Abstract
Bordetella parapertussis like B. pertussis, is a causal agent of whooping cough but is not a strictly human pathogen. Because its endotoxin, a major structural component of the Gram-negative outer membrane, is an important virulence factor, we have analyzed the structure of its toxic lipid domain, in one rough and two smooth bacterial strains. Chemical analyses and mass spectra obtained before and after recently developed mild-alkali treatments revealed that the lipids A have the common bisphosphorylated β-(1→6)-linked D-glucosamine disaccharide with hydroxytetradecanoic acid in amide linkages. All three strains have two major molecular species: a tetraacyl and a pentaacyl species. The rough strain is richer in a minor hexaacyl species. Acylation at the C-2, C-3, and C-3′ positions was different from that of the B. pertussis lipid A. The C-2 position carries a secondary hexadecanoic acid, the C-3 position is free, and the C-3′ position is substituted with hydroxydecanoic acid (not at C-3 as in B. pertussis), and the rough strain hexaacyl species carries a second secondary hexadecanoic acid. Like the lipid A of B. pertussis, the hydroxytetradecanoic acid at the C-2′ position was substituted by tetradecanoic acid.
Keywords: B. parapertussis, endotoxin, lipopolysaccharide, structure
The best-studied member of the Bordetella genus has understandably been B. pertussis, the pathogen responsible for whooping cough. Its endotoxin, an immunomodulator of the bacterial surface, has been antigenically defined and the structures of the constituent lipopolysaccharides (LPS) established (1–11). B. parapertussis, the other agent of whooping cough is not a strictly human pathogen and can often be isolated from late-stage pertussis patients as well as from infected sheep (6, 9, 12, 13). Like B. bronchiseptica, it has a smooth- type LPS, and the O-chains of these two species are similar homopolymers of 1,4 linked 2,3-diacetamido-2,3-dideoxy-α-L-galactopyranosyluronic acid with different substituents on the nonreducing terminal sugar, one having an alanine, and the other an O-methyl lactic acid residue (9, 14). This epitopic difference explains the early observed serological differences between the two species (15).
The ester-linked fatty-acid of Bordetella lipids A are highly variable (5, 16). In B. bronchiseptica, this variability, is found in the nature and the localization of its fatty acids and may be related to the multiplicity of its hosts (16).
The lipid A molecules can be modified via acylation; deacylation; secondary fatty acid hydroxylation; and /or phosphate-group substitutions with aminoarabinose, galactosamine, or phosphoethanolamine (17–19). We recently reported substitution with glucosamine on the phosphates of B. bronchiseptica and B. pertussis lipids A, causing on other examples a significant modulation of host responses to infection (11, 20).
The Bordetella genus shows a remarkable ability to modify lipid A structures by late-steps in their biosynthesis. This may represent adaptations of a putative ancestor of B. bronchiseptica, an animal pathogen, to diverse niches or hosts, leading to the relatively recent appearance of the human pathogens (21).
We report here, for the first time, the detailed structures of lipids A of one rough- and two smooth-type strains of B. parapertussis, structures obtained with the critical help of a method recently established to distinguish between primary and secondary esters (20). This structure modifies an earlier one proposed in a congress poster (22) also reproduced by others (23). It is in accord with the reported genetic and biochemical characteristics of Bordetella acyl transferases, which so far have not been shown to esterify lipid A backbones with unhydroxylated fatty acids (23).
MATERIALS AND METHODS
Bacterial strains
B. parapertussis smooth-type strains NRCC4364 and 4425, and the rough-type strain 4424 were from the National Research Council (NRC) collection (Canada).
B. pertussis strain 1414 was grown at the Institut Mérieux (Lyon, France). B. parapertussis cells were grown as described (9) and the cells killed in 2% phenol before harvesting.
LPS and lipid A preparation
The LPSs were extracted by the enzyme-phenol-water method (24) and sedimented by ultracentrifugation (105,000 g, 4°C, 12 h). Lipid A was prepared by mild, detergent-facilitated hydrolysis of LPS and purified as before (25). Alternatively, lipid A was obtained by direct hydrolysis of the lyophilized bacteria (26). Briefly, 10 mg of lyophilized bacteria were suspended in 400 μl of isobutyric acid and 1 M ammonium hydroxide (5:3, v:v), heated 2 h at 100°C with stirring, cooled to 4°C, and centrifuged (as before). The supernatant was diluted with water (1:1, v:v) and lyophilized. The material obtained was then washed twice with 400 μl of methanol and centrifuged (2,000 g for 15 min). Finally, the insoluble lipid A was extracted once in a 100 to 200 μl mixture of chloroform/methanol/water (3:1.5:0.25, v:v:v).
Identification of glycose absolute configurations
Lipids A (4 mg) were hydrolyzed with 0.5 ml of 4 M HCl at 100°C for 2 h. After cooling and extraction of fatty acids with chloroform, residual solutions were brought to neutrality by repeated evaporation under reduced pressure. After N-acetylation, the residue was treated with trifluoracetic acid - R-(-)-2-butanol, peracetylated, and analyzed by gas chromatography on a BP10 capillary (Scientific Glass Engineering) column using a program 160°C (1 min) to 220°C, 5°C min−1 at 0.6 kPa (27).
Sequential liberation of ester-linked fatty acids by mild alkali treatment
Sequential liberation of ester-linked fatty acids by mild alkali treatment was used to establish the lipid A acylation patterns (20). For the first-step liberation of primary ester-linked fatty acids, lipid A (200 μg) was suspended at 1 mg/ml in 35% ammonium hydroxide and stirred for 5 h at 50°C. To liberate the secondary ester-linked fatty acids, lipid A was suspended in 41% methylamine and stirred for 5 h at 37°C. The solutions were dried under a stream of nitrogen, the residues taken up in a mixture of chloroform/methanol/water (3: 1.5: 0.25, v:v:v) followed by MALDI/MS analysis. In this case, kinetics (15 min, 30 min, 1 h, 2 h, 3 h, 4 h, 5 h) were done to follow the complete process in parallel with the B. pertussis lipid A taken as a reference.
Mass spectrometry
Two different techniques were used in this work.
Plasma desorption mass spectrometry
Plasma desorption mass spectra were obtained with a Depil TOF 21 mass spectrometer as described, and lipid A samples were prepared as before (16). Spectra were recorded in the positive and negative-ion modes.
MALDI/MS
MALDI/MS was done in the linear mode with delayed extraction using a Perseptive Voyager STR (PE Biosystem, France) time-of-flight mass spectrometer (I.B.B.M.C., Orsay, France). A suspension of lipid A in chloroform/methanol/water (3:1.5:0.25, v/v/v) (1 mg/ml) was desalted with a few grains of Dowex 50W-X8 (H+), 1 μl was deposited on the target, mixed with one μl of the matrix suspended at 10 μg/μl in the same solvent or in 0.1 M aqueous citric acid (28) and dried. Analyte ions were desorbed from the gentisic acid (2,5-dihydroxybenzoic acid) matrix with pulses from a 337 nm nitrogen laser. Spectra were obtained in the negative-ion mode at 20 kV.
Chemical analyses
Hexosamines were assayed as in (29) and phosphate, as in (30).
Fatty acids were analyzed as in (31); GC-MS was done as before using a Finnigan Mat 95S mass spectrometer (5).
Nuclear magnetic resonance spectra
(1H and 31P nuclear magnetic resonance) were obtained on a Bruker AMX-500 spectrometer using standard Bruker software as previously described (5).
RESULTS AND DISCUSSION
Colorimetric tests showed that the lipids A contain D-glucosamine (GlcN) and phosphorus in a 0.9:1 ratio (29, 30). No pyrophosphate or additional sugars were detected. Plasma desorption mass and MALDI spectra had no signals indicating the presence of phosphorylethanolamine or any other substituents than fatty acids.
Total fatty-acid compositions
Total fatty-acid compositions (31) of the three strains lipid A was performed by GC-MS after strong acid treatment, extraction, and esterification with diazomethane. Strain 4364 lipid A had 3-hydroxydecanoic acid (C10-OH), tetradecanoic acid (C14), 3-hydroxytetradecanoic acid (C14-OH), and hexadecanoic acid (C16) in the proportions 0.5:1:2:0.9. Taking into account the heterogeneity of this lipid A preparation and the tendency toward underestimation of the short-chain fatty acids (31) these proportions translated to 1 unit of C10-OH, 1 unit of C14, 2 units of C14-OH, and 1 unit of C16, for a major molecular species (see later discussion). Strain 4425 gave similar results, but the rough-strain 4424 showed a larger amount of C16 fatty acid with the proportions: 0.7:1: 2:1.3.
As the three lipids A gave identical results in the different experiments apart from the extra C16, only one experiment of each is along this work.
Nuclear magnetic resonance analysis of the O-deacylated rough-type LPS indicated a β-1′,6-D-glucosamine disaccharide backbone substituted at C-4′ and C-1 by phosphomonoester groups and N-acylated at C-2′ and C-2. Characteristic values were similar to those obtained with B. pertussis lipid A (5). Data not shown.
Molecular heterogeneity and distribution of the fatty acids between the two D-glucosamine residues
A combination of the positive- and negative- ion mode plasma desorption mass spectrometry fragmentation patterns was used (32–34). This method although rarely employed nowadays gives in the high mass region molecular-ion peaks that are due to the natural heterogeneity of lipid A preparations. Peak intensities are proportional to the relative abundances of the molecular species.
In the negative-ion mode the X,Y,Z series of peaks, corresponding to well-defined fragments associated with GlcN-I, is usually observed while in the positive-ion mode, B1 fragments are associated with GlN-II, and both modes give the fatty acid distribution between the two GlcN residues (35).
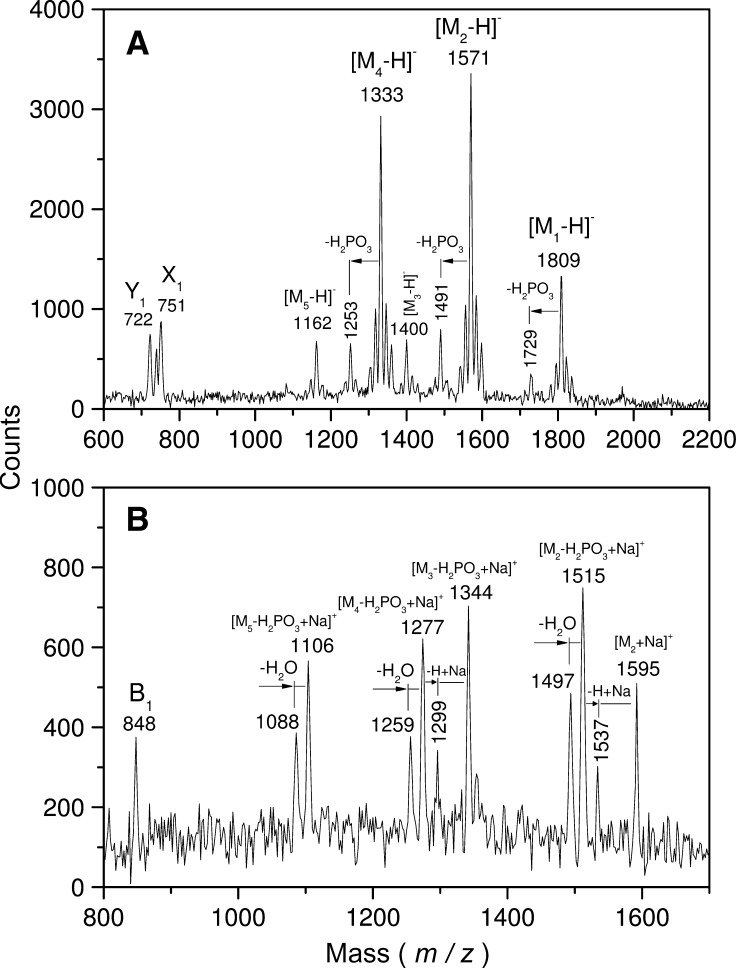
The negative-ion spectrum (Fig. 1A) of strain 4424 lipid A showed two main molecular-ion [M−H]− signals at m/z 1,333 and 1,571. Compositions of the corresponding molecular species attributed on the basis of the overall chemical composition given by colorimetric methods as well as GC-MS gave 2 GlcN, 2 phosphates, 2 C14-OH, 1 C14, 1 C10-OH with and without 1 C16 (Mr−H2O = 161.16, 80, 226.36, 210.36, 170.25, and 238.4, respectively). Smaller peaks at m/z 1,162 (1,333−C10-OH), 1,400 (1,571−C10-OH) 1,491 (1,571−phosphate), and 1,809 (1571+C16) were also found. Consistent with GC-MS data, the rough-type lipid A peak at m/z 1,809 was higher than that in the corresponding spectrum of each smooth type lipid A. It is thus richer in a molecular species having two residues of C16.
Fig. 1.
Plasma desorption spectra of B. parapertussis strain NRCC 4424 lipid A. A: Negative-ion mode; B: positive-ion mode.
Fragmentation is annotated according to the nomenclature presented in Ref. 35. In the lower field, there were peaks at m/z 751 (X1) and 722 (Y1) corresponding to the O-GlcN I moiety with and without CHO separated from GlcN II by double breakage (C5′-O′, C1′-C2′). A composition of 1 GlcN, 1 phosphate, 1 C14-OH, and 1 C16 was attributed to it. Lower in the spectrum, a peak at m/z 512 was interpreted to be m/z 751 minus C16, a confirmation of the incomplete secondary acylation at C-2. The absence of other peaks indicated the absence of substitution at C-3 and C-4.
Positive-ion mass spectrum of the same lipid A preparation is presented in Fig. 1B. A peak at m/z 848 corresponded to B1 fragments associated with GlcN-II and containing 1 GlcN, 1 phosphate, 1 C14-OH, 1 C14, and 1 C10-OH. In the higher mass region, multiple peaks were observed resulting from the loss of phosphate groups (H2PO3 or H3PO4) and attachments of one or two atoms of sodium by the different lipid A molecules present in the mixture. These data taken together allows to define the fatty acid distribution for the major molecular species as follows:
m/z 1571: GlcN-I, C14-OH, C16; GlcN-II, C14-OH, C14, C10-OH
m/z 1333: GlcN-I, C14-OH; GlcN-II, C14-OH, C14, C10-OH
The C14 would then have to be on the C14-OH at C-2′ as in B. pertussis, and the second hexadecanoic acid, on the C10-OH, which would be at C-3′. Interpretation for other peaks is given in the spectrum (Fig. 1A, B).
Liberation of primary ester-linked fatty acids
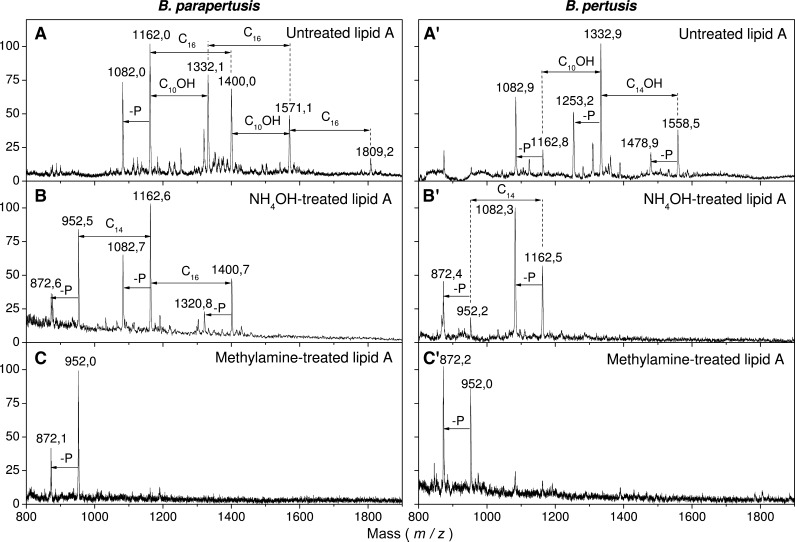
Liberation of primary ester-linked fatty acids was also applied to B.parapertussis and B.pertussis lipids A isolated directly from bacteria (Fig. 2A, A′) (26). The kinetics of B. pertussis lipid A deacylation showed a total release of the fatty acid at C-3 (C10-OH) in the first 15 min of treatment (not shown). The first 15 min of treatment applied to B. parapertussis lipids A did not change the general aspect of the spectrum, which could be explained by a free position at C-3.
Fig. 2.
Matrix-assisted laser-desorption/ionisation mass spectrometry of: B. parapertussis lipid A NRCC4323 (A); lipid A obtained after a first primary deO-acylation step (at 50°C for 5 h with ammonium hydroxide) (B); lipid A obtained after complete deO-acylation (at 37°C for 5 h with methyl-amine) (C). A′, B′, C′: same treatments for B. pertussis strain1414 lipid A.
In the conditions used to liberate esterified substituents at the C-3 and C-3′ positions (NH4OH 5 h at 50°C), (Fig. 2B, B′), C10-OH, C10-O-C16 were completely released together with a small amount of C16 in B. parapertussis lipids A. After a 5 h treatment, the C14 and a C16 fatty acid were still present as indicated by peaks at m/z 1,162 and 1,400. It was concluded that this C16 was present in secondary acylation at C-2 as for the C14 at C-2′, which was confirmed by the fragmentation data. The appearance of two peaks, one at m/z 1,162 (952+ C14) and the other at +C16 demonstrated that the C14 substituted all the C14-OH at C2′, whereas the C16 only substituted some of the C14-OH at C-2. In the same conditions C10-OH and C14-OH were liberated from B. pertussis lipid A.
In the conditions used to liberate secondary esterified substituents at C-2 and C-2′ (methylamine 5 h at 37°C) the C14 and C16 fatty acids were liberated giving rise to a single molecular species at m/z 952 and some of its dephosphorylated form (Fig. 2C, C′).
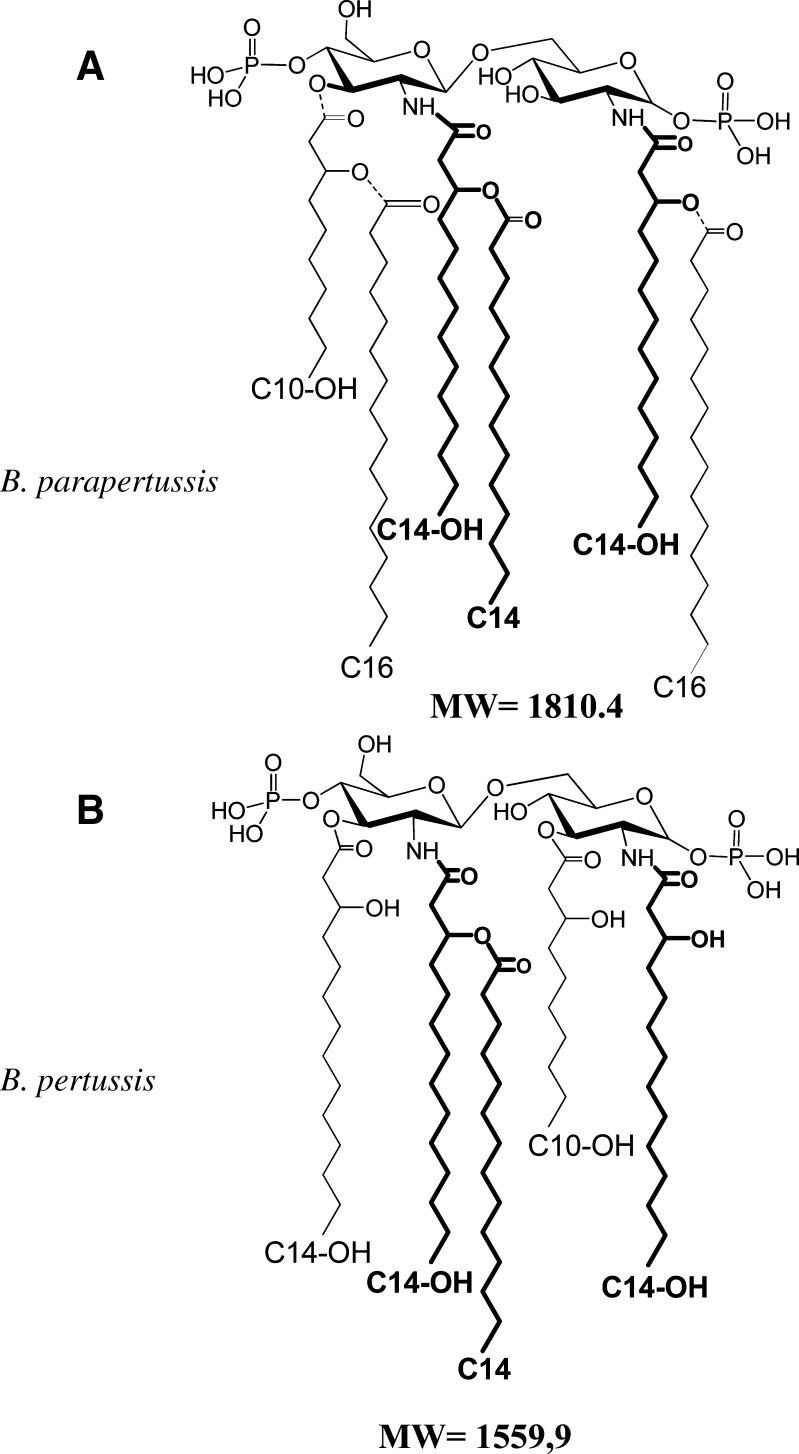
The ensemble of these results defined the structures presented in Fig. 3 with the higher mass molecular species of each lipid A, MW 1810 for B. parapertussis (Fig. 3A) and the basic structure found in B. pertussis (Fig. 3B). It should be noted that the peak at m/z 1,333, although identical and corresponding to the same overall composition as that seen in B. pertussis lipid A, differs in that the hydroxydecanoic acid at C-3 in B. pertussis lipid A, is at C-3′ in B. parapertussis (Fig. 3A). This could indicate a different biosynthetic pathway and illustrates the usefulness of spectra taken in both tension modes and the use of fragmentation patterns for detecting such differences.
Fig. 3.
Structures corresponding to the higher mass molecular species present in the negative-ion spectra of B. parapertussis (A) and B. pertussis (B). Doted lines indicate incomplete fatty acid substitution. Bold lines represent common fatty acids in both lipid A structures.
The presence of C16 fatty acids in lipid A structures was always associated with late biosynthetic steps, these fatty acids originating from the outer membrane phospholipids (36). In the present case, an ester-linked C10-OH and a C14-OH amide-linked fatty acid would both be substituted in secondary linkage.
C14-O-C16 substitutions on C-2 of the reducing GlcN are particularly common in Salmonella typhimurium, and first thought to be a characteristic of Salmonellae. In Bordetellae, this PagP modification was first detected in secondary acylation at C-3′ on GlcN II (37). In B. bronchiseptica, pagP expression is Bvg-dependent and impacts colonization in mice and serum resistance in vitro (37). With B. parapertussis we have the expression of both secondary acylations due to PagP. No C16 was ever found at C-2 of the lipid A of any Bordetella species examined by our group.
The lipids A of some strains of B. bronchiseptica, B. hinzii, and B. trematum are substituted with hexadecanoic acid in secondary linkage at C3′ (M. Caroff, unpublished observations). Recently, the occurrence of a novel secondary acyl chain in B. pertussis LPS and its negative effect on infection of human macrophages has been reported (19).
At this stage, the situation could be tentatively explained for the two human pathogens by the following scenario: the two species could follow a common biosynthetic pathway for their lipids A up to the formation of a more common symmetric GlcN disaccharide bearing a C10-OH at both C-3 and C-3′. Then, this lipid could undergo an enzymatic deO-acylation, one at C-3 for B. parapertussis and one at C-3′ for B. pertussis, leading to the two different structures. DeO-acylation would then have to be asymmetric, but the presence of specific deacylases has been reported in other genera (38, 39).
The lipids A of the three strains of B. parapertussis differed only in degree of acylation as seems to be the case with various species of some genera such as Haemophilus and Neisseria (33). Some investigators consider the 3 Bordetella species that infect human beings as variants of a single species. B. pertussis and B. parapertussis still maintain the short C10-OH in primary acylation. B. bronchiseptica displays a high degree of heterogeneity and variability. In all mass spectra, each major peak representing a molecular species is surrounded by smaller peaks corresponding to molecular species having fatty acids differing by 2 carbons or by an oxygen atom showing the capacity of the bacteria to shift to other structures and the relaxed enzyme specificity already described (23). The relation of this flexibility and the relatively recent human niches together with the potential of late-stage alterations could explain the adaptation of earlier Bordetella pathogens to humans and the reemergence of resistant strains. With the present strong interest in the biosynthesis of Bordetella LPS by various groups, an understanding of this phenomenon may soon be achieved.
Abbreviations
C12, dodecanoic acid
C14, tetradecanoic acid
C16, hexadecanoic acid
C10-OH, hydroxydecanoic acid
C12-OH, hydroxydodecanoic acid
C14-OH, hydroxytetradecanoic acid
GlcN, D-glucosamine
LPS, lipopolysaccharide
Published, JLR Papers in Press, November 17, 2008.
References
- 1.Ayme G., M. Caroff, R. Chaby, N. Haeffner-Cavaillon, A. Le Dur, M. Moreau, M. Muset, M. C. Mynard, M. Roumiantzeff, D. Schulz, et al. 1980. Biological activities of fragments derived from Bordetella pertussis endotoxin: isolation of a nontoxic, Shwartzman-negative lipid A possessing high adjuvant properties. Infect. Immun. 27 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caroff M., J. M. Cavaillon, C. Fitting, and N. Haeffner-Cavaillon. 1986. Inability of pyrogenic, purified Bordetella pertussis lipid A to induce interleukin-1 release by human monocytes. Infect. Immun. 54 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caroff M., R. Chaby, D. Karibian, J. Perry, C. Deprun, and L. Szabo. 1990. Variations in the carbohydrate regions of Bordetella pertussis lipopolysaccharides: electrophoretic, serological, and structural features. J. Bacteriol. 172 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amano K., K. Fukushi, and M. Watanabe. 1990. Biochemical and immunological comparison of lipopolysaccharides from Bordetella species. J. Gen. Microbiol. 136 481–487. [DOI] [PubMed] [Google Scholar]
- 5.Caroff M., C. Deprun, J. C. Richards, and D. Karibian. 1994. Structural characterization of the lipid A of Bordetella pertussis 1414 endotoxin. J. Bacteriol. 176 5156–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Blay K., M. Caroff, J. C. Richards, M. B. Perry, and R. Chaby. 1994. Specific and cross-reacting monoclonal antibodies to Bordetella parapertussis and Bordetella bronchiseptica lipopolysaccharides. Microbiology. 140 2459–2465. [DOI] [PubMed] [Google Scholar]
- 7.Lebbar S., M. Caroff, L. Szabo, C. Merienne, and L. Szilogyi. 1994. Structure of a hexasaccharide proximal to the hydrophobic region of lipopolysaccharides present in Bordetella pertussis endotoxin preparations. Carbohydr. Res. 259 257–275. [DOI] [PubMed] [Google Scholar]
- 8.Le Blay K., M. Caroff, F. Blanchard, M. B. Perry, and R. Chaby. 1996. Epitopes of Bordetella pertussis lipopolysaccharides as potential markers for typing of isolates with monoclonal antibodies. Microbiology. 142 971–978. [DOI] [PubMed] [Google Scholar]
- 9.Di Fabio J. L., M. Caroff, D. Karibian, J. C. Richards, and M. B. Perry. 1992. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol. Lett. 76 275–281. [DOI] [PubMed] [Google Scholar]
- 10.Caroff M., J. Brisson, A. Martin, and D. Karibian. 2000. Structure of the Bordetella pertussis 1414 endotoxin. FEBS Lett. 477 8–14. [DOI] [PubMed] [Google Scholar]
- 11.Marr N., A. Tirsoaga, D. Blanot, R. Fernandez, and M. Caroff. 2008. Glucosamine found as a substituent of both phosphate groups in Bordetella lipid A backbones: role of a BvgAS-activated ArnT ortholog. J. Bacteriol. 190 4281–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter J. F., K. Connor, and W. Donachie. 1994. Isolation and characterization of Bordetella parapertussis-like bacteria from ovine lungs. Microbiology. 140 255–261. [DOI] [PubMed] [Google Scholar]
- 13.Kawai H., T. Aoyama, Y. Murase, C. Tamura, and A. Imaizumi. 1996. A causal relationship between Bordetella pertussis and Bordetella parapertussis infections. Scand. J. Infect. Dis. 28 377–381. [DOI] [PubMed] [Google Scholar]
- 14.Kubler-Kielb J., E. Vinogradov, G. Ben-Menachem, V. Pozsgay, J. B. Robbins, and R. Schneerson. 2008. Saccharide/protein conjugate vaccines for Bordetella species: preparation of saccharide, development of new conjugation procedures, and physico-chemical and immunological characterization of the conjugates. Vaccine. 26 3587–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Blay K., P. Gueirard, N. Guiso, and R. Chaby. 1997. Antigenic polymorphism of the lipopolysaccharides from human and animal isolates of Bordetella bronchiseptica. Microbiology. 143 1433–1441. [DOI] [PubMed] [Google Scholar]
- 16.Zarrouk H., D. Karibian, S. Bodie, M. B. Perry, J. C. Richards, and M. Caroff. 1997. Structural characterization of the lipids A of three Bordetella bronchiseptica strains: variability of fatty acid substitution. J. Bacteriol. 179 3756–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller S. I., R. K. Ernst, and M. W. Bader. 2005. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 3 36–46. [DOI] [PubMed] [Google Scholar]
- 18.Dixon D. R., and R. P. Darveau. 2005. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J. Dent. Res. 84 584–595. [DOI] [PubMed] [Google Scholar]
- 19.Geurtsen J., E. Angevaare, M. Janssen, H. J. Hamstra, J. ten Hove, A. de Haan, B. Kuipers, J. Tommassen, and P. van der Ley. 2007. A novel secondary acyl chain in the lipopolysaccharide of Bordetella pertussis required for efficient infection of human macrophages. J. Biol. Chem. 282 37875–37884. [DOI] [PubMed] [Google Scholar]
- 20.Tirsoaga A., A. El Hamidi, M. B. Perry, M. Caroff, and A. Novikov. 2007. A rapid, small-scale procedure for the structural characterization of lipid A applied to Citrobacter and Bordetella strains: discovery of a new structural element. J. Lipid Res. 48 2419–2427. [DOI] [PubMed] [Google Scholar]
- 21.Parkhill J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, et al. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35 32–40. [DOI] [PubMed] [Google Scholar]
- 22.Caroff, M., D. Karibian, H. Zarrouk, C. Deprun, J. C. Richards, and M. B. Perry. 1994. Comparison of endotoxin structures in 3 Bordetella species. In 17th International Carbohydrate Symposium. Poster A1.56.
- 23.Sweet C. R., A. Preston, E. Toland, S. M. Ramirez, R. J. Cotter, D. J. Maskell, and C. R. Raetz. 2002. Relaxed acyl chain specificity of Bordetella UDP-N-acetylglucosamine acyltransferases. J. Biol. Chem. 277 18281–18290. [DOI] [PubMed] [Google Scholar]
- 24.Johnson K. G., and M. B. Perry. 1976. Improved techniques for the preparation of bacterial lipopolysaccharides. Can. J. Microbiol. 22 29–34. [DOI] [PubMed] [Google Scholar]
- 25.Caroff M., A. Tacken, and L. Szabo. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175 273–282. [DOI] [PubMed] [Google Scholar]
- 26.El Hamidi A., A. Tirsoaga, A. Novikov, A. Hussein, and M. Caroff. 2005. Microextraction of bacterial lipid A: easy and rapid method for mass spectrometric characterization. J. Lipid Res. 46 1773–1778. [DOI] [PubMed] [Google Scholar]
- 27.Gerwig G. J., J. P. Kamerling, and J. F. Vliegenthart. 1979. Determination of the absolute configuration of mono-saccharides in complex carbohydrates by capillary G.L.C. Carbohydr. Res. 77 10–17. [DOI] [PubMed] [Google Scholar]
- 28.Therisod H., V. Labas, and M. Caroff. 2001. Direct microextraction and analysis of rough-type lipopolysaccharides by combined thin-layer chromatography and MALDI mass spectrometry. Anal. Chem. 73 3804–3807. [DOI] [PubMed] [Google Scholar]
- 29.Rondle C. J., and W. T. Morgan. 1955. The determination of glucosamine and galactosamine. Biochem. J. 61 586–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen P. S., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorus. Anal. Chem. 28 1756–1758. [Google Scholar]
- 31.Wollenweber H.-W., and E. T. Rietschel. 1990. Analysis of lipopolysaccharide (lipid A) fatty acids. J. Microbiol. Methods. 11 195–211. [Google Scholar]
- 32.Costello C. E., and J. E. Vath. 1990. Tandem mass spectrometry of glycolipids. Methods Enzymol. 193 738–768. [DOI] [PubMed] [Google Scholar]
- 33.Karibian D., C. Deprun, L. Szabo, Y. Le Beyec, and M. Caroff. 1991. 252Cf plasma desorption mass spectrometry applied to the analysis of endotoxin lipid A preparations. Int. J. Mass Spectrom. Ion Process. 111 273–286. [Google Scholar]
- 34.Aussel L., H. Therisod, D. Karibian, M. B. Perry, M. Bruneteau, and M. Caroff. 2000. Novel variation of lipid A structures in strains of different Yersinia species. FEBS Lett. 465 87–92. [DOI] [PubMed] [Google Scholar]
- 35.Karibian D., A. Brunelle, L. Aussel, and M. Caroff. 1999. 252Cf plasma desorption mass spectrometry of unmodified Lipid A: fragmentation patterns and localization of fatty acids. Rapid Commun. Mass Spectrom. 13 2252–2259. [DOI] [PubMed] [Google Scholar]
- 36.Bishop R. E., H. S. Gibbons, T. Guina, M. S. Trent, S. I. Miller, and C. R. Raetz. 2000. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 19 5071–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston A., E. Maxim, E. Toland, E. J. Pishko, E. T. Harvill, M. Caroff, and D. J. Maskell. 2003. Bordetella bronchiseptica PagP is a Bvg-regulated lipid A palmitoyl transferase that is required for persistent colonization of the mouse respiratory tract. Mol. Microbiol. 48 725–736. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds C. M., A. A. Ribeiro, S. C. McGrath, R. J. Cotter, C. R. Raetz, and M. S. Trent. 2006. An outer membrane enzyme encoded by Salmonella typhimurium lpxR that removes the 3′-acyloxyacyl moiety of lipid A. J. Biol. Chem. 281 21974–21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geurtsen J., L. Steeghs, H. J. Hamstra, J. Ten Hove, A. de Haan, B. Kuipers, J. Tommassen, and P. van der Ley. 2006. Expression of the lipopolysaccharide-modifying enzymes PagP and PagL modulates the endotoxic activity of Bordetella pertussis. Infect. Immun. 74 5574–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]