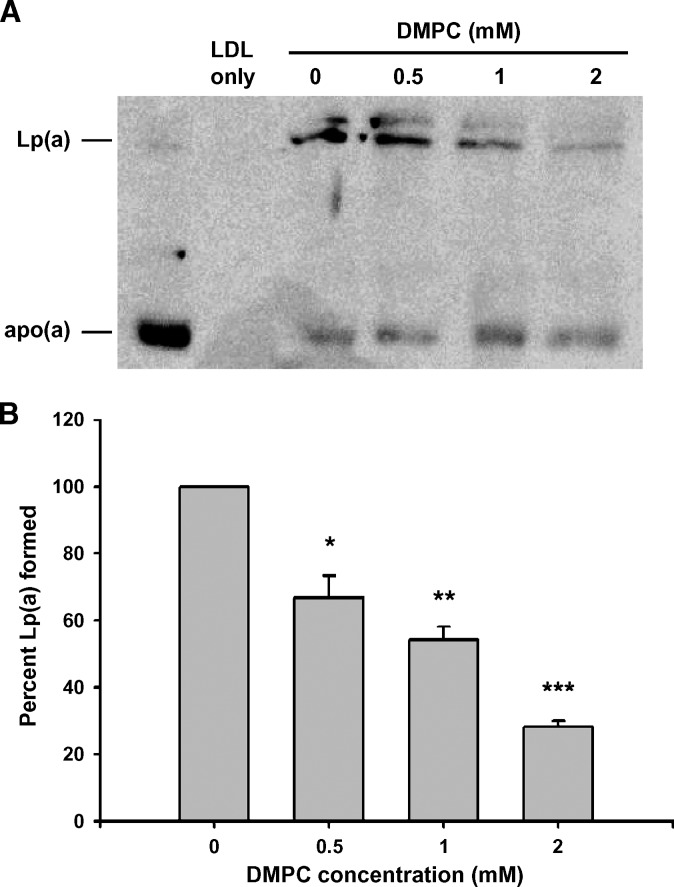
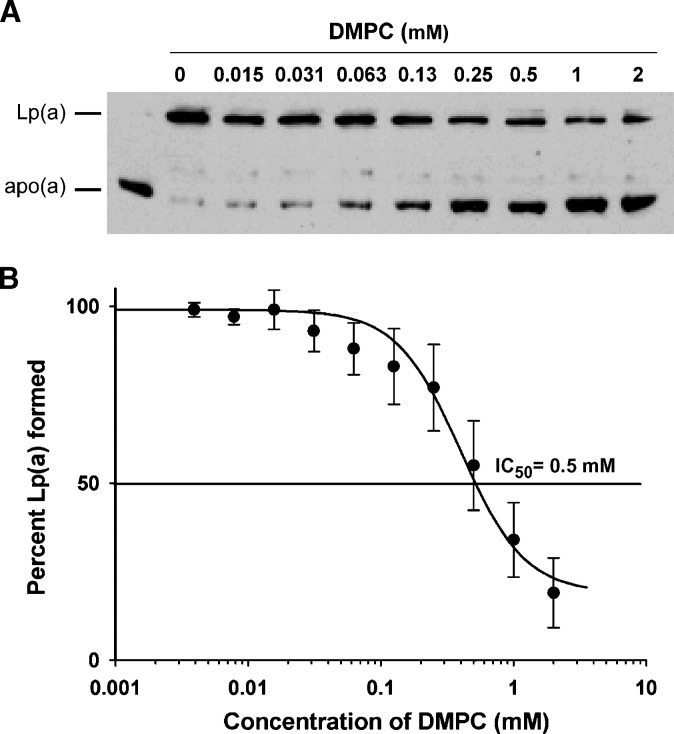
Fig. 4.
Inhibition of Lp(a) formation by DMPC using human LDL. Increasing concentrations of the DMPC vesicles were added to incubations containing 1 μl human apo(a) and 275 μg/ml protein of isolated human LDL at 37°C for 3 h. A: The amount of Lp(a) formed in each incubation was assessed by the separation of the incubation mix on 4% nonreducing SDS-PAGE gels and Western blotting with the MAb-a-5-hp antibody. B: The amount of Lp(a) formed in each incubation was measured in an Lp(a) ELISA. Each incubation was measured in triplicate and the average value expressed as a percentage of Lp(a) formed compared with incubations containing no DMPC. All values are expressed as mean ± SEM from three independent experiments (*P < 0.01, **P < 0.001, and ***P < 0.0001, compared with incubations containing no DMPC).
Effect of DMPC on LDL size and electrophoretic mobility
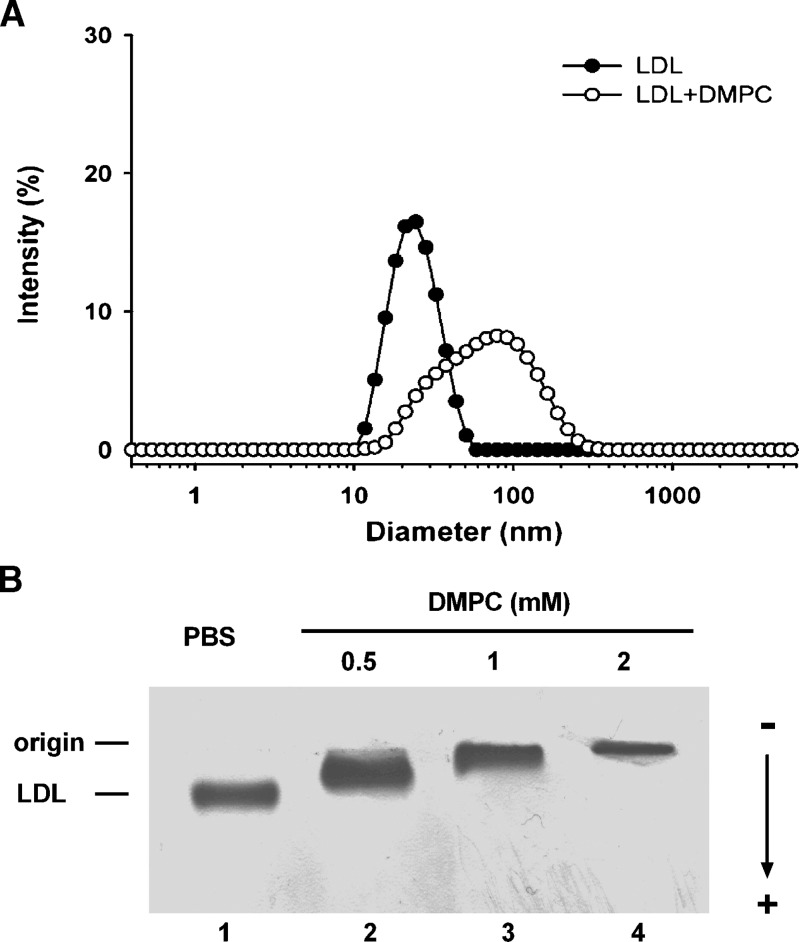
Addition of 1 mM DMPC vesicles to LDL increased the average particle size significantly from 22.4 ± 0.1 nm to 56.2 ± 3.9 nm (P < 0.001) as determined by dynamic light scattering (Fig. 1A). A wider distribution of LDL particles sizes was apparent in the DMPC-treated sample (polydispersity index of 0.251 ± 0.003) compared with untreated LDL (0.095 ± 0.002, P < 0.0001). Agarose gel electrophoresis of DMPC-treated LDL showed a reduction in the electrophoretic mobility of the LDL with the addition of increasing amounts of DMPC from 0.5 to 2 mM (Fig. 1B).
Fig. 1.
Particle size distribution and electrophoretic mobility of DMPC-treated LDL. A: The particle size of LDL in PBS was determined by dynamic light scattering. The LDL was incubated with DMPC (1 mM) at 25°C for 5 min. The particle size distribution was expressed as the intensity of light scattering as a function of particle size. B: Agarose gel electrophoresis of DMPC-treated LDL. Increasing amounts of DMPC vesicles (0.5 to 2 mM) in PBS were incubated with LDL (275 μg/ml protein) at 37°C for 2 h. Samples were subjected to separation with the Helena TITAN™ lipoprotein electrophoresis system and stained with Fat Red 7B.
Effect of DMPC on apoB-100 secondary structure
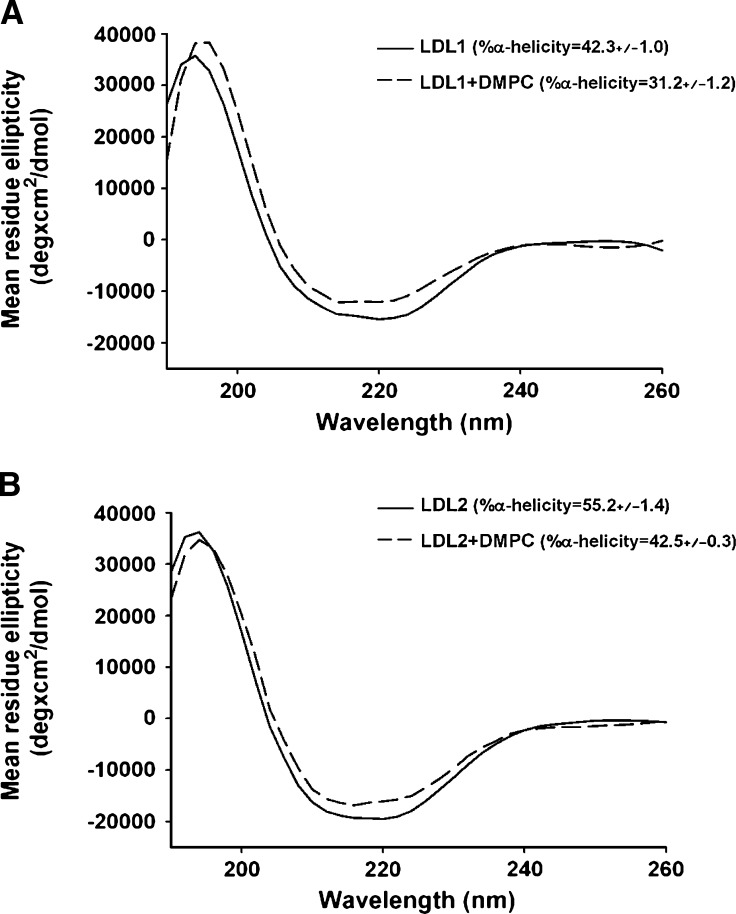
The secondary structure of the apoB on both LDL samples before and after treatment with DMPC was examined by CD spectroscopy. Both samples showed spectra typical of proteins with significant α-helical content with minima at 222 nm (Fig. 2A, B). The calculated α-helical content of both LDL samples was similar to values previously reported for normal LDL (30, 31). Addition of DMPC elicited a significant decrease in the α-helical content of both samples from 42.3 ± 1.0% to 31.2 ± 1.2% (P < 0.001) for LDL1 (Fig. 2A) and from 55.2 ± 1.4% to 42.5 ± 0.3% (P < 0.001) for LDL2 (Fig. 2B).
Fig. 2.
CD spectra of DMPC-treated LDL. The CD spectra of the LDL1 (A) and LDL2 (B) samples with and without DMPC were recorded at 37°C on an Olis™ DSM 10 spectrophotometer using a 1 mm path length quartz cuvette. The LDL samples were analyzed in 10 mM sodium phosphate buffer, pH 7.4, at a protein concentration of 30 μg/ml. The ellipticities were recorded over the wavelengths 190–260 nm, with a step size of 1 nm. The mean residue ellipticity ([θ]) is plotted as a function of wavelength. The spectra of each sample were obtained by signal averaging of four separate scans with baseline corrected. The percentage of α-helix in apoB-100 was calculated from the mean residue ellipticity at 222 nm using a mean residue weight of 113.
Effect of DMPC on apoB immunoreactivity
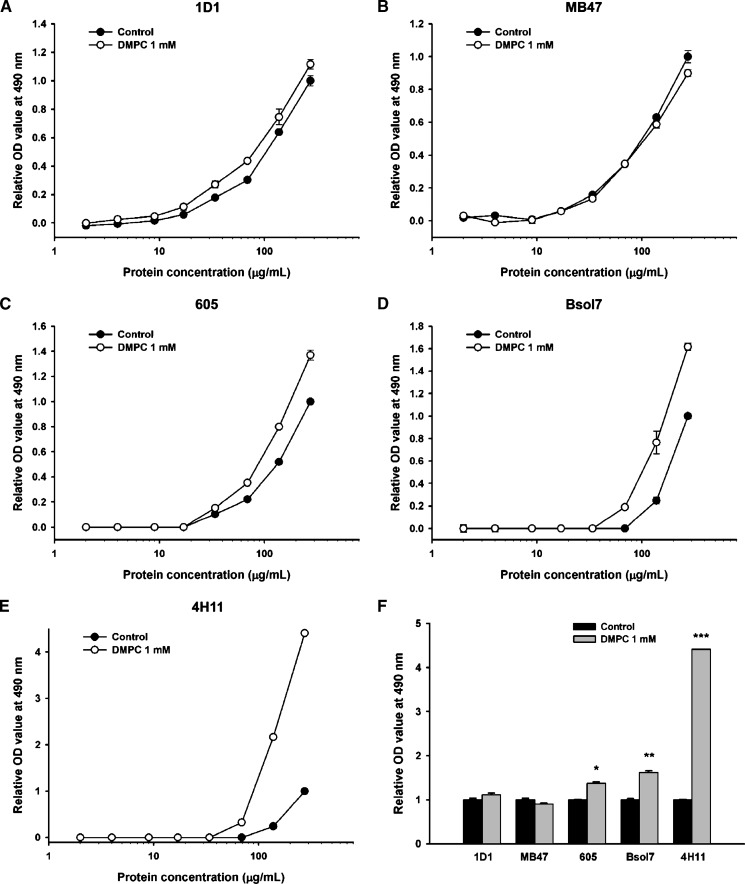
The binding of five different monoclonal antibodies to increasing amounts of LDL treated with 1 mM DMPC was tested in a sandwich ELISA. Both the control untreated LDL and the DMPC-treated LDL showed a similar immunoreactivity to the 1D1 and MB47 antibodies (Fig. 3A, B). In contrast, the DMPC-treated LDL showed an increased reactivity to the 605, Bsol7, and 4H11 antibodies (Fig. 3C–E). A comparison of the immunoreactivities at the highest LDL concentration (275 μg/ml) showed a significantly increased reactivity of the DMPC-treated LDL toward the 605, Bsol7, and 4H11 antibodies compared with untreated LDL (P < 0.01, 0.001, and 0.0001, respectively) (Fig. 3F). The 605 and 4H11 antibodies have epitopes between apoB amino acids 4342 and 4536, while the Bsol7 antibody has an epitope between 4521 and 4536.
Fig. 3.
Immunoreactivity of DMPC-treated LDL to apoB MAbs. Increasing concentrations of human LDL were incubated with DMPC (1 mM) at 37°C for 2 h. A to E: The immunoreactivity of DMPC-treated LDL to human apoB monoclonal antibodies 1D1, MB47, 605, Bsol7, and 4H11 was monitored by an apoB-specific ELISA in triplicate. F: The immunoreactivity of DMPC-treated LDL with each monoclonal antibody was compared with control LDL at 275 μg/ml of LDL (*P < 0.01, **P < 0.001, and ***P < 0.0001, compared with control LDL).
In vitro Lp(a) formation with DMPC-treated LDL
We investigated whether the changes in LDL induced by DMPC would affect the ability of the LDL to bind to apo(a) and form Lp(a). An Lp(a) formation assay containing DMPC-treated LDL showed a reduction in Lp(a) formation with increasing amounts of DMPC as assessed by both Western blot analysis to detect Lp(a) (Fig. 4A) and an Lp(a) ELISA that measured the amount of Lp(a) formed in each incubation (Fig. 4B). The amount of Lp(a) formed was significantly reduced in the presence of 0.5, 1.0, and 2.0 mM DMPC (P < 0.01, 0.001, and 0.0001, respectively). Subsequent agarose gel electrophoresis of the incubations showed a reduced mobility of LDL with increasing amounts of DMPC similar to that seen in Fig. 1B.
In vitro Lp(a) formation with DMPC-treated plasma
To test whether DMPC could inhibit Lp(a) assembly in plasma, increasing concentrations of the DMPC vesicles were incubated with fixed amounts of human apoB and human apo(a) in plasma taken from human apoB and human apo(a) transgenic mice. Western blot analysis of the resulting incubation mixes showed a reduction in Lp(a) formation with increasing concentrations of DMPC (Fig. 5A) as indicated by a gradual decrease in the intensity of the Lp(a) band and a corresponding increase in the intensity of the free apo(a) band. To quantify the inhibitory effect of DMPC on Lp(a) formation, an Lp(a) ELISA was used to measure the amounts of Lp(a) formed in the incubations and an inhibition curve derived from the data (Fig. 5B). An IC50 value for DMPC of 500 μM was determined from the inhibition curve. Agarose gel electrophoresis of incubations containing DMPC to visualize plasma lipoproteins showed a reduction in the electrophoretic mobility of LDL and HDL (data not shown).
Fig. 5.
Inhibition of Lp(a) formation by DMPC using human apoB transgenic mouse plasma. Increasing concentrations of the DMPC vesicles were added to incubations containing 1 μl human apo(a) and 2 μl human apoB transgenic mouse plasma at 37°C for 3 h. A: The amount of Lp(a) formed in each incubation was assessed by the separation of the incubation mix on 4% nonreducing SDS-PAGE gels and Western blotting with the MAb-a-5-hp antibody. B: The amount of Lp(a) formed in each incubation was measured in an Lp(a) ELISA. Each incubation was measured in triplicate and the average value expressed as a percentage of Lp(a) formed compared with incubations containing no DMPC. All values are expressed as mean ± SEM from four independent experiments.
In vivo Lp(a) lowering effect
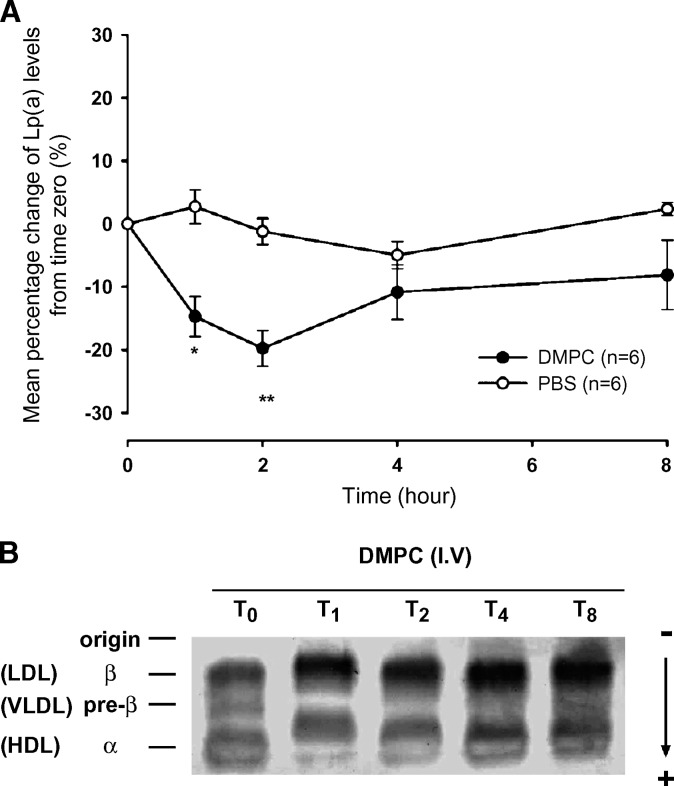
Based on the inhibitory effect of DMPC on Lp(a) formation in vitro, we tested whether DMPC could lower Lp(a) levels in vivo. Intravenous injection of DMPC vesicles in PBS (0.2 mg/g body weight) to Lp(a) transgenic mice (Fig. 6A) resulted in a significant decrease of plasma Lp(a) levels at 1 and 2 h compared with the PBS-treated animals (−14.7 ± 3.2% versus +2.7 ± 2.7%, P < 0.01 for the 1 h and −19.8 ± 2.9% versus −1.2 ± 2.1%, P < 0.001 for the 2 h). There was no significant difference in plasma Lp(a) levels between the DMPC- and PBS-treated animals at the 4 or 8 h time point. To evaluate the effect of the DMPC on plasma lipoproteins in the treated mice, plasma samples from the various time points were subjected to agarose gel electrophoresis. This showed a transient reduction in LDL mobility at the 1 h time point returning to normal mobility by 2 h (Fig. 6B). A similar mobility shift was also seen in the HDL of the DMPC-treated animals. In contrast, the electrophoretic mobility of plasma lipoproteins in PBS-treated animals remained unchanged.
Fig. 6.
Effect of DMPC vesicles on plasma Lp(a) levels and electrophoretic mobility of LDL in Lp(a) transgenic mouse plasma. A: DMPC vesicles in PBS were administered to six Lp(a) transgenic mice via tail vein injection and 50 μl blood was obtained at various time points up to 8 h. Six control mice were injected with PBS only. Plasma Lp(a) was measured in triplicate by an Lp(a) ELISA. Data were normalized by expressing values as percentage change of Lp(a) levels from time zero after injection. Each point represents the mean ± SEM from each group (*P < 0.01 and *P < 0.001, compared with PBS). B: Plasma samples from the DMPC-treated mice at the various time points were subjected to separation with the Helena TITAN™ lipoprotein electrophoresis system and stained with Fat Red 7B. A representative gel is presented.
DISCUSSION
Elevated levels of Lp(a) are an independent risk factor for developing heart disease (2–4); however, there is currently no safe and effective therapy available for lowering Lp(a) (32). As Lp(a) levels are mainly determined by the rate of synthesis, factors affecting assembly are of interest. Lp(a) assembly involves interactions between weak lysine binding sites in apo(a) (9, 10) and apoB-100 lysine residues on the LDL surface (11, 12) and the formation of a disulfide bond between apo(a)Cys4057 (7, 13) and apoBCys4326 (14, 15). Factors affecting the presentation of these sequences could potentially alter the efficiency of Lp(a) formation in vivo. ApoB is of particular interest because it is known to change conformation with changing lipid composition of the associated lipoprotein (17, 20, 31). The effects of changes in apoB conformation on Lp(a) assembly has not been well analyzed. In this study, we investigated the effect of phospholipid addition to LDL on Lp(a) assembly. We hypothesized that the addition of phospholipid content to LDL would alter the conformation of apoB and reduce the ability of the LDL to form Lp(a).
To alter the surface phospholipid content of LDL, we incubated LDL with DMPC vesicles. DMPC was chosen because it is a well-studied phospholipid with no net charge known to interact with apolipoproteins above its phase transition temperature (24°C) (33, 34). Addition of DMPC to isolated LDL increased the size of the particle and reduced its electrophoretic mobility (Fig. 1). While the reduced electrophoretic mobility may in part be due to an increase in LDL size, it is apparent that there is a reduction in the net negative charge of the particle. The lower net negative charge on LDL is likely due to changes in the microenvironment of lysine residues on the apoB surface. Previous studies of LDL subclasses have shown that changes in surface charges are accompanied by changes in apoB conformation that alter the ionization state and location of lysine residues (35). This is of particular interest for Lp(a) assembly since the initial step in Lp(a) assembly involves the binding of apoB lysine residues to apo(a) lysine binding sites (11, 12) and alteration in lysine location on the surface of apoB may alter Lp(a) formation.
It has been predicted that as LDL size decreases, the total surface area of lipid associated amphipathic α-helices in apoB increases (36). Conversely, one would expect an increase in LDL size on addition of DMPC to decrease the content of lipid associated apoB amphipathic α-helices. Indeed, CD spectroscopy analysis of LDL (Fig. 2) showed a significant decrease in the α-helical content of apoB after DMPC treatment. This decrease in α-helical content could induce conformational changes in apoB that alter the exposure of sequences required for Lp(a) assembly. A conformational change in apoB with the addition of DMPC was detected with immunoreactivity studies (Fig. 3) that showed an increase in reactivity to three monoclonal antibodies containing epitopes within the apoB C-terminal region. The increased immunoreactivity was particularly noticeable with the 4H11 antibody that binds an epitope between 4342 and 4536 (17). This region lies in the α3 domain of the pentapartite model of apoB100 proposed by Segrest et al. (36). It contains multiple repeats of amphipathic α-helices that have reversible lipid affinity and is thought to represent a flexible domain that alters with changes in lipid composition. It is of interest that conformational changes in apoB on addition of phospholipids occur in this region because an α-helical lysine-rich sequence (apoB amino acids 4372–4392) implicated in the noncovalent binding to apo(a) (12, 25) is located within this domain. Conformational changes in this region would be expected to alter the exposure of these sequences and interfere with Lp(a) assembly.
Having gained data to support conformational changes in apoB following the addition of phospholipids, we then tested the ability of DMPC-treated LDL to form Lp(a). Treatment of isolated LDL with increasing amounts of DMPC impaired the ability of the LDL to form Lp(a) in vitro. A similar result was seen in Lp(a) assembly assays performed with DMPC-treated plasma. Interestingly, DMPC showed an IC50 of 500 μM in these assays, which is well below that of lysine analogs that are routinely used as Lp(a) assembly inhibitors in vitro (37) and have been tested in vivo (38). These results suggest that the changes in apoB conformation elicited by the addition of DMPC do alter the ability of the LDL to form Lp(a) presumably by altering the conformation of the apoB sequences that are required for efficient Lp(a) formation. Exactly which of the two steps of Lp(a) assembly is most affected by these conformational changes is not clear. Future experiments to determine the accessibility of apoB lysines and the Cys4326 residue to labeling after DMPC treatment might be useful to identify the exact regions of altered conformation and the step in Lp(a) assembly most likely to be affected.
Based on our in vitro Lp(a) assembly results, we tested the effect of intravenous administration of DMPC to Lp(a) transgenic mice to investigate whether DMPC could reduce Lp(a) assembly in vivo. A significant reduction in Lp(a) levels was evident at 1 and 2 h (17.4 and 18.6%, respectively) after administration of a single dose of DMPC. This level of reduction in Lp(a) levels suggests a robust response given that the association of LDL with DMPC in plasma would only affect Lp(a) that is being newly assembled in circulation and not that already formed. As the half-life of Lp(a) is known to be around 4–6 h, our result would suggest that virtually no Lp(a) formation occurred for the 2 h following DMPC injection. It is also possible that the DMPC treatment might enhance the clearance of Lp(a) contributing to the transient reduction; however, kinetic studies would be required to investigate this further. The Lp(a)-lowering effect coincided with a reduction in LDL mobility at the 1 h time point indicative that conformational changes in apoB are most likely responsible for the reduction in Lp(a) levels. Furthermore, these conformational changes are short lived with the LDL returning to normal mobility by 2 h, and this coincides with the subsequent return of Lp(a) levels to normal by 4 h. The short-term effect of DMPC is probably due to its relatively short half-life in plasma (39). Our results suggest that DMPC only transiently associates with LDL before being rapidly metabolized. A longer-term multiple dose study with DMPC may be warranted since a more constant level of DMPC would maintain conformational changes and allow for a bigger effect on Lp(a) levels. Ideally, the DMPC vesicles should be modified to increase their half-life and enhance their interaction with LDL over HDL. Incorporation of cholesterol into the DMPC vesicles may help achieve these aims (39, 40).
In conclusion, our study shows that changes in apoB conformation on the LDL surface, particularly in the C-terminal region, alter its ability to form Lp(a). The phospholipid DMPC effectively induces changes in apoB conformation that inhibit Lp(a) assembly both in vitro and in vivo. Further investigation of DMPC as a potential Lp(a)-lowering agent may be warranted.
Acknowledgments
The authors thank Prof. Ross Milne for supplying the 1D1, 4H11, 605, and Bsol7 apoB monoclonal antibodies; Prof. Santica Marcovina for supplying the MAb a-6 and MAb a-5 apo(a) monoclonal antibodies; Prof. Stephen Young for supplying the MB47 apoB monoclonal antibody and human apoB100 transgenic mice; and Prof. Robert Hammer for the human apo(a) transgenic mice.
Abbreviations
apo(a), apolipoprotein(a)
apoB, apolipoprotein B
CD, circular dichroism
DMPC, dimyristoylphosphatidylcholine
Lp(a), lipoprotein(a)
This work was supported by a grant from the National Heart Foundation (Grant 1051). Y-T.W. was supported by an International Postgraduate Scholarship and a Postgraduate Publishing Bursary from the University of Otago.
Published, JLR Papers in Press, December 19, 2008.
References
- 1.Utermann G. 1989. The mysteries of lipoprotein(a). Science. 246 904–910. [DOI] [PubMed] [Google Scholar]
- 2.Berg K., G. Dahlen, B. Christophersen, T. Cook, J. Kjekshus, and T. Pedersen. 1997. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin. Genet. 52 254–261. [DOI] [PubMed] [Google Scholar]
- 3.Seman L. J., C. DeLuca, J. L. Jenner, L. A. Cupples, J. R. McNamara, P. W. Wilson, W. P. Castelli, J. M. Ordovas, and E. J. Schaefer. 1999. Lipoprotein(a)-cholesterol and coronary heart disease in the Framingham Heart Study. Clin. Chem. 45 1039–1046. [PubMed] [Google Scholar]
- 4.Luc G., J. M. Bard, D. Arveiler, J. Ferrieres, A. Evans, P. Amouyel, J. C. Fruchart, and P. Ducimetiere. 2002. Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis. 163 377–384. [DOI] [PubMed] [Google Scholar]
- 5.Rader D. J., W. Cain, K. Ikewaki, G. Talley, L. A. Zech, D. Usher, and H. B. Brewer, Jr. 1994. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J. Clin. Invest. 93 2758–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieplinger H., and G. Utermann. 1999. The seventh myth of lipoprotein(a): where and how is it assembled? Curr. Opin. Lipidol. 10 275–283. [DOI] [PubMed] [Google Scholar]
- 7.Brunner C., H. G. Kraft, G. Utermann, and H. J. Muller. 1993. Cys4057 of apolipoprotein(a) is essential for lipoprotein(a) assembly. Proc. Natl. Acad. Sci. USA. 90 11643–11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trieu V. N., and W. J. McConathy. 1995. A two-step model for lipoprotein(a) formation. J. Biol. Chem. 270 15471–15474. [DOI] [PubMed] [Google Scholar]
- 9.Gabel B. R., and M. L. Koschinsky. 1998. Sequences within apolipoprotein(a) kringle IV types 6–8 bind directly to low-density lipoprotein and mediate noncovalent association of apolipoprotein(a) with apolipoprotein B-100. Biochemistry. 37 7892–7898. [DOI] [PubMed] [Google Scholar]
- 10.Becker L., P. M. Cook, T. G. Wright, and M. L. Koschinsky. 2004. Quantitative evaluation of the contribution of weak lysine-binding sites present within apolipoprotein(a) kringle IV types 6–8 to lipoprotein(a) assembly. J. Biol. Chem. 279 2679–2688. [DOI] [PubMed] [Google Scholar]
- 11.Becker L., R. S. McLeod, S. M. Marcovina, Z. Yao, and M. L. Koschinsky. 2001. Identification of a critical lysine residue in apolipoprotein B-100 that mediates noncovalent interaction with apolipoprotein(a). J. Biol. Chem. 276 36155–36162. [DOI] [PubMed] [Google Scholar]
- 12.Liu C. Y., R. Broadhurst, S. M. Marcovina, and S. P. McCormick. 2003. Mutation of lysine residues in apolipoprotein B-100 causes defective lipoprotein[a] formation. J. Lipid Res. 45 63–70. [DOI] [PubMed] [Google Scholar]
- 13.Koschinsky M. L., G. P. Cote, B. Gabel, and Y. Y. van der Hoek. 1993. Identification of the cysteine residue in apolipoprotein(a) that mediates extracellular coupling with apolipoprotein B-100. J. Biol. Chem. 268 19819–19825. [PubMed] [Google Scholar]
- 14.Callow M. J., and E. M. Rubin. 1995. Site-specific mutagenesis demonstrates that cysteine 4326 of apolipoprotein B is required for covalent linkage with apolipoprotein (a) in vivo. J. Biol. Chem. 270 23914–23917. [DOI] [PubMed] [Google Scholar]
- 15.McCormick S. P. A., J. K. Ng, S. Taylor, L. M. Flynn, R. E. Hammer, and S. G. Young. 1995. Mutagenesis of the human apolipoprotein B gene in a yeast artificial chromosome reveals the site of attachment for apolipoprotein(a). Proc. Natl. Acad. Sci. USA. 92 10147–10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker L., B. A. Webb, S. Chitayat, M. E. Nesheim, and M. L. Koschinsky. 2003. A ligand-induced conformational change in apolipoprotein(a) enhances covalent Lp(a) formation. J. Biol. Chem. 278 14074–14081. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., R. Pease, J. Bertinato, and R. W. Milne. 2000. Well-defined regions of apolipoprotein B-100 undergo conformational change during its intravascular metabolism. Arterioscler. Thromb. Vasc. Biol. 20 1301–1308. [DOI] [PubMed] [Google Scholar]
- 18.Chiesa G., H. H. Hobbs, M. L. Koschinsky, R. M. Lawn, S. D. Maika, and R. E. Hammer. 1992. Reconstitution of lipoprotein(a) by infusion of human low density lipoprotein into transgenic mice expressing human apolipoprotein(a). J. Biol. Chem. 267 24369–24374. [PubMed] [Google Scholar]
- 19.Steyrer E., S. Durovic, S. Frank, W. Giessauf, A. Burger, H. Dieplinger, R. Zechner, and G. M. Kostner. 1994. The role of lecithin: cholesterol acyltransferase for lipoprotein (a) assembly. Structural integrity of low density lipoproteins is a prerequisite for Lp(a) formation in human plasma. J. Clin. Invest. 94 2330–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinman Y., E. S. Krul, M. Burnes, W. Aronson, B. Pfleger, and G. Schonfeld. 1988. Lipolysis of LDL with phospholipase A2 alters the expression of selected apoB-100 epitopes and the interaction of LDL with cells. J. Lipid Res. 29 729–743. [PubMed] [Google Scholar]
- 21.Gieseg S. P., and H. Esterbauer. 1994. Low density lipoprotein is saturable by pro-oxidant copper. FEBS Lett. 343 188–194. [DOI] [PubMed] [Google Scholar]
- 22.Markwell M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87 206–210. [DOI] [PubMed] [Google Scholar]
- 23.New, R. R. C. 1990. Preparation of liposomes. In Liposomes: A Practical Approach. R. R. C. New, editor. Oxford University Press, Oxford, UK. 33–104.
- 24.Aggerbeck L. P., J. R. Wetterau, K. H. Weisgraber, C. S. Wu, and F. T. Lindgren. 1988. Human apolipoprotein E3 in aqueous solution. II. Properties of the amino- and carboxyl-terminal domains. J. Biol. Chem. 263 6249–6258. [PubMed] [Google Scholar]
- 25.Sharp R. J., M. A. Perugini, S. M. Marcovina, and S. P. A. McCormick. 2003. A synthetic peptide that inhibits lipoprotein(a) assembly. Arterioscler. Thromb. Vasc. Biol. 23 502–507. [DOI] [PubMed] [Google Scholar]
- 26.Pease R. J., R. W. Milne, W. K. Jessup, A. Law, P. Provost, J. C. Fruchart, R. T. Dean, Y. L. Marcel, and J. Scott. 1990. Use of bacterial expression cloning to localize the epitopes for a series of monoclonal antibodies against apolipoprotein B100. J. Biol. Chem. 265 553–568. [PubMed] [Google Scholar]
- 27.Young S. G., J. L. Witztum, D. C. Casal, L. K. Curtiss, and S. Bernstein. 1986. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis. 6 178–188. [DOI] [PubMed] [Google Scholar]
- 28.Linton M. F., R. V. Farese, G. Chiesa, D. S. Grass, P. Chin, R. E. Hammer, H. H. Hobbs, and S. G. Young. 1993. Transgenic mice expressing high plasma concentrations of human apolipoprotein B100 and lipoprotein(a). J. Clin. Invest. 92 3029–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcovina S. M., J. J. Albers, B. Gabel, M. L. Koschinsky, and V. P. Gaur. 1995. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a). Clin. Chem. 41 246–255. [PubMed] [Google Scholar]
- 30.Chauhan V., X. Wang, T. Ramsamy, R. W. Milne, and D. L. Sparks. 1998. Evidence for lipid-dependent structural changes in specific domains of apolipoprotein B100. Biochemistry. 37 3735–3742. [DOI] [PubMed] [Google Scholar]
- 31.Chen G. C., W. Liu, P. Duchateau, J. Allaart, R. L. Hamilton, C. M. Mendel, K. Lau, D. A. Hardman, P. H. Frost, M. J. Malloy, et al. 1994. Conformational differences in human apolipoprotein B-100 among subspecies of low density lipoproteins (LDL). Association of altered proteolytic accessibility with decreased receptor binding of LDL subspecies from hypertriglyceridemic subjects. J. Biol. Chem. 269 29121–29128. [PubMed] [Google Scholar]
- 32.Kostner K. M., and G. M. Kostner. 2005. Therapy of hyper-Lp(a). Handb. Exp. Pharmacol. 170 519–536. [DOI] [PubMed] [Google Scholar]
- 33.Tall A. R., and Y. Lange. 1978. Interaction of cholesterol, phospholipid and apoprotein in high density lipoprotein recombinants. Biochim. Biophys. Acta. 513 185–197. [DOI] [PubMed] [Google Scholar]
- 34.Pownall H., Q. Pao, D. Hickson, J. T. Sparrow, S. K. Kusserow, and J. B. Massey. 1981. Kinetics and mechanism of association of human plasma apolipoproteins with dimyristoylphosphatidylcholine: effect of protein structure and lipid clusters on reaction rates. Biochemistry. 20 6630–6635. [DOI] [PubMed] [Google Scholar]
- 35.Lund-Katz S., P. M. Laplaud, M. C. Phillips, and M. J. Chapman. 1998. Apolipoprotein B-100 conformation and particle surface charge in human LDL subspecies: implication for LDL receptor interaction. Biochemistry. 37 12867–12874. [DOI] [PubMed] [Google Scholar]
- 36.Segrest J. P., M. K. Jones, H. De Loof, and N. Dashti. 2001. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 42 1346–1367. [PubMed] [Google Scholar]
- 37.Frank S., S. Durovic, K. Kostner, and G. M. Kostner. 1995. Inhibitors for the in vitro assembly of Lp(a). Arterioscler. Thromb. Vasc. Biol. 15 1774–1780. [DOI] [PubMed] [Google Scholar]
- 38.Frank S., A. Hrzenjak, K. Kostner, W. Sattler, and G. M. Kostner. 1999. Effect of tranexamic acid and delta-aminovaleric acid on lipoprotein(a) metabolism in transgenic mice. Biochim. Biophys. Acta. 1438 99–110. [DOI] [PubMed] [Google Scholar]
- 39.Semple S. C., A. Chonn, and P. R. Cullis. 1996. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry. 35 2521–2525. [DOI] [PubMed] [Google Scholar]
- 40.Zakharova T. S., A. S. Ivanov, A. P. Echkalov, A. T. Beriozov, E. M. Khalilov, and A. I. Archakov. 1993. Interaction of cholesterol containing liposomes with blood serum lipoproteins. Biochem. Mol. Biol. Int. 31 315–324. [PubMed] [Google Scholar]