Abstract
Lipid trafficking in the brain is essential for the maintenance and repair of neuronal membranes, especially after neurotoxic insults. However, brain lipid metabolism is not completely understood. In plasma, LCAT catalyses the esterification of free cholesterol on circulating lipoproteins, a key step in the maturation of HDL. Brain lipoproteins are apolipoprotein E (apoE)-containing, HDL-like particles secreted initially as lipid-poor discs by glial cells. LCAT is synthesized within the brain, suggesting that it may play a key role in the maturation of these lipoproteins. Here we demonstrate that astrocytes are the primary producers of brain LCAT. This LCAT esterifies free cholesterol on nascent apoE-containing lipopoproteins secreted from glia. ApoE is the major LCAT activator in glia-conditioned media (GCM), and both the cholesterol transporter ABCA1 and apoE are required to generate glial LCAT substrate particles. LCAT deficiency leads to the appearance of abnormal ∼8 nm particles in GCM, and exogenous LCAT restores the lipoprotein particle distribution to the wild-type (WT) pattern. In vivo, complete LCAT deficiency results in a dramatic increase in apoE-HDL and reduced apolipoprotein A-I (apoA-I)-HDL in murine cerebrospinal fluid (CSF). These data show that brain LCAT esterifies cholesterol on glial-derived apoE-lipoproteins, and influences CSF apoE and apoA-I levels.
Keywords: central nervous system, HDL, apoE, apoA-I, ABCA1, mouse, lipoprotein metabolism
In plasma, LCAT is the sole enzyme capable of esterifying cholesterol in the circulation. LCAT is a 416 amino acid protein that circulates in plasma predominately bound to lipoproteins, where it catalyses the transfer of an unsaturated fatty acid from phosphatidylcholine, or lecithin, to the free β-hydroxyl residue of cholesterol to generate cholesterol esters (CE) and lysoPC (lysolecithin) (1). Esterification of lipoprotein cholesterol results in the segregation of CE into the lipoprotein core, an essential step in peripheral HDL maturation. Mutations in the human LCAT gene underlie two distinct metabolic diseases, Familial LCAT Deficiency and Fish Eye Disease, both of which present with low HDL levels (2).
The preferred plasma substrate for circulating LCAT is free cholesterol found on HDL, and apolipoprotein A-I (apoA-I), the primary protein constituent of HDL, is considered the major physiological activator of LCAT (3). In vitro experiments show that other plasma apolipoproteins, including apolipoprotein E (apoE), apoC-I, and apoA-IV, are capable of activating LCAT, albeit less efficiently than apoA-I (3). Moreover, apoA-I, and to a lesser extent, apoE appear to be the predominant in vivo activators of LCAT, as a recent analysis of apoA-I-, apoE-, and double apoA-I/apoE-deficient mice shows that the percentage of free cholesterol esterified in plasma drops to less than 2% of wild-type (WT) values after deletion of apoA-I and apoE (4).
LCAT is synthesized mainly in liver, but is also abundant in brain and testes (5–8). Indeed, brain exhibits the second highest LCAT mRNA level after liver in rats and rhesus monkeys (6, 9). Brain LCAT mRNA expression has been demonstrated in cortex, cerebellum, hippocampus, and brain stem. In situ hybridization assays show that LCAT mRNA is found in neurons and glial cells, as determined by nuclear morphology (9), and LCAT activity can be detected in conditioned media of two-thirds of 25 neuronal and gliomal cell lines of human and rodent origin (10). LCAT derived from these cell lines responds to the same inhibitor (DTNB) and activator (apoA-I) as plasma LCAT (10). In humans, LCAT protein has been found in cerebrospinal fluid (CSF) at levels that also suggest local synthesis within the central nervous system (CNS) rather than import from the circulation (7). For example, human CSF contains LCAT at levels corresponding to ∼2.5% that of plasma LCAT (11), roughly comparable to that of apoE, which is present in CSF at approximately 4% of plasma apoE. However, the relevance of LCAT function in brain cholesterol metabolism has not been addressed.
As apoB is not found within the CNS, brain lipoprotein metabolism is based entirely on HDL-like particles, which are initially generated as lipid-poor discoidal particles by both astrocytes and microglia (12). CNS HDL particles differ from plasma HDL particles primarily in that apoE, rather than apoA-I, constitutes their main apolipoprotein component. In vitro, the discoidal, lipid-poor, apoE- and apoJ-containing lipoproteins secreted by astrocytes and microglia contain only 0% to 18% of their cholesterol as esters (13–16), whereas mature lipoprotein particles isolated from human CSF contain 64% of their cholesterol as esters. In addition, CSF HDL particles are spherical and associated with several other apolipoproteins, including apoA-I, A-IV, C-II, C-III, and apoD (17–21), similar to the properties of mature plasma HDL. These observations suggest that brain LCAT may participate in the maturation of nascent glial-derived lipoproteins into the particles found in CSF, much like LCAT functions in plasma HDL metabolism.
The most important CSF apolipoproteins to CNS lipid and lipoprotein metabolism are apoE and apoA-I, found at approximately 4% and 0.5% of their plasma concentrations, respectively (17, 18). In situ hybridization studies have documented mRNA expression of apoE but not apoA-I within the CNS, although apoA-I is synthesized in cultured brain capillary endothelial cells and in porcine choroid plexus extracts (22). These observations suggest that apoA-I is imported into the brain from the circulation or released into the brain from cerebrovascular endothelial cells. ApoA-I and apoE are the physiological activators of LCAT in mouse plasma as evaluated by ex vivo cholesterol esterification assays (4); however, little is known about their respective roles as LCAT activators on glia-derived lipoproteins. In addition to apoA-I and apoE, the cholesterol transporter ABCA1 plays a crucial role in plasma lipoprotein maturation, as deficiency of ABCA1 results in lipid-poor preβ HDL particles that are ineffective LCAT substrates (23). For instance, ABCA1-deficient plasma requires the presence of cells that express both ABCA1 and LCAT to rescue the in vitro formation of mature α-migrating lipoproteins (23). Similarly, ABCA1-deficient mice do not form mature HDL even in the presence of excess, adenoviral-delivered recombinant human apoE4 and LCAT (24), suggesting that ABCA1 activity is crucial to create LCAT substrates.
Taken together, these data suggest the hypothesis that LCAT plays a key role in the maturation of glial-derived, nascent lipoproteins in brain. Here we provide the first systematic analysis of the function of LCAT derived from primary glia and neurons on nascent glial lipoproteins. We identify astrocytes as the predominant LCAT-secreting cell type in the brain. We demonstrate that astrocyte-derived LCAT has cholesterol esterification activity and that glia-derived apoE-containing nascent lipoproteins are LCAT substrates. We also show that complete murine LCAT deficiency leads to changes in both nascent and mature lipoprotein particles in glia-conditioned medium and CSF, respectively. Our results suggest that brain-derived LCAT plays a significant role in the metabolism of nascent glial lipoproteins.
EXPERIMENTAL PROCEDURES
Animals
LCAT-deficient, ABCA1-deficient, apoE-deficient, and WT C57Bl/6 mice were maintained on a chow diet (PMI LabDiet 5010). All animal procedures were in accordance with the Canadian Council of Animal Care and the University of British Columbia Committee on Animal Care.
qRT-PCR
RNA was extracted using Trizol (Invitrogen, Burlington, Ontario, Canada) and treated with DNaseI. cDNA was generated using oligo-dT primers and Taqman Reverse transcription reagents (Applied Biosystems, Foster City). Quantitative real-time PCR primers were designed using PrimerExpress (Applied Biosystems, Foster City) to detect murine LCAT. Primer sequences and cycling conditions are provided upon request. Real-time quantitative PCR was done with Sybr green reagents (Applied Biosystems, Foster City) on an ABI 7000 (Applied Biosystems, Foster City). Each sample was assayed at least in duplicate, normalized to β-actin and analyzed with 7000 system SDS software v1.2 (Applied Biosystems, Foster City) using the relative standard curve method.
Cell culture and culture media conditioning
Primary glia: cultures were prepared as previously described (25) with the following modifications: the entire forebrain was used as tissue source for primary glia, and forebrain cell suspensions were plated in T150 flasks. After 10 days in vitro, cells were washed twice with 1× PBS (Invitrogen Canada, Burlington) to remove residual FBS components. Then, 20 ml of serum-free DMEM/F12 (Invitrogen Canada, Burlington) containing 2 mM L-glutamine (Invitrogen Canada, Burlington), and 100 units/ml of penicillin-streptomycin (Invitrogen Canada, Burlington) were added for a 72 h conditioning period. Conditioned media were then concentrated 100-fold using a 10 kDa molecular mass cut-off spin concentrator (Sartorius Mechatronics Canada, Mississauga, Ontario, Canada).
Primary neurons
Cells were isolated at embryonic day 16 (E16)–E18. Forebrain tissue was dissected, gently trypsinized, and triturated with fire polished Pasteur pipettes. Neurons were then seeded onto poly-D-lysine-coated (Sigma-Aldrich, Oakville, Ontario, Canada) plates at a density of one embryo per 6-well plate. Cultures were maintained in Neurobasal media (Invitrogen Canada, Burlington) with B27 supplement (Invitrogen Canada, Burlington), 0.5 mM glutamine (Invitrogen Canada, Burlington), and 100 units/ml of penicillin-streptomycin (Invitrogen Canada, Burlington), and 50% of the culture media was changed every 4–5 d. Media was collected over a period of 10–14 days and concentrated 100-fold with 10 kDa molecular mass cut-off spin concentrator (Sartorius Mechatronics Canada, Mississauga).
BHK and BHK-LCAT
Stably transfected baby hamster kidney (BHK) with the human LCAT cDNA BHK-LCAT cells were generously provided by Dr. John Hill (University of British Columbia, Canada). Two hundred fifty micrometers Methotrexate (Sigma-Aldrich, Oakville) was used as the selection agent for the BHK-LCAT cells (26). Nontransfected control BHK and BHK-LCAT cells were cultured in DMEM (Invitrogen Canada, Burlington) with 10% FBS (Invitrogen Canada, Burlington), 2 mM L-glutamine (Invitrogen Canada, Burlington), and 100 units/ml of penicillin-streptomycin (Invitrogen Canada, Burlington). After reaching confluence, cells were washed twice with 1× PBS and incubated for 24 h in serum-free DMEM/F12 without methotrexate to condition media, which was subsequently concentrated 60-fold as above.
Determination of LCAT activity and cholesterol esterification rates
Exogenous LCAT activity on glial-derived lipoproteins
This assay evaluates cholesterol esterification on glia-derived, [3H]cholesterol-labeled lipoproteins by exogenous, recombinant human LCAT as previously described (27) with modifications. Briefly, concentrated glia-conditioned media (GCM) was equilibrated overnight with [3H]cholesterol (Perkin Elmer, Waltham, MA) at 4°C by adding at least 200 μl of concentrated GCM to a glass test tube containing [3H]cholesterol-labeled filter paper discs, cut to size from Whatman #1 filter paper (GE Healthcare). The labeled GCM was then incubated with excess recombinant LCAT in BHK conditioned media at ratios of 1:1 or 2:1 (GCM/BHK-LCAT), which corresponds to final LCAT concentrations of approximately 130 μg/ml and 87 μg/ml, respectively (26). The rate of [3H]cholesterol esterification at 37°C was determined over a time period of 0 to 24 h. The reaction was terminated by adding 1.5 ml of chloroform/methanol (2:1 v/v). After centrifugation at 3,750 rpm for 15 min, the cholesterol and cholesteryl ester in the supernatant were separated by TLC on Merck silica gel 60 glass plates (Sigma-Aldrich, Oakville) with the solvent system petroleum ether-diethyl ether-acetic acid 70:12:1 (v/v). The radioactivity associated with cholesterol and cholesteryl ester was determined by liquid scintillation counting after TLC. The results are expressed as either fractional esterification rate [(CE ×100)/(TC + CE)] or molar esterification rate (MER) of cholesterol (% esterified cholesterol × nmoles of free cholesterol at the start of the reaction per ml of media per h).
Glial- and neuronal-derived LCAT activity on human apoA-I containing phosphatidylcholine vesicles
This LCAT activity assay was performed using radioactively labeled vesicles composed of egg yolk phosphatidylcho1ine (EYPC) (Sigma-Aldrich, Oakville), unesterified cholesterol (UC) (Sigma-Aldrich, Oakville), [3H]cholesterol (Perkin Elmer, Waltham, MA) (in a molar ratio of 4:1 = EYPC/UC) and human apoA-I (0.0167 μg/ml) purified from plasma as previously described (27) with minor changes. Incubations were carried out over a time period of 0 to 5 h, and the reaction was terminated by the addition of 1 ml of absolute ethanol. Unesterified cholesterol and cholesteryl ester were separated by TLC, counted, and quantified as above.
Cholesterol esterification assay during glial culture
To evaluate whether glial-derived LCAT esterifies cholesterol on glial-derived lipoproteins, confluent mixed glial cultures where rinsed twice with 1× PBS, and incubated for 18 h in serum free DMEM/F12 with 200 nCi/ml [3H]cholesterol. After incubation, conditioned media were concentrated 100-fold as above, and the reaction was terminated by the addition of 2 ml of chloroform:methanol (2:1 v/v). Cholesterol and CE were determined using TLC as above.
CSF collection and Western blotting
CSF collection
As previously described (28).
Western Blotting
Fifteen microliters of conditioned media or 10 μl of CSF were electrophoresed through 6% nondenaturing or 10% SDS-polyacrylamide gels. After electrophoresis, proteins were electrophoretically transferred to polyvinylidene fluoride membrane (Millipore), and immunodetected using antibodies against apoE (Chemicon, 1:500), apoA-I (Biodesign, 1:250), or murine LCAT (1:1000) (29). Blots were developed using enhanced chemiluminescence (Amersham Biosciences) according to the manufacturer's recommendations.
Total cellular protein was measured using a BioRad protein detection kit.
ApoE ELISA
Murine apoE levels in GCM were determined by ELISA as described previously (30) with minor modifications. Plates were coated with anti-apoE antibody (WUE-4) (a gift from Dr. David Holtzman, Washington University). Samples were diluted in 0.5% BSA and 0.025% Tween-20 in PBS. Standards were based on murine apoE purified from plasma (1.3 μg/μl, a gift from Dr. Mary Sorci-Thomas, Wake Forest University). After an overnight incubation at 4°C, plates were incubated with goat anti-apoE (EMD Biosciences, San Diego, CA) followed by biotinylated anti-goat antibody (Vector Laboratories, Burlingame, CA). Plates were developed with poly-horseradish peroxidase streptavidin (Pierce, Rockford, IL) and ultra-slow 3,3′,5,5′-tetramethylbenzidine (Sigma, St. Louis, MO), stopped with 1 N HCl, and read at 450 nm.
Determination of total CSF cholesterol
Total CSF cholesterol was measured by an isotope dilution method using 2H6-cholesterol as internal standard (Medical Isotopes Inc., Pelham, NH). After alkaline hydrolysis, the trimethylsilylated sterols were separated and determined by highly sensitive GC-MS-selected ion monitoring (31).
Statistical analysis
Data is shown as mean ± SEM and analyzed by two-tailed unpaired Student's t-tests or one-way ANOVA followed by either Tukey (compares all pairs of groups) or Dunnett (compares all groups vs a control group) posttests. Analyses were performed using Graphpad Prism (version 5.0, San Diego).
RESULTS
LCAT is synthesized in primary murine astrocytes and neurons
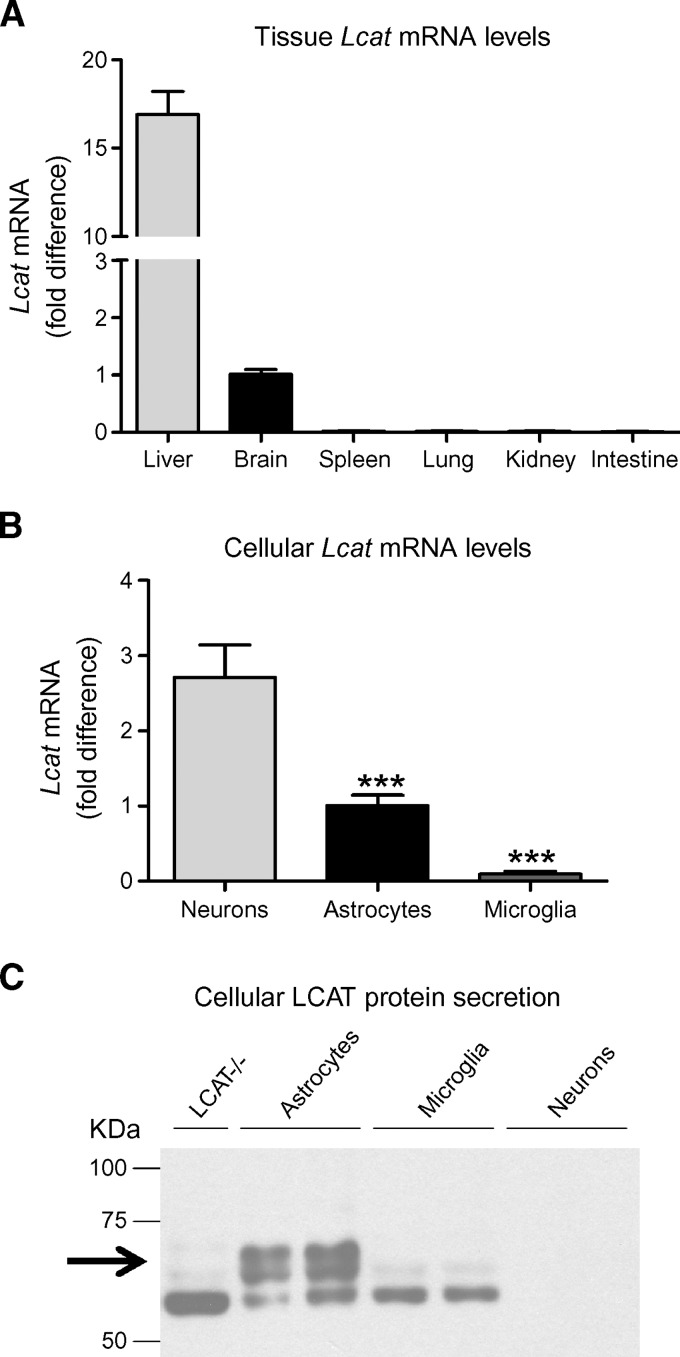
Previous studies identified the brain as one of three organs that synthesize LCAT, together with liver and testes (6, 9, 32–35). In situ hybridization experiments showed that LCAT mRNA is present in neuronal and nonneuronal cell types in rhesus monkeys (9). However, neither the identity of the nonneuronal cells that express LCAT nor the relative contribution of neurons, astrocytes, and microglia to the possible brain pool of LCAT has been evaluated. To address these questions, we first confirmed the tissue distribution of endogenous murine Lcat mRNA using quantitative RT-PCR (qRT-PCR). In accordance with previous reports, brain and liver exhibited robust Lcat mRNA expression, whereas spleen, lung, kidney, and intestine were devoid of Lcat mRNA (Fig. 1A). Brain LCAT mRNA was approximately 6% as abundant as liver LCAT mRNA (N = 12, Fig. 1A).
Fig. 1.
Endogenous murine Lcat mRNA is found in brain and in primary astrocytes and neurons. A: Total Lcat mRNA levels in various tissues were determined by quantitative RT-PCR (qRT-PCR) and normalized to actin mRNA. Graph summarizes the results of four independent experiments with a total of N = 12 samples for brain and liver, and N = 4 for spleen, lung, kidney and intestine, all measured in duplicate. Lcat mRNA expression levels are expressed as fold difference over brain. B: Total mRNA of primary neurons, astrocytes, and microglia was purified using Trizol and tested for the presence and levels of murine Lcat mRNA by qRT-PCR. Individual samples were normalized to actin mRNA to correct for cell number variability. Graph summarizes the results of two independent experiments with a total of N = 10 individual cultures for neurons, N = 7 for astrocytes, and N = 8 for microglia, all measured in duplicate. Lcat mRNA expression levels are expressed as fold difference over astrocytes. Error bars represent SEM. One-way ANOVA with a Tukey posttest was used for statistical analysis. *** P < 0.0001. C: Fifteen microliters of 100-fold concentrated conditioned media of LCAT-deficient glia (LCAT−/−, negative control) (N = 1), wild-type (WT) astrocytes (N = 2), and microglia (N = 2), and 1.5 microliters of neuronal-conditioned media (N = 2) were evaluated for LCAT protein levels by Western blot. The arrow demarcates LCAT protein, and the 55 kDa species detected in several lanes is a nonspecific cross-reactive band.
To identify the CNS cell types that may express LCAT in murine brain, endogenous Lcat mRNA levels were evaluated in primary neuronal, astrocytic, and microglial cultures by qRT-PCR. Neurons and astrocytes showed high levels of Lcat mRNA (Fig. 1B). After correcting for differences in total cell number using actin mRNA abundance, neurons were found to express almost three times as much Lcat mRNA as astrocytes (N ⩾ 7, P < 0.0001, Fig. 1B). The very low levels of Lcat mRNA seen in microglial cultures may correspond to residual contamination (less than 1%) with astrocytes, given the sensitivity of the qRT-PCR methodology and absence of Lcat mRNA signal in macrophage-rich organs, such as lung, spleen and intestine (6, 9, 32). These data suggest that astrocytes and neurons are the major sites of LCAT synthesis in brain.
Western blots of murine LCAT in astrocyte, microglial and neuronal conditioned media (NCM) (N = 2 samples per cell type) show that astrocyte-conditioned media (ACM) contains two proteins of 63 and 67 kDa that are not present in LCAT-deficient GCM (Fig. 1C) and that correspond to the expected molecular weight of glycosylated LCAT observed in human plasma. These bands were absent in conditioned media obtained from neurons and microglia. These data suggest that despite high Lcat mRNA levels in neurons, of the three brain-derived cell types, astrocytes are the primary producers of LCAT protein.
Astrocyte-derived LCAT esterifies cholesterol
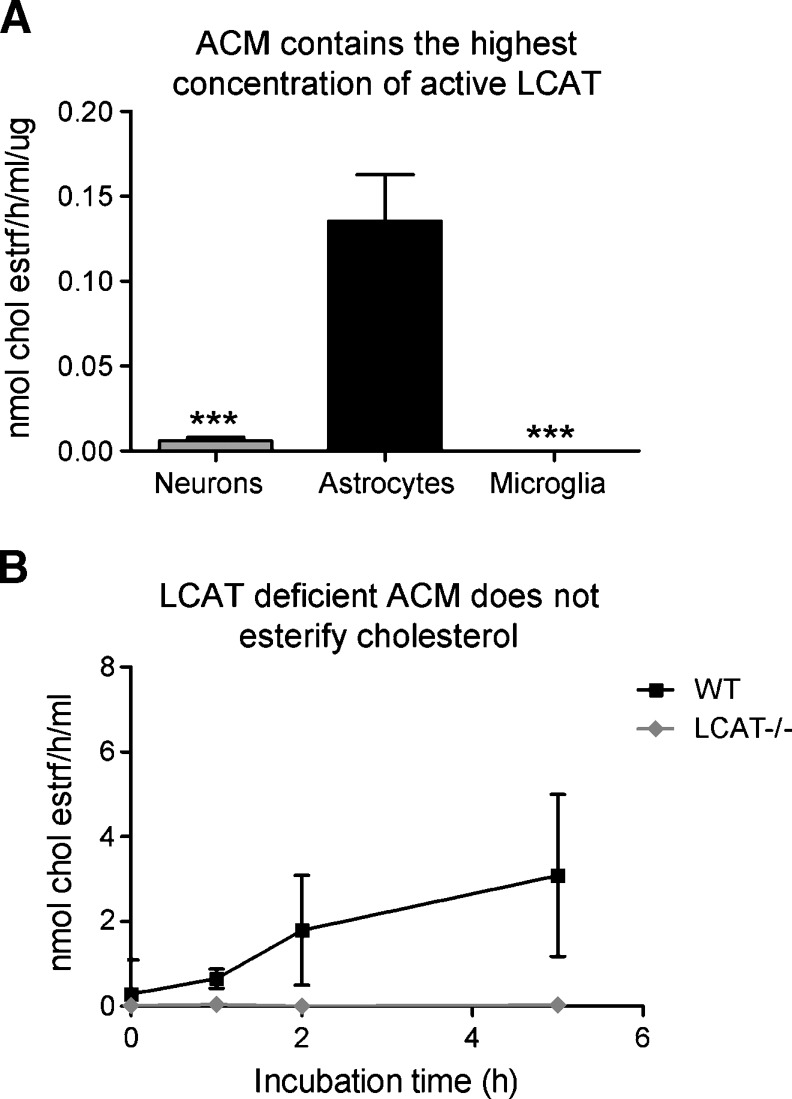
To determine whether LCAT secreted by astrocytes is enzymatically active, we performed exogenous esterification assays with conditioned media from primary astrocytes, neurons, and microglia as sources of brain-derived LCAT, and exogenous apoA-I-containing EYPC vesicles as substrates. When corrected for cellular protein, ACM exhibited the highest MER with 0.1354 nmoles of cholesterol esterified per h per ml of concentrated media normalized to μg of total cellular protein (Fig. 2A). Unlike microglia, where no cholesterol esterification could be detected, NCM did exhibit esterification activity, albeit at less than 5% of the levels detected in ACM (N = 5, P < 0.0001, Fig. 2A). This level of activity is consistent with the absence of detectable LCAT protein in NCM and may be due to the presence of low levels of astrocytes in neuronal cultures.
Fig. 2.
Primary astrocytes are the main source of active LCAT in vitro. A: Astrocyte conditioned media (ACM), neuronal conditioned media (NCM), and microglia conditioned media were evaluated for the ability to esterify cholesterol on [3]Hcholesterol-labeled egg yolk phosphatidylcho1ine (EYPC)/unesterified cholesterol (UC) vesicles. Graph summarizes two independent experiments with N = 5 individual cultures per cell type. Cholesterol esterification is expressed as molar esterification rate of cholesterol (MER) (nmoles of cholesterol esterified per h per ml of concentrated media) per microgram for cellular protein, to correct for differences in cell number between cultures. B: Cholesterol esterification detected in ACM is LCAT dependent. ACM from WT and LCAT-deficient (LCAT−/−) glia cultures were evaluated for the ability to esterify cholesterol as above. Graph summarizes the results of four independent experiments. Cholesterol esterification is expressed as MER. One-way ANOVA with a Tukey posttest was used for statistical analysis. *** P < 0.0001.
To evaluate whether NCM could have an inhibitory effect on LCAT function and explain the discrepancy between low NCM LCAT activity and the high neuronal Lcat mRNA, a known amount of recombinant human LCAT was added to ACM and NCM, and cholesterol esterification was evaluated over a 5 h period. Compared with ACM, NCM was found to inhibit the activity of recombinant LCAT by up to 30% (data not shown). However, even when corrected for this inhibitory effect, LCAT activity in NCM does not match the levels of Lcat mRNA. This observation suggests that cultured neurons may have mechanisms that suppress translation or secretion of LCAT protein. We believe that the suppression of LCAT activity in NCM is nonspecific and due mainly to the viscosity of concentrated NCM compared with ACM.
Because astrocytes appear to be the main source of active LCAT in vitro, cholesterol esterification activity was compared in 72 h-conditioned WT and LCAT-deficient ACM using EYPC/UC vesicles. In WT ACM, cholesterol esterification was linear up to 5 h of incubation and occurs at a catalytic rate of 78.05 ± 9 pmoles per h per ml of concentrated media (Fig. 2B). As expected, ACM obtained from LCAT-deficient glia did not esterify cholesterol above background levels (Fig. 2B). These data show that astrocyte-secreted LCAT is capable of catalyzing cholesterol esterification and that no other astrocyte-derived protein has this functional activity.
ApoE is required for LCAT activation in GCM
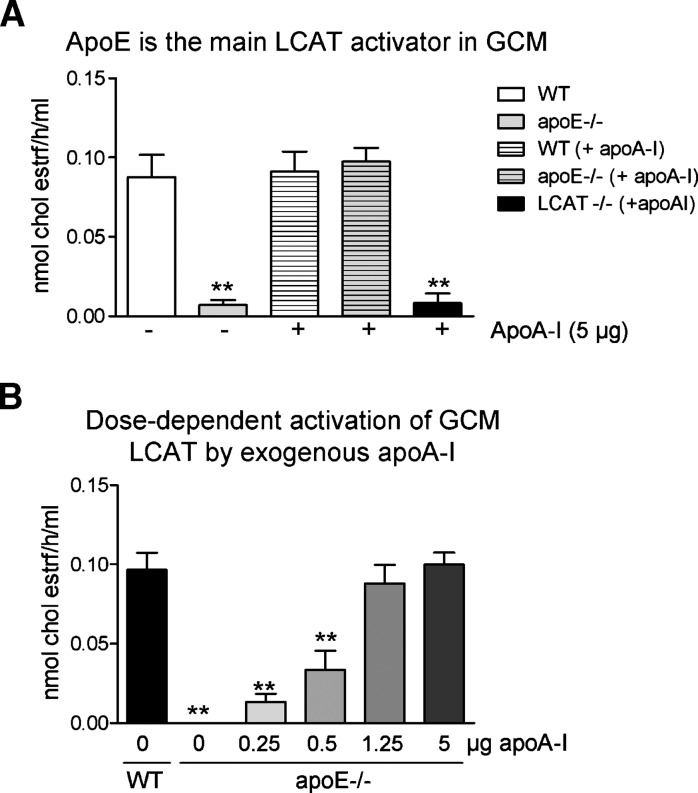
ApoA-I has been shown to be the primary activator of plasma LCAT (36). ApoE can also activate LCAT, albeit less efficiently than apoA-I. Astrocytes and microglia are the major sources of CNS apoE, and also secrete other apolipoproteins that have been reported to act as LCAT activators in vitro, such as apoD (37) and apoC-I (38). Because no apoA-I is synthesized in brain parenchyma, it is of interest to evaluate whether glial apoE can act as the sole activator of LCAT in the CNS or if other glial apolipoproteins are also relevant. We therefore determined whether the absence of apoE in GCM affects LCAT activity by performing exogenous esterification assays using WT and apoE-deficient GCM in the presence or absence of exogenous apoA-I.
In this set of experiments, WT and apoE-deficient GCM samples were divided into two aliquots. One aliquot was incubated only with EYPC/UC vesicles (no apoA-I group) to reveal the esterification potential of endogenous lipid poor apolipoproteins present in WT and apoE-deficient GCM. The other aliquot was incubated in the presence of EYPC/UC vesicles premixed with apoA-I (+ apoA-I group) to reveal the maximum esterfication potential in the presence of the most effective LCAT activator known.
ApoE-deficient GCM samples exhibited a 90% reduction in cholesterol esterification when compared with WT GCM (9.3 ± 5.5 and 87.7 ± 28 pmoles per h per ml, respectively, P < 0.001, N = 3) (Fig. 3A). Supplementation of apoE-deficient GCM with 5 μg of apoA-I completely restored cholesterol esterification to WT levels (94.71 ± 23 pmoles per h per ml, N = 6) (Fig. 3A), suggesting that exogenous apoA-I is sufficient to activate LCAT in the absence of apoE. Notably, this finding also suggests that secretion of LCAT by apoE-deficient cells is equal to that of WT cells, as cholesterol esterification rates were equivalent in WT and apoE-deficient samples supplemented with exogenous apoA-I.
Fig. 3.
Apolipoprotein E (apoE) is the main LCAT activator in glia-conditioned media (GCM). A: Cholesterol esterification was evaluated in conditioned media from WT, apoE-deficient (apoE−/−) and LCAT-deficient (LCAT−/−) mixed glial cultures. The first two bars on the graph represent the level of esterification seen in conditioned media supplemented only with [3]Hcholesterol-labeled EYPC/UC vesicles, whereas the last three bars correspond to the effects seen when [3]Hcholesterol-labeled EYPC/UC vesicles were complexed with 5 μg of human apolipoprotein A-I (apoA-I) purified from plasma and then added to the conditioned media. Graph represents at least four independent experiments with an N ⩾ 4 independent cultures for each group. B: Cholesterol esterification was evaluated in conditioned media from WT and apoE-deficient (apoE−/−) mixed glial cultures after addition of [3]Hcholesterol-labeled EYPC/UC vesicles and increasing amounts of purified human apoA-I (added only to apoE-deficient samples). Graph represents the MER of two independent experiments. One-way ANOVA with a Dunnett posttest was used for statistical analysis. ** P < 0.05 compared with first WT group (A) and WT (B).
Importantly, the rate of cholesterol esterification in WT GCM samples (87.7 ± 28 pmoles per h per ml, N = 4) (Fig. 3A) was not increased further by the addition of apoA-I (92.7 ± 40 pmoles per h per ml, N = 8), suggesting that, in the absence of apoA-I, the apoE present in concentrated GCM is fully capable of maximally activating LCAT in the presence of exogenous EYPC/UC vesicles as optimized substrates.
As expected, cholesterol esterification was nearly absent when conditioned media from LCAT-deficient glia was used even when supplemented by apoA-I (Fig. 3A), demonstrating that cholesterol esterification in this assay is LCAT-dependent.
To evaluate how much apoA-I is required to obtain the same amount of LCAT activation as with endogenous levels of apoE, an apoA-I titration experiment was performed. Approximately 1.25 μg of human apoA-I was required to obtain the same activation of LCAT seen with 30 μg of endogenous apoE found in GCM (Fig. 3B). Further experiments showed that the minimal amount of apoE required to obtain WT activation levels of LCAT is 5 μg (data not shown). This suggests that unpurified GCM apoE has 25% of the LCAT activation potential of purified human apoA-I from plasma. This is roughly consistent with the reported 15–19% activation potential of purified human plasma apoE when compared with purified human plasma apoA-I in in vitro cholesterol esterification assays using EYPC vesicles and proteoliposomes (39, 40).
ApoE and ABCA1 are required for the generation of LCAT substrate particles by glia
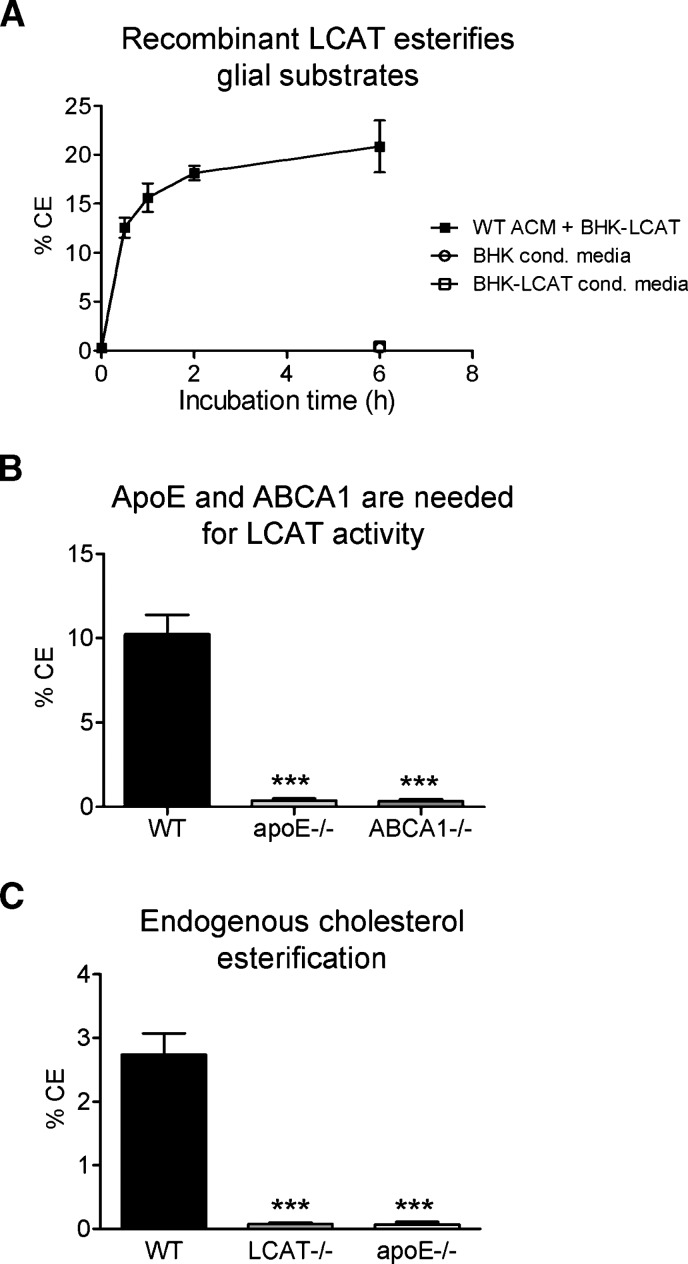
The results above demonstrate that astrocyte-derived LCAT is active on exogenous EYPC/UC vesicles complexed with either endogenous murine apoE or exogenous human apoA-I. To determine whether glia-derived nascent lipoproteins are suitable LCAT substrates, WT GCM lipoproteins were equilibrated with [3H]cholesterol and incubated with recombinant human LCAT obtained from stably transfected BHK-LCAT as described in Experimental Procedures. Up to ∼20% of the labeled unesterified cholesterol on glial-derived lipoproteins had been esterified by exogenous LCAT in a time-dependent manner after 5 h of incubation (Fig. 4A). As expected, no cholesterol esterification was seen with conditioned media from nontransfected BHK cells (Fig. 4A). Importantly, no esterification was seen when conditioned media from stably transfected BHK-LCAT media was labeled with [3H]cholesterol in the absence of GCM, thus ensuring that esterification occurred on glia-derived particles and not on nonspecific substrates. Together, these data show that LCAT-dependent cholesterol esterification occurs on glia-derived lipoproteins.
Fig. 4.
ApoE and ABCA1 are required for the generation of LCAT substrates by glia cells. A: Time-dependent esterification of cholesterol on nascent lipoproteins from WT mixed glia in the presence of recombinant LCAT derived from baby hamster kidney cells stably transfected with human LCAT (BHK-LCAT cells). No esterification activity is observed in the absence of nascent GCM lipoproteins in either BHK or BHK-LCAT control media. Graph represents two independent experiments and expresses % of cholesterol esters (CE). B: Cholesterol esterification by recombinant LCAT on glia-derived lipoproteins was evaluated as above over a period of 5 h in WT, apoE-deficient (apoE−/−), and ABCA1-deficient (ABCA1−/−) glia conditioned media. Graph represents two independent experiments. C: In situ cholesterol esterification in mixed glial conditioned media. WT, LCAT−/−, and apoE−/− glial cultures were labeled with [3H]cholesterol for 18 h prior to concentration and separation of UC and CE by TLC. Graph represents two independent experiments. *** P < 0.0001 with respect to WT, as determined by one-way ANOVA with a Dunnett posttest analysis.
Our results above demonstrate that apoE is the main LCAT activator secreted by glia (Fig. 3A). We and others have shown that the cholesterol transporter ABCA1 as a key regulator of apoE lipidation in the CNS (25, 30). Intriguingly, ABCA1 is required to generate functional LCAT substrates in plasma, as lipid-poor apolipoproteins found in ABCA1-deficient animals are weak activators of LCAT (23). To determine the importance of apoE and ABCA1 activity for LCAT function on GCM-derived lipoproteins, GCM from WT, apoE-, and ABCA1-deficient glia were incubated with recombinant human LCAT. The generation of CE on GCM lipoproteins derived from apoE- and ABCA1-deficient glia was reduced by more than 95% compared with WT GCM (P < 0.0001, N = 4) (Fig. 4B), indicating that both ABCA1 and apoE are required for the generation of functional LCAT lipoprotein substrates in the CNS.
Finally, we performed in situ cholesterol esterification assays to determine whether astrocyte-derived LCAT could esterify cholesterol on endogenous glial nascent lipoproteins. In these experiments, unesterified [H3]cholesterol was added directly to serum-free medium during conditioning, in order to efficiently incorporate the radioactive label into nascent lipoprotein particles. Although no increase in CE was detected after concentration of the conditioned media and subsequent incubation at 37°C (data not shown), we noted that approximately 2.8% of the radiolabeled cholesterol had been esterified after an 18 h conditioning period (Fig. 4C), possibly representing endogenous esterification in GCM. To confirm this hypothesis, WT, LCAT-, and apoE-deficient glial cultures were conditioned for 18 h in serum-free media labeled directly with [H3]unesterified cholesterol. Under these conditions, CE were detected only in WT GCM. The absence of detectable CE in the absence of endogenous enzyme (LCAT−/− GCM) or endogenous substrates (apoE−/− GCM) suggests that WT GCM is capable of supporting cholesterol esterification on endogenous apoE-containing nascent lipoprotein substrate particles. However, the low endogenous levels of enzyme and substrate even in concentrated GCM may preclude rate measurements under our conditions.
LCAT deficiency results in altered GCM and CSF lipid composition
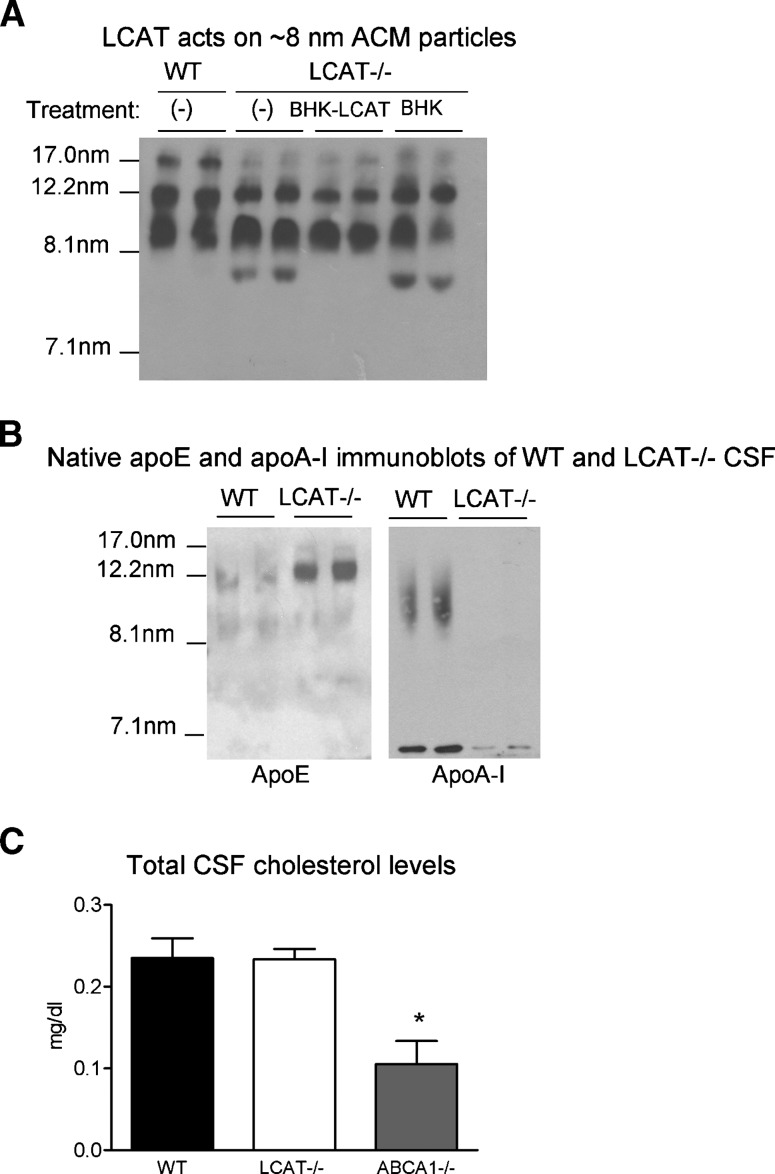
To evaluate the effects of LCAT deficiency on the size distribution of lipoprotein particles in GCM, concentrated WT and LCAT−/− GCM were subjected to nondenaturing Western blotting and probed for murine apoE. Compared with the pattern of nascent particle size in WT samples, LCAT −/− media displayed a reduced level of large 17.0 nm particles, and contained a small ∼8.0 nm particle not present in WT samples (Fig. 5A). These observations suggest that these small particles may correspond to LCAT substrates, whereas the bigger ones may represent the products of the LCAT reaction. To test this hypothesis, we incubated LCAT−/− samples with recombinant human BHK-LCAT or vehicle alone, followed by native gel electrophoresis. Supplementation with exogenous LCAT completely eliminated the appearance of the small 8 nm particles, supporting our hypothesis that these small particles are the preferred LCAT substrates.
Fig. 5.
LCAT-deficiency leads to altered lipoprotein metabolism in GCM and cerebrospinal fluid (CSF). A: Distribution of apoE-containing particles in WT and LCAT-deficient (LCAT−/−) mixed glial cultures was evaluated by native gel electrophoresis. WT(−) and LCAT−/−(−) lanes represent WT and LCAT-deficient media with no treatment. Incubation of LCAT−/− media with exogenous LCAT (BHK-LCAT media) at 37C for 1 h results in the disappearance of an 8 nm particle observed in LCAT−/− media. This effect is not seen when LCAT−/− media is incubated with vehicle alone (control BHK media), suggesting that this particle may represent the preferred LCAT substrate. B: Distribution of apoE and apoA-I-containing particles in the CSF of WT and LCAT−/− animals was evaluated by native gel electrophoresis. Blots are representative of two independent experiments and at least four animals per genotype. C: Total cholesterol levels were evaluated in CSF from WT, LCAT−/−, and ABCA1−/− mice by mass spectrometry. Data represent two independent experiments with N ⩾ 6 animals per group. * P < 0.05 with respect to WT, as determined by one-way ANOVA with a Dunnett posttest analysis.
WT and LCAT−/− CSF samples were then evaluated for apoE and apoA-I particle distribution as well as cholesterol and CE levels. Compared with WT CSF, apoE levels were dramatically increased in LCAT−/− CSF (Fig. 5B). Furthermore, apoA-I particles were not detected in LCAT-deficient CSF compared with WT samples (Fig. 5B). Although the significance of these findings in CNS lipoprotein metabolism is unclear, reduced apoA-I plasma levels is a consistent finding in patients and animals with complete LCAT deficiency (2, 3, 41, 42).
We attempted to determine cholesterol and CE levels in WT and LCAT-deficient murine CSF using mass spectrometry. Measurements of total cholesterol were reliable and showed that LCAT deficiency did not alter total cholesterol levels in CSF (Fig. 5C). However, we found that CE measurements were below the detection limit of this assay even using pooled CSF samples from N ⩾ 8 mice, suggesting that direct demonstration of the effect of LCAT deficiency on CSF CE levels will require an assay on a scale that is not possible in mice at this time. We also confirm the expected decrease in CSF cholesterol levels in ABCA1−/− mice (Fig. 5C). Of note, in our hands, WT C57Bl/6 mice have an average plasma cholesterol level of approximately 110 mg/dl (43), indicating that the average murine CSF cholesterol concentration (0.23 mg/dl, Fig. 5C) corresponds to not more than 0.2% of that of plasma.
DISCUSSION
In this manuscript, we describe the first in-depth exploration of the expression and function of LCAT in primary cultures of brain-derived cell types. With respect to LCAT production by these cells, our major findings are that astrocytes are the primary source of active LCAT and that astrocyte-derived LCAT is the only enzyme capable of esterifying cholesterol on exogenous or endogenous lipoprotein particles, similar to LCAT's unique enzymatic activity in plasma.
With respect to substrates, we observed that glial ABCA1 and apoE are each required to generate nascent lipoprotein particles that serve as LCAT substrates, again similar to the requirement of peripheral ABCA1 and apoA-I for the LCAT reaction on plasma HDL. Furthermore, the almost complete abolishment of cholesterol esterification in experiments with apoE−/− GCM demonstrate that other apolipoproteins secreted by glia, such as apoJ, do not play a significant role in GCM LCAT-dependent cholesteryl esterification under our in vitro conditions. Which CSF lipoproteins can act as LCAT activators in WT, apoE-deficient, and ABCA1-deficient mice are important questions to address in the future. The low CSF cholesterol concentration (0.2% that of plasma) together with the very low yield of CSF per mouse (8 μl on average) and the suboptimal lipoprotein labeling techniques preclude these analyses in mice at this time.
The regulation of brain LCAT expression and activity now becomes an important question. For example, it is intriguing that neurons express high levels of Lcat mRNA in mice and rhesus monkeys (9). However, LCAT activity in NCM is low, and LCAT protein levels in NCM are below the detection limit of Western blots. These observations suggest that neurons may contain posttranscriptional regulatory mechanisms that suppress translation or secretion of LCAT, at least under the in vitro conditions used here. Additional experiments will be required to test this hypothesis and furthermore to determine whether neurons have the capability to derepress LCAT translation or secretion under physiologic conditions and conditions of acute or chronic stress.
Patients with LCAT deficiency do not exhibit overt neurological dysfunction (2). However, to our knowledge, such patients have not been assessed by detailed neurological examinations, nor are there any indications of whether such patients may be more or less resistant to neuronal injury. The brain RCT system is upregulated after neuronal injury in order to capture lipids that are released by degenerating axons and supply lipids to neurons during regeneration and restoration of synaptic connections (44). It is therefore interesting to speculate on how LCAT's function in brain HDL maturation may influence recovery from neuronal injury. On one hand, the inability to produce mature CE-containing HDL particles may compromise brain RCT and increase the severity of injury. On the other hand, we observed that the levels of apoE-containing lipoproteins in CSF are dramatically increased in LCAT−/− mice, suggesting the possibility that LCAT deficiency may lead to compensatory actions that prime the brain for increased protection from neuronal injury. Additional experiments in animal models, and careful evaluation of patient case histories, will be required to shed light on this question.
Acknowledgments
We are grateful to Dr. John Hill for supplying the BHK and BHK-LCAT cells, Dr. Mary Sorci-Thomas for the murine apoE standard used in the apoE ELISA, Dr. David Holtzman for the apoE ELISA coating antibody, Anja Kerksiek for technical assistance, and all of the members of our research teams for their critical input.
Abbreviations
ACM, astrocyte-conditioned media
apoA-I, apolipoprotein A-I
apoE, apolipoprotein E
BHK, baby hamster kidney cells
CE, cholesterol esters
CNS, central nervous system
CSF, cerebrospinal fluid
EYPC, egg yolk phosphatidylcho1ine
GCM, glia-conditioned media
MER, molar esterification rate of cholesterol
NCM, neuronal conditioned media
qRT-PCR, quantitative RT-PCR
UC, unesterified cholesterol
WT, wild-type
JD is supported by the Arthur and June Willms Postdoctoral fellowship at the University of British Columbia, JP is supported by NIH grants HL54176 and HL49373, JAK is supported by a grant of the European Community (FP6-2005-LIFESCIHEALTH-6; STREP contract number 037631), and CLW is supported by a Canadian Institutes of Health Research New Investigator Award. Operating funding for this work was provided by the Heart and Stroke Foundation of Canada to HP and CLW.
Published, JLR Papers in Press, December 8, 2008.
References
- 1.Jonas A. 1998. Regulation of lecithin cholesterol acyltransferase activity. Prog. Lipid Res. 37 209–234. [DOI] [PubMed] [Google Scholar]
- 2.Glomset, J. A., G. Assmann, E. Gjone, and K. R. Norum. Lecithin:cholesterol acyltransferase deficiency and fish eye disease. In The Metabolic & Molecular Bases of Inherited Disease. C.R. Scriver, A. L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill, New York. 1933–1951.
- 3.Jonas A. 1991. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim. Biophys. Acta. 1084 205–220. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y., F. E. Thorngate, K. H. Weisgraber, D. L. Williams, and J. S. Parks. 2005. Apolipoprotein E is the major physiological activator of lecithin-cholesterol acyltransferase (LCAT) on apolipoprotein B lipoproteins. Biochemistry. 44 1013–1025. [DOI] [PubMed] [Google Scholar]
- 5.McLean J., K. Wion, D. Drayna, C. Fielding, and R. Lawn. 1986. Human lecithin-cholesterol acyltransferase gene: complete gene sequence and sites of expression. Nucleic Acids Res. 14 9397–9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warden C. H., C. A. Langner, J. I. Gordon, B. A. Taylor, J. W. McLean, and A. J. Lusis. 1989. Tissue-specific expression, developmental regulation, and chromosomal mapping of the lecithin: cholesterol acyltransferase gene. Evidence for expression in brain and testes as well as liver. J. Biol. Chem. 264 21573–21581. [PubMed] [Google Scholar]
- 7.Albers J. J., S. M. Marcovina, and R. H. Christenson. 1992. Lecithin cholesterol acyltransferase in human cerebrospinal fluid: reduced level in patients with multiple sclerosis and evidence of direct synthesis in the brain. Int. J. Clin. Lab. Res. 22 169–172. [DOI] [PubMed] [Google Scholar]
- 8.Hengstschlager-Ottnad E., K. Kuchler, and W. J. Schneider. 1995. Chicken lecithin-cholesterol acyltransferase. Molecular characterization reveals unusual structure and expression pattern. J. Biol. Chem. 270 26139–26145. [DOI] [PubMed] [Google Scholar]
- 9.Smith K. M., R. M. Lawn, and J. N. Wilcox. 1990. Cellular localization of apolipoprotein D and lecithin:cholesterol acyltransferase mRNA in rhesus monkey tissues by in situ hybridization. J. Lipid Res. 31 995–1004. [PubMed] [Google Scholar]
- 10.Collet X., O. Francone, F. Besnard, and C. J. Fielding. 1999. Secretion of lecithin:cholesterol acyltransferase by brain neuroglial cell lines. Biochem. Biophys. Res. Commun. 258 73–76. [DOI] [PubMed] [Google Scholar]
- 11.Demeester N., G. Castro, C. Desrumaux, C. De Geitere, J. C. Fruchart, P. Santens, E. Mulleners, S. Engelborghs, P. P. De Deyn, J. Vandekerckhove, et al. 2000. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer's disease. J. Lipid Res. 41 963–974. [PubMed] [Google Scholar]
- 12.Ladu M. J., C. Reardon, L. Van Eldik, A. M. Fagan, G. Bu, D. Holtzmann, and G. S. Getz. 2000. Lipoproteins in the central nervous system. Ann. N. Y. Acad. Sci. 903 167–175. [DOI] [PubMed] [Google Scholar]
- 13.Ladu M. J., S. M. Gilligan, J. R. Lukens, V. G. Cabana, C. A. Reardon, L. J. Van Eldik, and D. A. Holtzman. 1998. Nascent astrocyte particles differ from lipoproteins in CSF. J. Neurochem. 70 2070–2081. [DOI] [PubMed] [Google Scholar]
- 14.Fagan A. M., D. M. Holtzman, G. Munson, T. Mathur, D. Schnieder, L. K. Chang, G. S. Getz, C. A. Reardon, J. Lukens, J. A. Shah, et al. 1999. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/−), and human apoE transgenic mice. J. Biol. Chem. 274 30001–30004. [DOI] [PubMed] [Google Scholar]
- 15.DeMattos R. B., R. P. Brendza, J. E. Heuser, M. Kierson, J. R. Cirrito, J. Fryer, P. M. Sullivan, A. M. Fagan, X. Han, and D. A. Holtzman. 2001. Purification and characterization of astrocyte-secreted apolipoprotein E and J-containing lipoproteins from wild-type and human apoE transgenic mice. Neurochem. Int. 39 415–425. [DOI] [PubMed] [Google Scholar]
- 16.Pitas R. E., J. K. Boyles, S. H. Lee, D. Foss, and R. W. Mahley. 1987. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta. 917 148–161. [DOI] [PubMed] [Google Scholar]
- 17.Roheim P. S., M. Carey, T. Forte, and G. L. Vega. 1979. Apolipoproteins in human cerebrospinal fluid. Proc. Natl. Acad. Sci. USA. 76 4646–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitas R. E., J. K. Boyles, S. H. Lee, D. Hui, and K. H. Weisgraber. 1987. Lipoproteins and their receptors in the central nervous system. J. Biol. Chem. 262 14352–14360. [PubMed] [Google Scholar]
- 19.Koch S., N. Donarski, K. Goetze, M. Kreckel, H. J. Sturernburg, C. Buhmann, and U. Beisiegel. 2001. Characterization of four lipoprotein classes in human cerebrospinal fluid. J. Lipid Res. 42 1143–1151. [PubMed] [Google Scholar]
- 20.Borghini I., F. Barja, D. Pometta, and R. W. James. 1995. Characterization of subpopulations of lipoprotein particles isolated from human cerebrospinal fluid. Biochim. Biophys. Acta. 1255 192–200. [DOI] [PubMed] [Google Scholar]
- 21.Koudinov A. R., N. V. Koudinova, A. Kumar, R. C. Beavis, and J. Ghiso. 1996. Biochemical characterization of Alzheimer's soluble amyloid beta protein in human cerebrospinal fluid: association with high density lipoproteins. Biochem. Biophys. Res. Commun. 223 592–597. [DOI] [PubMed] [Google Scholar]
- 22.Beffert U., M. Danik, P. Krzywkowski, C. Ramassamy, F. Berrada, and J. Poirier. 1998. The neurobiology of apolipoproteins and their receptors in the CNS and Alzheimer's disease. Brain Res. Brain Res. Rev. 27 119–142. [DOI] [PubMed] [Google Scholar]
- 23.Francone O. L., P. V. Subbaiah, A. van Tol, L. Royer, and M. Haghpassand. 2003. Abnormal phospholipid composition impairs HDL biogenesis and maturation in mice lacking Abca1. Biochemistry. 42 8569–8578. [DOI] [PubMed] [Google Scholar]
- 24.Kypreos K. E., and V. I. Zannis. 2007. Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and LCAT. Biochem. J. 403 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch-Reinshagen V., S. Zhou, B. L. Burgess, L. Bernier, S. A. McIsaac, J. Y. Chan, G. H. Tansley, J. S. Cohn, M. R. Hayden, and C. L. Wellington. 2004. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 279 41197–41207. [DOI] [PubMed] [Google Scholar]
- 26.Hill J. S., K. O, X. Wang, S. Paranjape, D. Dimitrijevich, A.G. Lacko, and P.H. Pritchard. 1993. Expression and characterization of recombinant human lecithin:cholesterol acyltransferase. J. Lipid Res. 34 1245–1251. [PubMed] [Google Scholar]
- 27.O, K, J.S. Hill, X. Wang, and P.H. Pritchard. 1993. Recombinant lecithin:cholesterol acyltransferase containing a Thr123→Ile mutation esterifies cholesterol in low density lipoprotein but not in high density lipoprotein. J. Lipid Res. 34: 81–88. [PubMed]
- 28.DeMattos R. B., K. R. Bales, M. Parsadanian, M. A. O'Dell, E. M. Foss, S. M. Paul, and D. M. Holtzman. 2002. Plaque-associated disruption of CSF and plasma amyloid-β (Aβ) equilibrium in a mouse model of Alzheimer's disease. J. Neurochem. 81 229–236. [DOI] [PubMed] [Google Scholar]
- 29.Lee J. Y., R. M. Badeau, A. Mulya, E. Boudyguina, A. K. Gebre, T. L. Smith, and J. S. Parks. 2007. Functional LCAT deficiency in human apolipoprotein A-I transgenic, SR-BI knockout mice. J. Lipid Res. 48 1052–1061. [DOI] [PubMed] [Google Scholar]
- 30.Wahrle S. E., H. Jiang, M. Parsadanian, J. Legleiter, X. Han, J. D. Fryer, T. Kowalewski, and D. M. Holtzman. 2004. ABCA1 is required for normal CNS apoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 279 40987–40993. [DOI] [PubMed] [Google Scholar]
- 31.Lutjohann D., O. Breuer, G. Ahlborg, L. Nennesmo, A. Siden, U. Diczfalusy, and I. Bjorkhem. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 93 9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francone O. L., E. L. Gong, D. S. Ng, C. J. Fielding, and E. M. Rubin. 1995. Expression of human lecithin-cholesterol acyltransferase in transgenic mice. Effect of human apolipoprotein AI and human apolipoprotein all on plasma lipoprotein cholesterol metabolism. J. Clin. Invest. 96 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaisman B. L., H. G. Klein, M. Rouis, A. M. Berard, M. R. Kindt, G. D. Talley, S. M. Meyn, R. F. Hoyt, Jr., S. M. Marcovina, and J. J. Albers. 1995. Overexpression of human lecithin cholesterol acyltransferase leads to hyperalphalipoproteinemia in transgenic mice. J. Biol. Chem. 270 12269–12275. [DOI] [PubMed] [Google Scholar]
- 34.Mehlum A., B. Staels, N. Duverger, A. Tailleux, G. Castro, C. Fievet, G. Luc, J. C. Fruchart, G. Olivecrona, and G. Skretting. 1995. Tissue-specific expression of the human gene for lecithin: cholesterol acyltransferase in transgenic mice alters blood lipids, lipoproteins and lipases towards a less atherogenic profile. Eur. J. Biochem. 230 567–575. [DOI] [PubMed] [Google Scholar]
- 35.Hoeg J. M., B. L. Vaisman, S. J. Demosky, Jr., S. M. Meyn, G. D. Talley, R. F. Hoyt, Jr., S. Feldman, A. M. Berard, N. Sakai, D. Wood, et al. 1996. Lecithin:cholesterol acyltransferase overexpression generates hyperalpha-lipoproteinemia and a nonatherogenic lipoprotein pattern in transgenic rabbits. J. Biol. Chem. 271 4396–4402. [DOI] [PubMed] [Google Scholar]
- 36.Parks J. S., H. Li, A. K. Gebre, T. L. Smith, and N. Maeda. 1995. Effect of apolipoprotein A-I deficiency on lecithin:cholesterol acyltransferase activation in mouse plasma. J. Lipid Res. 36 349–355. [PubMed] [Google Scholar]
- 37.Patel S. C., K. Asotra, Y. C. Patel, W. J. McConathy, R. C. Patel, and S. Suresh. 1995. Astrocytes synthesize and secrete the lipophilic ligand carrier apolipoprotein D. Neuroreport. 6 653–657. [DOI] [PubMed] [Google Scholar]
- 38.Petit-Turcotte C., S. M. Stohl, U. Beffert, J. S. Cohn, N. Aumont, M. Tremblay, D. Dea, L. Yang, J. Poirier, and N. S. Shachter. 2001. Apolipoprotein C–I expression in the brain in Alzheimer's disease. Neurobiol. Dis. 8 953–963. [DOI] [PubMed] [Google Scholar]
- 39.Zorich N., A. Jonas, and H. J. Pownall. 1985. Activation of lecithin cholesterol acyltransferase by human apolipoprotein E in discoidal complexes with lipids. J. Biol. Chem. 260 8831–8837. [PubMed] [Google Scholar]
- 40.Anantharamaiah G. M. 1986. Synthetic peptide analogs of apolipoproteins. Methods Enzymol. 128 627–647. [DOI] [PubMed] [Google Scholar]
- 41.Ng D. S., O. L. Francone, T. M. Forte, J. Zhang, M. Haghpassand, and E. M. Rubin. 1997. Disruption of the murine lecithin:cholesterol acyltransferase gene causes impairment of adrenal lipid delivery and up-regulation of scavenger receptor class B type I. J. Biol. Chem. 272 15777–15781. [DOI] [PubMed] [Google Scholar]
- 42.Asztalos B. F., E. J. Schaefer, K. V. Horvath, S. Yamashita, M. Miller, G. Franceschini, and L. Calabresi. 2007. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J. Lipid Res. 48 592–599. [DOI] [PubMed] [Google Scholar]
- 43.Burgess B. L., S. A. McIsaac, K. E. Naus, J. Y. Chan, G. H. K. Tansley, J. Yang, F. Miao, C. J. D. Ross, M. Van Eck, M. R. Hayden, et al. 2006. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer's Disease mouse models with abundant Aβ in plasma. Neurobiol. Dis. 24 114–127. [DOI] [PubMed] [Google Scholar]
- 44.Poirier J. 1994. Apolipoprotein E in animal models of CNS injury and Alzheimer's disease. Trends Neurosci. 17 525–530. [DOI] [PubMed] [Google Scholar]