Abstract
Apolipoprotein AV (apoAV) overexpression causes a decrease in plasma triglyceride (TG) levels, while deficiency of apoAV causes hypertriglyceridemia in both men and mice. However, contrary to what would be expected, plasma apoAV and TG levels in humans are positively correlated. To address this apparent paradox, we determined plasma apoAV levels in various mouse models with median TG levels ranging from 30 mg/dl in wild-type mice to 2089 mg/dl in glycosylphosphatidylinositol-anchored HDL binding protein 1-deficient mice. The data show that apoAV and TG levels are positively correlated in mice (r = +0.798, P < 0.001). In addition, we show that LPL gene transfer caused a simultaneous decrease in TG and apoAV in LPL-deficient mice. The combined data suggest that apoAV levels follow TG levels due to an intimate link between the apoAV molecule and TG-rich lipoproteins, comprising both secretion and removal of these lipoproteins. Taken together, the data suggest that higher plasma apoAV levels reflect an increased demand for plasma TG hydrolysis under normal physiological conditions.
Keywords: lipoproteins, lipolysis, VLDL, chylomicrons
Since its discovery in 2001, apolipoprotein AV (apoAV) has been postulated as a key regulator of plasma triglyceride (TG) levels (1). Relevance for TG homeostasis was initially demonstrated using genetically engineered mice: Overexpression of human apoAV decreased plasma TG by 64%, while apoa5-deficient mice had 4-fold increased plasma TG levels (1). In humans, it was furthermore shown that variation at the APOA5 gene locus was associated with TG levels (2–4), while complete apoAV deficiency was associated with severe hypertriglyceridemia (5, 6). Combined, these data suggested that plasma levels of apoAV are in general inversely correlated with TG levels. However, we and others subsequently showed that plasma apoAV and TG levels are, unexpectedly, positively correlated in patients with type 2 diabetes and in the general population (7–11). Considering the suggested negative correlation between plasma apoAV and TG in mice, based on the overexpression models, these subsequent observations in humans have led several investigators to suggest an inherent difference between humans and mice (10–12). This despite the fact that the phenotypic presentation of complete apoAV deficiency in mice and humans is similar (1, 5). Moreover, in mice with endotoxemia (13) or in rats fed PUFAs (14), semiquantification of apoAV levels by immunoblotting also suggested a positive correlation with TG levels. In addition, Nelbach et al. (15) recently showed a positive correlation between plasma apoAV and TG concentrations in mice overexpressing human apoAV. In this study, we further addressed this hypothesis by determining the relationship between plasma apoAV and TG levels in wild-type mice and various genetically engineered mouse models that are characterized by a wide range of elevated levels of plasma triglycerides.
METHODS
Mouse models
To determine the association between plasma apoAV and TG levels in mice, we collected plasma samples from different mouse models on a C57BL/6 background with variable plasma TG levels. The etiology of increased TG levels in these mice and the collaborators who provided plasma samples are given below. Human APOCI-transgenic-APOE3Leiden mice develop moderately elevated plasma triglyceride levels due to an inhibition of LPL activity [n = 16, courtesy of Dr. P. Rensen, Leiden University, Leiden, the Netherlands (16)]. Glycosylphosphatidylinositol-anchored HDL binding protein-deficient (Gpihbp1−/−) mice are severely hypertriglyceridemic because the lack of GPIHBP1 prevents binding of LPL to the vascular endothelium, resulting in an inability to lipolyse triglycerides in apoB48- and apoB100-containing lipoproteins [n = 26, courtesy of Dr. S. Young and M. Weinstein, University of California, Los Angeles, CA (17)]. Both murine apoAII-transgenic (n = 60) and murine apoAII-transgenic-apoE-deficient mice (n = 38) develop moderately elevated plasma TG levels due to both an overproduction of VLDL by the liver as well as an impaired LPL-mediated TG hydrolysis [both strains are courtesy of Dr. L. Castellani, University of California, Los Angeles, CA (18)]. Lpl−/− mice develop severe hypertriglyceridemia due to the inability to hydrolyze plasma triglycerides (19). Plasma samples of heterozygous and homozygous lpl−/− mice (both n = 5) were provided by Dr. C. Ross [University of British Columbia, Vancouver, Canada (19)]. To determine whether an induced change in TG levels in mice affects apoAV levels, we used plasma samples of lpl−/− mice that were injected intramuscularly with adeno-associated virus encoding the natural variant LPL447×, as previously described (19). Wild-type mice (n = 66), housed in our institute, were obtained from Harlan (Horst, The Netherlands). Fasting (4–12 h) plasma samples of all mice indicated above were prepared and immediately frozen at −80°C at the respective institutions. The samples were sent on dry ice to the laboratory in Amsterdam where they were stored at −80°C until use for measurements.
Plasma TG and apoAV analyses
Plasma samples were analyzed for TG using a standard enzymatic assay (Roche, Basel, Switzerland). Murine apoAV levels were determined using a newly developed ELISA. A rabbit-anti-rat apoAV polyclonal antibody (20), with strong cross-reactivity to murine apoAV, was used both as capture and biotinylated secondary antibody. Purified recombinant murine apoAV was used to generate standard curves. Plates (Maxisorb, Nunc, Thermo Fisher Scientific, Roskilde, Denmark) were coated overnight with the capture antibody (100 μl, 5 μg/ml in 0.1 M carbonate buffer, pH 9.6) at 4°C. After washing with PBX (PBS containing 0.1% Triton X-100), wells were blocked for 1 h at room temperature with PBXC (PBX containing 1% casein; Hammerstan grade, Merck, Darmstadt, Germany). After extensive washing, wells were incubated for 2 h with 100 μl of standard or sample (1:10) all diluted in PBXC. Next, wells were washed and incubated with 100 μl of the biotinylated secondary antibody (2 μg/ml in PBXC) for 1 h. Following washing, wells were incubated with streptavidin-horseradish peroxidase (1:5,000 in PBXC; DAKO, Glostrup, Denmark) for 1 h. After extensive washing, the plate was incubated with o-phenylenediamine dihydrochloride substrate (Sigma, St. Louis, MO). The color reaction was stopped after exactly 10 min with 2 M sulfuric acid, and absorbance was read at 490 nm (Easia reader, Medgenix Diagnostics, Springfield, MO). The inter-assay variation coefficient was 3.7 ± 1.6%.
Statistics
Statistics were performed using SPSS (version 16.0; Chicago, IL). Concentrations are expressed as mean ± SD. Plasma TG levels show a skewed distribution; therefore, logarithmically transformed values were used. Correlations between plasma apoAV levels and TG levels were calculated using Pearson correlation. Comparisons between pre- and posttreatment TG values of Lpl−/− mice were performed using the Student's t-test. P values < 0.05 were considered significant.
RESULTS
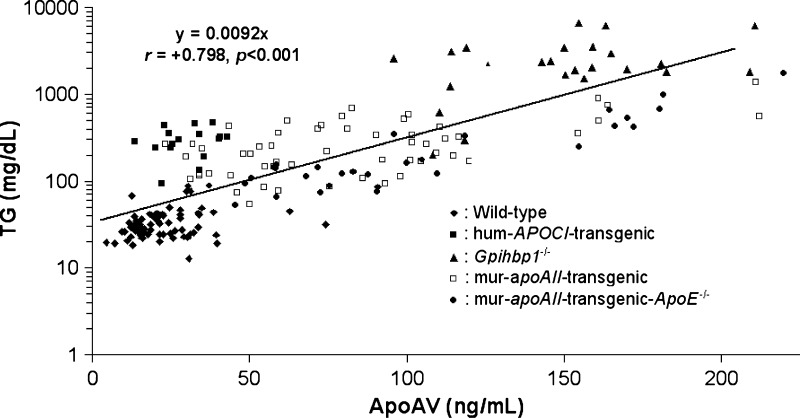
To determine the association between plasma apoAV and TG levels in mice, we tested plasma samples from mouse models with median TG levels ranging from 30 mg/dl (range 13–88) in wild-type mice to 2089 mg/dl (range 200–6677) in gpihbp1−/− mice (Table 1). Figure 1 shows that plasma apoAV and TG are positively correlated in mice (r = +0.798; P < 0.001) over the entire TG range. Table 1 shows that this positive correlation was observed in each of the following subgroups: wild-type mice (r = +0.368; P < 0.01), gpihbp1−/− mice (r = +0.455; P < 0.001), murine-apoAII-transgenic mice (r = +0.596; P < 0.001), and murine-apoAII-transgenic-apoE−/− mice (r = +0.882; P < 0.001). This correlation did not reach statistical significance for the human APOCI-transgenic mice (r = +0.2; P = 0.45), most likely due to the relative low number of animals in this group. Thus, irrespective of the molecular cause of hypertriglyceridemia, apoAV plasma levels are positively correlated with plasma TG in mice.
TABLE 1.
Plasma TG and apoAV levels in the studied mouse models and their correlations
| Mouse Model | TG (mg/dl)a | ApoAV (ng/ml)b | Pearson Correlation (r) | Significance (P) |
|---|---|---|---|---|
| Wild-type (n = 66) | 30 (13; 88) | 24 ± 14 | +0.368 | <0.010 |
| Human-APOCI-transgenic (n = 16) | 297 (94; 464) | 30 ± 9 | +0.200 | =0.450 |
| Gpihbp1−/− (n = 26) | 2089 (200; 6677) | 147 ± 30 | +0.455 | <0.010 |
| Murine-apoAII-transgenic (n = 60) | 258 (54; 1360) | 87 ± 43 | +0.596 | <0.001 |
| Murine-apoAII-transgenic-apoE−/− (n = 38) | 182 (53; 1739) | 111 ± 47 | +0.882 | <0.001 |
Data are presented as median (minimum; maximum).
Data are represented as mean ± SD.
Fig. 1.
Plasma apoAV and TG levels are positively correlated in mice. Correlation between log-transformed plasma TG and apoAV levels in wild-type (n = 66), human APOCI-transgenic (n = 16), Gpihbp1−/− (n = 26), murine apoAII-transgenic (n = 60), and murine apoAII-transgenic-apoE−/− mice (n = 38) (all on a C57Bl/6 background). Correlations within each group are presented in Table 1.
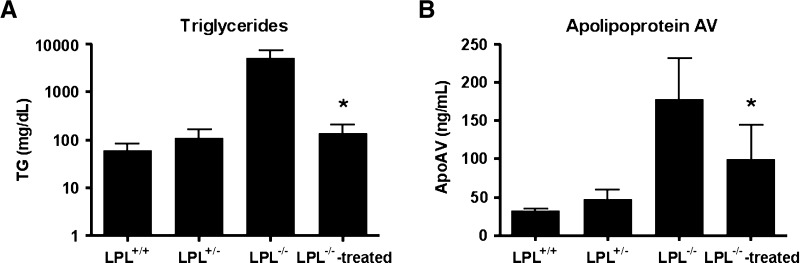
To study apoAV in a context of changing TG levels, we assessed plasma apoAV levels in lpl−/− mice before and after LPL gene transfer (19). We observed that apoAV was elevated in lpl+/− mice (TG of 110 ± 52 mg/dl) and even more so in lpl−/− mice (TG levels of 5033 ± 2141 mg/dl), confirming our finding in the other mouse models (Fig. 2). LPL gene transfer in lpl−/− mice led to simultaneous significant reductions of both TG and apoAV concentrations (Fig. 2), showing that induction of TG lowering is closely followed by a decrease of apoAV levels.
Fig. 2.
LPL gene transfer simultaneously reduces TG and apoAV levels in Lpl-deficient mice. Plasma TG (A) and apoAV (B) levels in lpl+/+, lpl+/−, lpl−/−-untreated, and adeno-associated virus-LPL-treated lpl−/− mice 4 weeks after treatment (19). * Significantly different (P < 0.05) compared with lpl−/− mice that were not treated with LPL gene transfer.
DISCUSSION
This study provides unequivocal evidence for a positive correlation between plasma apoAV and TG in mice, underlining that humans and mice are not different in this regard as has been suggested by other investigators (10–12). The current data also show that measuring apoAV plasma levels does not provide direct biological insight into the function of apoAV in vivo. It has been shown that apoAV functions as a regulator for LPL to improve TG hydrolysis, at least in vitro (21, 22), and that apoAV may be involved in remnant uptake (23, 24), both suggestive of a role for apoAV in regulating TG levels. However, our current data suggest that apoAV plasma levels change according to changes in plasma TG levels.
The current data show that apoAV levels are positively correlated with TG levels irrespective of the different genetic causes of hypertriglyceridemia that were studied. This correlation was present in mouse models with primary defects in LPL-mediated TG hydrolysis: human APOCI-transgenic mice in which overexpression of human apoCI inhibits LPL-mediated TG hydrolysis (16), gpihbp1−/− mice that lack the ability to bind LPL in the capillaries of the heart, skeletal muscle and adipose tissue resulting in the accumulation of chylomicrons and VLDL (17), and, finally, lpl−/− mice that cannot lipolyze plasma triglycerides in the absence of LPL. In addition, the positive correlation was also observed in animals with increased hepatic VLDL-TG secretion in addition to a decreased LPL-mediated TG hydrolysis. Specifically, the two murine apoAII-transgenic strains have increased hepatic VLDL-TG secretion due to increased hepatic lipogenesis and a reduced LPL-mediated TG hydrolysis because the presence of apoAII makes VLDL a poorer substrate for hydrolysis (18). A closer look into our data furthermore revealed that apoAV plasma levels vary strongly, especially relative to the observed TG levels, in the different strains of mice. Specifically and relative to TG levels, apoAV levels are only moderately elevated in the gpihbp1−/− and lpl−/− models, which present with the highest TG levels. On the other hand, apoAV levels are more strongly increased in the APOAII-transgenic lines that present with less severe hypertriglyceridemia. It thus appears that plasma apoAV levels are most intimately linked to VLDL secretion and that the moderately elevated plasma apoAV levels in models with primary defects in LPL hydrolysis reflect a delayed catabolism of chylomicrons and VLDL particles. In line with this hypothesis, targeted reduction of plasma TG through LPL gene therapy, i.e., increasing TG hydrolysis in lpl−/− mice, caused a concomitant decrease in plasma apoAV levels This finding indicates that plasma apoAV levels are thus also related to TG removal, as this effect may be seen in the context of an apparently unchanged VLDL-TG secretion in these animals (25). It is difficult to speculate on the cause for this phenomenon; however, we wish to refer to the idea that apoAV-containing lipoproteins are rapidly removed from the circulation after LPL-mediated lipolysis that is induced upon binding to GPIHBP1 (17). Nilsson et al. (26) recently showed that apoAV binds to and is internalized via peripheral receptors SorLA/LR11 and sortilin. It is thus conceivable that upon increased TG hydrolysis after LPL gene therapy, apoAV internalization is also increased, offering a possible explanation for the decrease of plasma apoAV in our experiment.
Taken together, it appears that the positive correlation of plasma apoAV with plasma TG levels is the result of an intimate link between the apoAV molecule and TG-rich lipoproteins that comprises both secretion and removal of these lipoproteins. Current lines of evidence suggest that apoAV is only synthesized in the liver, from which it is secreted into the circulation, a process that seems to be directly associated with the secretion of TG-rich VLDL. Upon TG hydrolysis in VLDL in the plasma compartment, apoAV is redistributed over all TG-rich lipoproteins, i.e., VLDL and chylomicrons, as well as HDL (27) and, in mice, possibly also LDL (15). HDL is thought to serve as a plasma reservoir for apoAV as previously described for apoCs (28, 29) and apoAII (18). In the postprandial state, this apoAV can transfer to TG-rich lipoproteins to again facilitate TG hydrolysis. Upon adequate TG hydrolysis and effective removal of remnant lipoproteins from the circulation, apoAV is also likely to be internalized and routed for degradation (26), putatively after being reused as previously suggested (30).
In this light, we propose that increased plasma apoAV levels may reflect an increased demand for plasma TG hydrolysis. However, it does not explain the observation that mice overexpressing human apoAV have very low plasma TG levels. In the latter situation, however, the physiological regulation of plasma apoAV levels is overruled, and under these conditions apoAV emerges as a primary regulator of plasma TG levels due to its intrinsic TG-lowering capacity (in the presence of active LPL). On the other hand, a recent study also showed a positive relationship between plasma apoAV and TG in mice that overexpress the human APOA5 gene, yet lack the murine apoa5 gene (15). However, this correlation was observed with plasma human apoAV levels 50- to 100-fold higher compared with normal human plasma apoAV concentrations. It will be interesting to see if a similar correlation will be found in the context of lower apoAV expression levels. On the other hand, previous murine studies suggesting a negative correlation between plasma apoAV and TG were all performed under conditions of human apoAV overexpression in the presence of the murine apoAV protein (1, 21). However, whether this difference contributes to the observed discrepancies between the murine overexpression studies is unclear.
In conclusion, we show that plasma TG and apoAV levels in mice are positively correlated, as previously observed in humans, due to an intimate link between apoAV and TG-rich lipoproteins that comprises both secretion and removal pathways of these lipoproteins. The current data suggest that apoAV levels follow TG levels, leading to the hypothesis that increased plasma apoAV levels reflect an increased demand for plasma TG hydrolysis under physiological conditions.
Acknowledgments
The authors would like to thank Prof. Stephen G. Young and Michael Weinstein (Division of Cardiology, Department of Medicine, David Geffen School of Medicine, UCLA) for providing the plasma samples of the GPIHBP1-deficient mice.
Abbreviations
apoAV, apolipoprotein AV
GPIHBP, glycosylphosphatidylinositol-anchored HDL binding protein-deficient
TG, triglyceride
Published, JLR Papers in Press, January 13, 2009.
References
- 1.Pennacchio L. A., M. Olivier, J. A. Hubacek, J. C. Cohen, D. R. Cox, J. C. Fruchart, R. M. Krauss, and E. M. Rubin. 2001. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 294 169–173. [DOI] [PubMed] [Google Scholar]
- 2.Pennacchio L. A., M. Olivier, J. A. Hubacek, R. M. Krauss, E. M. Rubin, and J. C. Cohen. 2002. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum. Mol. Genet. 11 3031–3038. [DOI] [PubMed] [Google Scholar]
- 3.Mar R., P. Pajukanta, H. Allayee, M. Groenendijk, G. Dallinga-Thie, R. M. Krauss, J. S. Sinsheimer, R. M. Cantor, T. W. de Bruin, and A. J. Lusis. 2004. Association of the APOLIPOPROTEIN A1/C3/A4/A5 gene cluster with triglyceride levels and LDL particle size in familial combined hyperlipidemia. Circ. Res. 94 993–999. [DOI] [PubMed] [Google Scholar]
- 4.Lai C. Q., S. Demissie, L. A. Cupples, Y. Zhu, X. Adiconis, L. D. Parnell, D. Corella, and J. M. Ordovas. 2004. Influence of the APOA5 locus on plasma triglyceride, lipoprotein subclasses, and CVD risk in the Framingham Heart Study. J. Lipid Res. 45 2096–2105. [DOI] [PubMed] [Google Scholar]
- 5.Priore Oliva C., L. Pisciotta, V. G. Li, M. P. Sambataro, A. Cantafora, A. Bellocchio, A. Catapano, P. Tarugi, S. Bertolini, and S. Calandra. 2005. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 25 411–417. [DOI] [PubMed] [Google Scholar]
- 6.Priore Oliva C., F. Carubbi, F. G. Schaap, S. Bertolini, and S. Calandra. 2008. Hypertriglyceridaemia and low plasma HDL in a patient with apolipoprotein A-V deficiency due to a novel mutation in the APOA5 gene. J. Intern. Med. 263 450–458. [DOI] [PubMed] [Google Scholar]
- 7.Dallinga-Thie G. M., A. van Tol, H. Hattori, L. C. van Vark-van der Zee, H. Jansen, and E. J. Sijbrands. 2006. Plasma apolipoprotein A5 and triglycerides in type 2 diabetes. Diabetologia. 49 1505–1511. [DOI] [PubMed] [Google Scholar]
- 8.Vaessen S. F., F. G. Schaap, J. A. Kuivenhoven, A. K. Groen, B. A. Hutten, S. M. Boekholdt, H. Hattori, M. S. Sandhu, S. A. Bingham, R. Luben, et al. 2006. Apolipoprotein A-V, triglycerides and risk of coronary artery disease: the prospective Epic-Norfolk Population Study. J. Lipid Res. 47 2064–2070. [DOI] [PubMed] [Google Scholar]
- 9.Kahri J., J. Fruchart-Najib, N. Matikainen, J. C. Fruchart, J. Vakkilainen, and M. R. Taskinen. 2007. The increase of apolipoprotein A-V during postprandial lipemia parallels the response of triglyceride-rich lipoproteins in type 2 diabetes: no relationship between apoA-V and postheparin plasma lipolytic activity. Diabetes Care. 30 2083–2085. [DOI] [PubMed] [Google Scholar]
- 10.Talmud P. J., J. A. Cooper, H. Hattori, I. P. Miller, G. J. Miller, and S. E. Humphries. 2006. The apolipoprotein A-V genotype and plasma apolipoprotein A-V and triglyceride levels: prospective risk of type 2 diabetes. Results from the Northwick Park Heart Study II. Diabetologia. 49 2337–2340. [DOI] [PubMed] [Google Scholar]
- 11.Alborn W. E., M. J. Prince, and R. J. Konrad. 2007. Relationship of apolipoprotein A5 and apolipoprotein C3 levels to serum triglycerides in patients with type 2 diabetes. Clin. Chim. Acta. 378 154–158. [DOI] [PubMed] [Google Scholar]
- 12.Wong K., and R. O. Ryan. 2007. Characterization of apolipoprotein A-V structure and mode of plasma triacylglycerol regulation. Curr. Opin. Lipidol. 18 319–324. [DOI] [PubMed] [Google Scholar]
- 13.Becker S., L. Schomburg, K. Renko, M. Tolle, M. van der Giet, and U. J. Tietge. 2006. Altered apolipoprotein A-V expression during the acute phase response is independent of plasma triglyceride levels in mice and humans. Biochem. Biophys. Res. Commun. 339 833–839. [DOI] [PubMed] [Google Scholar]
- 14.Hagerty B. P., F. G. Schaap, M. Hermann, B. Krenn, C. Eder, B. Dorfmeister, H. Stangl, W. Patsch, and W. Strobl. 2008. Changes in hepatic ApoAV expression are not required for the rapid triglyceride lowering effect of fish oil diet in rats. Horm. Metab. Res. 40 69–71. [DOI] [PubMed] [Google Scholar]
- 15.Nelbach L., X. Shu, R. J. Konrad, R. O. Ryan, and T. M. Forte. 2008. Effect of apolipoprotein A-V on plasma triglyceride, lipoprotein size, and composition in genetically engineered mice. J. Lipid Res. 49 572–580. [DOI] [PubMed] [Google Scholar]
- 16.Berbee J. F., C. C. van der Hoogt, D. Sundararaman, L. M. Havekes, and P. C. Rensen. 2005. Severe hypertriglyceridemia in human APOC1 transgenic mice is caused by apoC-I-induced inhibition of LPL. J. Lipid Res. 46 297–306. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein M. M., A. P. Beigneux, B. S. Davies, P. Gin, L. Yin, K. Estrada, K. Melford, J. R. Bishop, J. D. Esko, L. G. Fong, et al. 2008. Abnormal patterns of lipoprotein lipase release into the plasma in GPIHBP1-deficient mice. J. Biol. Chem. 283 34511–34518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellani L. W., C. N. Nguyen, S. Charugundla, M. M. Weinstein, C. X. Doan, W. S. Blaner, N. Wongsiriroj, and A. J. Lusis. 2008. Apolipoprotein AII is a regulator of very low density lipoprotein metabolism and insulin resistance. J. Biol. Chem. 283 11633–11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross C. J., J. Twisk, J.M. Meulenberg, G. Liu, K. van den Oever, E. Moraal, W. T. Hermens, J. Rip, J. J. Kastelein, J. A. Kuivenhoven, et al. 2004. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPL(S447X) beneficial mutation. Hum. Gene Ther. 15 906–919. [DOI] [PubMed] [Google Scholar]
- 20.van der Vliet H. N., M. G. Sammels, A. C. Leegwater, J. H. Levels, P. H. Reitsma, W. Boers, and R. A. Chamuleau. 2001. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 276 44512–44520. [DOI] [PubMed] [Google Scholar]
- 21.Schaap F. G., P. C. Rensen, P. J. Voshol, C. Vrins, H. N. van der Vliet, R. A. Chamuleau, L. M. Havekes, A. K. Groen, and K. W. van Dijk. 2004. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 279 27941–27947. [DOI] [PubMed] [Google Scholar]
- 22.Merkel M., B. Loeffler, M. Kluger, N. Fabig, G. Geppert, L. A. Pennacchio, A. Laatsch, and J. Heeren. 2005. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan bound lipoprotein lipase. J. Biol. Chem. 280 21553–21560. [DOI] [PubMed] [Google Scholar]
- 23.Lookene A., J. A. Beckstead, S. Nilsson, G. Olivecrona, and R. O. Ryan. 2005. Apolipoprotein A-V-heparin interactions: implications for plasma lipoprotein metabolism. J. Biol. Chem. 280 25383–25387. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson S. K., A. Lookene, J. A. Beckstead, J. Gliemann, R. O. Ryan, and G. Olivecrona. 2007. Apolipoprotein A-V interaction with members of the low density lipoprotein receptor gene family. Biochemistry. 46 3896–3904. [DOI] [PubMed] [Google Scholar]
- 25.Weinstock P. H., C.L. Bisgaier, K. Aalto-Setala, H. Radner, R. Ramakrishnan, S. Levak-Frank, A. D. Essenburg, R. Zechner, and J. L. Breslow. 1995. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. Mild hypertriglyceridemia with impaired very low density lipoprotein clearance in heterozygotes. J. Clin. Invest. 96 2555–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson S. K., S. Christensen, M. K. Raarup, R. O. Ryan, M. S. Nielsen, and G. Olivecrona. 2008. Endocytosis of apolipoprotein A-V by members of the low density lipoprotein receptor and the VPS10p domain receptor families. J. Biol. Chem. 283 25920–25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien P. J., W. E. Alborn, J. H. Sloan, M. Ulmer, A. Boodhoo, M. D. Knierman, A. E. Schultze, and R. J. Konrad. 2005. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin. Chem. 51 351–359. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg I. J., C. A. Scheraldi, L. K. Yacoub, U. Saxena, and C. L. Bisgaier. 1990. Lipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IV. J. Biol. Chem. 265 4266–4272. [PubMed] [Google Scholar]
- 29.Havel R. J., J. P. Kane, and M. L. Kashyap. 1973. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J. Clin. Invest. 52 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merkel M., and J. Heeren. 2005. Give me A5 for lipoprotein hydrolysis! J. Clin. Invest. 115 2694–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]