Abstract
This report describes studies in hyperlipidemic New Zealand White (NZW) rabbits investigating the impact of the liver-selective thyromimetic T-0681 on lipoprotein metabolism and the development of atherosclerosis. Prolonged treatment with T-0681 increased the hepatic expression of both LDL receptor and scavenger receptor class B, type I without affecting cholesteryl ester transfer protein activity. Upregulation of hepatic lipoprotein receptors was accompanied by a marked decrease of apolipoprotein B-containing lipoproteins, reflected by a 60% reduction of plasma cholesterol and a >70% reduction of plasma triglyceride levels. Most importantly, T-0681 reduced the development of atherosclerosis by 80% in NZW rabbits on high-cholesterol chow. Our data suggest that liver-selective thyromimetics, such as T-0681, may prove to be useful therapeutic agents against the development of atherosclerosis in humans.
Keywords: liver-selective thyromimetic; low density lipoprotein receptor; scavenger receptor class B, type I; potential side effects
Aggressive reduction of LDL-cholesterol (LDL-C) is a cornerstone of preventive cardiovascular care, but additional therapeutic approaches to reduce atherogenesis are still needed.
Thyroid hormones (THs) have been known to influence plasma levels of both LDL-C and HDL-cholesterol (1). TH-mediated lowering of LDL-C was shown to be brought about by increased hepatic expression of the LDL receptor (LDLr) (1–5). More recently, TH were demonstrated to upregulate scavenger receptor class B, type I (SR-BI) in mice, an important component of reverse cholesterol transport (RCT) (6). By their dual action on LDLr and SR-BI, THs could be expected to potently counteract the process of atherosclerosis. However, clinical use of these substances has been hampered mainly by their cardiotoxic effects (7). Recently, TH receptors were shown to occur in different isoforms where the α-variant regulates heart rate, and the β-isoform, abundant in the liver, mediates the effect of TH on lipids (7, 8).
Liver-selective thyromimetics have been shown to be useful lipid-lowering compounds in preclinical studies (6, 9, 10), resulting in phase 1 clinical trials (11). However, to our knowledge, no thyromimetic agent has yet been demonstrated to prevent the atherosclerotic process per se (6, 9, 10). Therefore, we investigated the impact of the liver-selective thyromimetic compound T-0681 (formerly KAT-681) (12) on the development of atherosclerosis using the animal model of hyperlipidemic New Zealand White (NZW) rabbits for the following reasons: First, in contrast to rodents, the lipoprotein pattern of cholesterol-fed rabbits is more similar to that found in humans. Second, rabbits express cholesteryl ester transfer protein (CETP) in their plasma. Finally, rabbits develop atherosclerosis when fed a high-cholesterol diet (13, 14).
MATERIALS AND METHODS
Reagent
The liver-selective thyromimetic T-0681, formerly KAT-681 (12), was kindly provided by Kissei Pharmaceutical Co., Nagano, Japan.
Animal studies
Male NZW rabbits were obtained from Charles River Laboratories, Kisslegg, Germany, and housed under protocols approved by the Austrian Animal Care and Use Committee. The animals were subcutaneously implanted with Alzet osmotic pumps (model 2ML4; Durect Corporation, Cupertino, CA) carrying T-0681 in 1% DMSO/PBS or 1% DMSO/PBS alone as control for the entire duration of the studies. Rabbits were fed a 0.2% cholesterol and 3.5% fat diet or a 2% cholesterol and 5% fat diet (both from Ssniff, Soest, Germany). Food consumption was restricted to 100 g/day/animal. At the end of the studies, animals were fasted 5 h before the collection of blood samples, killed by a threefold overdose of pentobarbital, and organ biopsies snap-frozen.
Lipoprotein parameters
Total cholesterol and triglycerides were measured in whole plasma of each animal employing Roche commercial kits (Mannheim, Germany). Additionally, pooled plasma of each group was subjected to fast-protein liquid chromatography (FPLC) fractionation analysis with two tandem Superose 6 columns (GE Healthcare, Vienna, Austria) as described previously (15). Apolipoprotein measurements were performed by an immunonephelometric assay as described (16).
Measurement of CETP, hepatic lipase, and LPL plasma activity
Plasma activities of CETP, hepatic lipase, and LPL were measured as described (15, 17).
Protein extraction and Western blot analysis
Preparation of hepatic proteins and Western blot analysis were performed as described (15). Rabbit SR-BI was detected using a previously described polyclonal antibody (13). Anti-LDLr antibody was a generous gift from J. Herz (18). Anti-rabbit 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase was from Upstate (Millipore, Billerica, MA). Human SR-BI was detected using anti-CLA-1 from BD (BD Biosciences, Franklin Lakes, NJ) as described (19). The chemoluminescent reaction was performed using Super Signal West Dura Reagent (Pierce, Rockford, IL), and blots were visualized by Fluor-S-Imager using Quantity One V4.1 software (Bio-Rad, Hercules, CA).
RNA isolation, reverse transcription, and real-time PCR
Total RNA was extracted using RNA bee according to the manufacturer's protocol (Tel-Test, Friendswood, TX) and reverse transcribed with the Omniscript-RT kit (Qiagen, Hilden, Germany). Primers for rabbit adenosine 5′-triphosphate-binding cassette transporter 1 (ABCA1) were described previously (20). Primers for rabbit cholesterol 7 α-hydroxylase (CYP7A1) were designed using Primer3 software (21): forward 5′-CTTACAAGGCAAGACGCACA-3′ and reverse 5′-CTGAGATGTGGTCCCTGGTT-3′. Hypoxanthine-guanine phosphoribosyltransferase was used as reference (Applied Biosystems, Foster City, CA). SYBGR real-time PCR reactions were performed on an Mx4000® Multiplex Quantitative PCR system (Stratagene, Amsterdam, The Netherlands).
Dual-energy X-ray absorptiometry
Analysis of body composition was performed by dual-energy X-ray absorptiometry with a Hologic QDR-4500 Discovery device (Hologic, Bedford, MA) and the Hologic QDR-4500 software (22).
Heart rate measurements
The abdomen of anesthetized rabbits was shaved, and warmed acoustic coupling gel was applied. The heart rate was recorded by duplex sonography of aortic blood flow (23) using an Acuson Sequoia 512 device with a 15L8 ultrasound transducer (Siemens Medical Systems, Erlangen, Germany). Pulsed Doppler measurements were acquired in a longitudinal paravertebral view of the abdominal aorta.
Atherosclerosis studies
NZW rabbits were fed the 2% cholesterol and 5% fat diet for 8 weeks, and aortas were prepared and stained with Sudan IV as described (24). Atherosclerotic lesion area was determined as described (24) using Image Pro-Plus 5.1 software (Media Cybernetics, Bethesda, MD).
Other in vivo measurements
Free 3,3′,5′-triiodothyronine (fT3) and free 3,5,3′,5′-tetraiodothyronine (fT4) plasma levels were measured using an immunoassay kit (Roche). Serum alanine-aminotransferase (ALT) and aspartate-aminotransferase (AST) were measured in a Roche MODULAR Hitachi P800/Elecsys E170 apparatus. Thyroid stimulating hormone was not measured in rabbits because no reliable assay existed at the time these studies were performed.
In vitro experiments
HepG2 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultivated by standard procedures. At a confluency of 70%, HepG2 cells were incubated with 50 μg/ml of acetylated LDL (AcLDL) for 24 h and subsequently treated with indicated amounts of T-0681 in serum-free medium for another 24 h. Cellular protein extraction and Western blot analysis were performed as described (19).
Statistics
Results are presented as mean ± SEM. The statistical significance of the differences between the means of the experimental groups was tested by the Student's t-test for unpaired data. A difference was considered statistically significant when P < 0.05.
RESULTS
Effect of T-0681 on lipid metabolism in cholesterol-fed rabbits
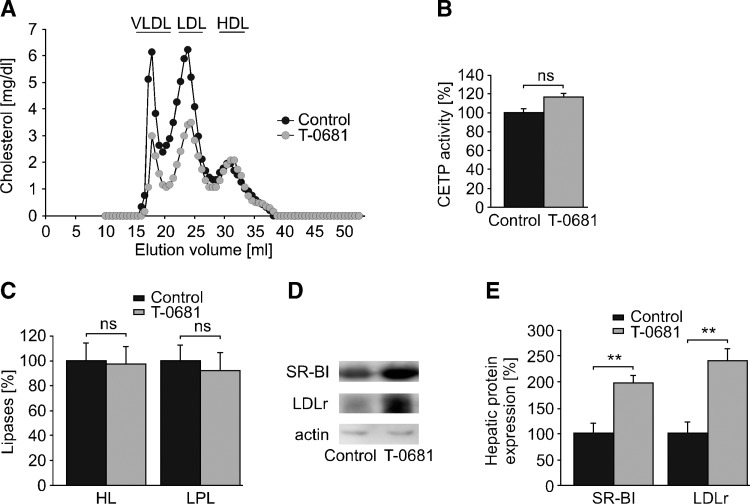
In preliminary dose-titration studies, we observed a marked decrease of plasma cholesterol at 36 nmoles/kg/day T-0681, whereas doses higher than 36 nmoles/kg/day showed no further lipid-lowering effect (data not shown). In the subsequent study (Fig. 1), NZW rabbits were fed a 0.2% cholesterol diet and dosed with 36 nmoles/kg/day T-0681 or a respective placebo control for 4 weeks. T-0681 treatment resulted in a 60% decrease of plasma cholesterol and a 70% decrease of plasma triglycerides (Table 1). FPLC analysis revealed a marked decrease of both atherogenic VLDL-cholesterol (VLDL-C) and LDL-C, with a trend to decreased apolipoprotein B (apoB) levels. No differences were observed for HDL-cholesterol, apolipoprotein A-I levels, CETP activity, or hepatic and lipoprotein lipase (Fig. 1A–C and Table 1). However, we detected a 2-fold increase in SR-BI and a 2.5-fold increase in LDLr protein expression in the liver of T-0681-treated animals (Fig. 1D, E).
Fig. 1.
Impact of T-0681 on lipoprotein metabolism. NZW rabbits on a 0.2% cholesterol diet were treated with T-0681 (36 nmoles/kg/day) or a respective control for 4 weeks. A: At study termination, plasma of each group (n = 5) was pooled and subjected to FPLC analysis. B: Plasma activity of CETP. C: Plasma activity of hepatic lipase (HL) and LPL (n = 5 for all). D: Representative Western blots showing hepatic expression of SR-BI and LDLr. E: The corresponding densitometric quantification (n = 4–5). Actin served as loading control. **P < 0.01 versus corresponding controls; ns, nonsignificant; data presented in percentages are normalized to the respective controls.
TABLE 1.
Plasma lipid parameters and markers of TH status of NZW rabbits fed a 0.2% cholesterol diet for 4 weeks
| Control (n = 5) | T-0681(36 nmol/kg/d) (n = 5) | P | |
|---|---|---|---|
| Plasma cholesterol (mg/dl) | 1393 ± 253 | 554 ± 94 | <0.01 |
| Plasma triglycerides (mg/dl) | 631 ± 94 | 205 ± 45 | <0.01 |
| apoA-I (%) | 100 ± 11 | 110 ± 6 | 0.47 |
| apoB (%) | 100 ± 22 | 50 ± 12 | 0.08 |
| ALT (U/l) | 5 | 9 | Pooled |
| AST (U/l) | 9 | 19 | Pooled |
| Plasma fT3 (pmol/l) | 9.7 ± 0.8 | 10.7 ± 1.5 | 0.54 |
| Plasma fT4 (pmol/l) | 18.9 ± 1.8 | 5.1 ± 1.5 | <0.001 |
| Heart rate (beats/min) | 142 ± 20 | 184 ± 28 | 0.25 |
| Rectal temperature (°C) | 38.9 ± 0.1 | 39.3 ± 0.3 | 0.26 |
Data presented in percentages are normalized to the respective controls. ALT and AST were measured in pooled samples (pooled). apoA-I, apolipoprotein A-I.
Regulation of hepatic SR-BI expression by T-0681
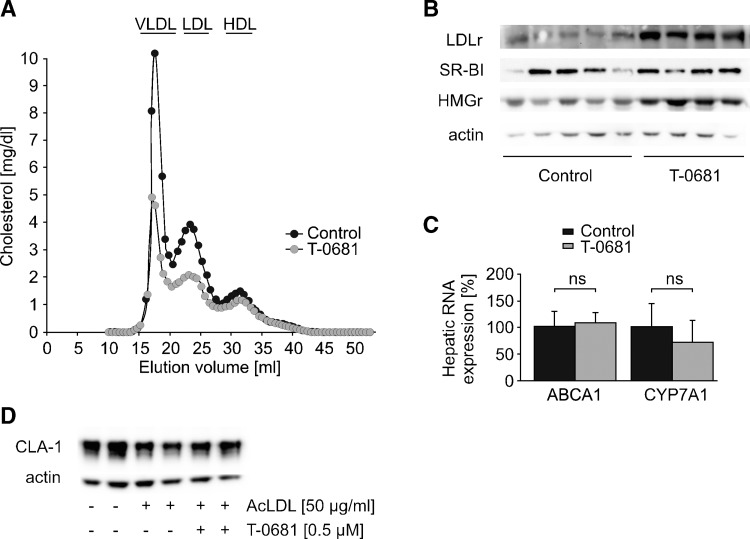
SR-BI is known to be under strong feedback inhibition of cholesterol (25–27). To understand whether the upregulation of hepatic SR-BI was directly mediated by T-0681, or secondary to decreased cholesterol levels, we next performed a short-term study. Again, rabbits were fed a 0.2% cholesterol diet and dosed with 36 nmoles/kg/day T-0681 or a respective control. After 10 days, VLDL-C and LDL-C were markedly decreased (Fig. 2A), and plasma total triacylglycerol was reduced by 70% in T-0681-treated animals (255 ± 29 vs. 93 ± 56 mg/dl, control vs. T-0681, P < 0.05), accompanied by a significantly increased hepatic LDLr protein expression (Fig. 2B). Interestingly, there was no change in SR-BI protein expression (Fig. 2B). Hepatic cholesterol concentrations were similar in both groups (11 ± 1 mg/g liver vs. 13 ± 1 mg/g liver, control vs. T-0681, P = 0.41), suggesting that SR-BI might be inducible only after significant cholesterol depletion of hepatocytes. To test this hypothesis, we subsequently performed in vitro experiments using the human hepatocyte cell line HepG2. SR-BI protein expression was markedly downregulated by incubation with 50 μg/ml AcLDL. This effect could not be reversed by addition of T-0681 (Fig. 2D).
Fig. 2.
Regulation of hepatic proteins involved in cholesterol homeostasis. To differentiate whether T-0681 directly influences certain proteins involved in cholesterol homeostasis or whether regulation of such hepatic proteins is secondary to cellular cholesterol depletion, a short-term in vivo study and in vitro studies were performed. A–C: Short-term study (10 days), in which NZW rabbits on a 0.2% cholesterol diet were treated with T-0681 (36 nmoles/kg/day) or a respective control. At study termination, plasma of each group (n = 4–5) was pooled and subjected to FPLC analysis (A). B: Western blot analysis showing hepatic expression of LDLr, SR-BI, and HMG-CoA reductase (HMGr); each lane shows the protein expression of an individual rabbit. Actin served as loading control. C: Real-time PCR measurement of hepatic ABCA1 and CYP7A1 RNA levels; ns, nonsignificant (n = 4–5). D: Western blot showing human SR-BI (CLA-1) expression in normal HepG2 cells and in HepG2 cells loaded with AcLDL and subsequently incubated with vehicle or T-0681. This representative experiment was run in duplicate, and actin served as loading control.
Finally, we screened the expression of further three hepatic proteins critically involved in whole-body cholesterol homeostasis and recently shown to be influenced by TH and TH analogs, namely, HMG-CoA reductase, ABCA1, and CYP7A1 (6, 10, 28). HMG-CoA reductase protein was markedly increased in the liver of treated rabbits (Fig. 2B), which is in accordance with our observation of increased cholesterol de novo synthesis in TH-treated animals (28), a mechanism apparently not counteracting the lipid-lowering effect of TH and T-0681. We found no change in hepatic mRNA of ABCA1, which is responsible for initial lipidation of nascent HDL particles (Fig. 2C). Contrary to reports on other thyromimetic compounds, such as GC-1 (6) and MB07811 (10), T-0681 did not influence the expression of hepatic CYP7A1, the rate-limiting enzyme of bile acid synthesis (Fig. 2C).
Prevention of atherosclerosis by T-0681
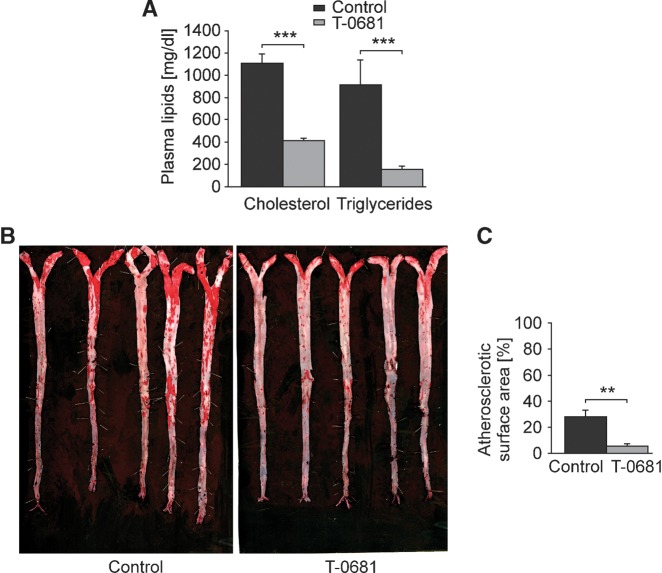
To study the impact of T-0681 on the development of atherosclerosis, NZW rabbits were fed a 2% cholesterol diet over 8 weeks. Concomitant T-0681 treatment did not cause any alterations of body weight (3210 ± 188 g vs. 3151 ± 195 g, control vs. T-0681, P = 0.83) or body fat mass (393 ± 55 g vs. 425 ± 50 g, control vs. T-0681, P = 0.68). However, inspection of the plasma predicted a massive impact of T-0681 on lipid metabolism, as plasma of T-0681 treated animals was noticeably translucent when compared with turbid milky controls (data not shown). Accordingly, plasma cholesterol levels were decreased by 60% and triglyceride levels by >80% (Fig. 3A). Most importantly, aortas of T-0681-treated rabbits exhibited an 80% reduction in atherosclerotic lesion area when compared with controls (Fig. 3B, C).
Fig. 3.
Prevention of atherosclerosis. NZW rabbits on a 2% cholesterol diet were treated with T-0681 (36 nmoles/kg/day) or a respective control for 8 weeks. A: Enzymatic analysis of plasma total cholesterol and triglycerides (n = 5); data presented in percentages are normalized to the respective controls. B, C: Aortas stained with Sudan IV (B) and corresponding analysis of atherosclerotic lesion area calculated as percentage of aortic surface (C). **P < 0.01 versus corresponding controls.
Potential toxicity of the thyromimetic compound
NZW rabbits treated with the 10-fold dose of T-0681, i.e., 360 nmoles/kg/day, exhibited reduced water and food intake and signs of constipation after 7 days. These symptoms also occur in the syndrome of overt hypothyroidism that was indeed ascertained by almost undetectable plasma levels of fT4 and fT3 in the treated rabbits. Moreover, four out of six animals showed increased serum levels of ALT and/or AST. Both overt hypothyroidism and elevation of liver enzymes were reversed by withdrawal of T-0681.
In contrast, at 36 nmoles/kg/day of T-0681, no symptoms of hypothyroidism were observed. At this dosage, T-0681 induced a reduction of T4 comparable to that described for the thyromimetic KB-141 (9), whereas no change of the active metabolite T3 was observed, indicating the existence of an intact thyroid status (Table 1). Moreover, no differences in heart rate or rectal temperature were observed, both sensitive indicators of thyroid dysfunction (Table 1).
Both high-cholesterol diets of 0.2 and 2% led to increased serum levels of ALT and AST. However, treatment with 36 nmoles/kg/day T-0681 did not aggravate steatohepatitis; liver enzymes were slightly increased when compared with controls but did not reach pathological levels (data not shown).
DISCUSSION
We used the model of hyperlipidemic NZW rabbits to investigate the influence of a liver-selective thyromimetic for potential use as a therapeutic antiatherosclerotic agent. In this animal model, 36 nmoles/kg/day T-0681 was found to reduce plasma levels of both VLDL-C and LDL-C, which could be attributed to the induction of hepatic LDLr expression. Whereas LDLr was increased by T-0681 independently from the duration of the study, the expression of SR-BI was found to be inducible only after a prolonged treatment with T-0681, suggesting dependence on whole-body cholesterol concentration, i.e., cellular cholesterol content. This hypothesis is strengthened by the finding that T-0681 failed to induce hepatic SR-BI in apolipoprotein E knockout mice on a Western-type diet, whereas it markedly upregulated SR-BI in the liver of chow-fed Balb/c and C57/B6 mice as soon as after 14 days (I. Tancevski and A. Ritsch, unpublished observations). In addition, in these in vitro studies, cholesterol loading of human hepatocytes led to a strong decrease of SR-BI, which could be minimally enhanced by the subsequent addition of T-0681. However, cholesterol-loaded and T-0681-treated cells did not reach SR-BI expression levels of normal HepG2, suggesting that cholesterol depletion may indeed be a stronger trigger for SR-BI upregulation than direct stimulation by T-0681.
Upregulation of SR-BI by the use of thyromimetics may promote RCT, as suggested by others (6). Employment of a thyromimetic such as T-0681 may lead, either directly and/or indirectly through cholesterol depletion, to hepatic upregulation of SR-BI and may well promote the reverse transport of cholesterol from atherosclerotic plaque macrophages to the liver for fecal excretion. RCT in humans is different from that found in rodents in that cholesterol from the periphery can be transported to the liver either directly via HDL particles or, after transfer to VLDL and LDL mediated by CETP, via apoB-containing lipoproteins (14). Interestingly, CETP transgenic mice require hepatic expression of LDLr to counterbalance accumulation of apoB-containing lipoproteins in plasma (29). In line with this finding, adenoviral overexpression of SR-BI in rabbits, which naturally express CETP in plasma, led to accumulation of VLDL and LDL-C (15). These data suggest hepatic stimulation of SR-BI expression to necessitate a concomitant, appropriate clearance of apoB lipoproteins to guarantee maintenance of RCT in CETP-expressing species like humans. Adequate upregulation of both hepatic SR-BI and LDLr may therefore represent a rational approach to direct excessive cholesterol from the periphery to the liver in humans, and selective thyromimetics may prove useful to promote this mechanism. As a result and most importantly, the use of the thyromimetic T-0681 effectively reduced the development of atherosclerosis in our experimental setting.
It has been known since 1930 that hyperthyroidism is associated with reduced plasma cholesterol levels (30), and since then, many efforts were made to exploit the ability of TH to lower cholesterol. In the late 1960s, a large clinical trial of D-T4 therapy was conducted as part of The Coronary Drug Project by the National Institutes of Health, which aimed to answer the question as to whether cholesterol reduction may prevent coronary heart disease (31). However, the unfavorable recruitment of patients together with the accidental employment of preparations contaminated with the enantiomer of D-T4 resulted in a higher proportion of deaths in the D-T4-treated group, leading to the discontinuation of clinical studies with TH analogs in the 1970s (7, 32). With the introduction into clinical practice of HMG-CoA reductase inhibitors, usually known as “statins,” to lower plasma cholesterol in the mid 1980s, efforts on the development of TH analogs slowed. However, the last 20 years saw the development of liver-selective TH analogs that all were shown to efficiently lower plasma cholesterol. To our knowledge, this is the first report demonstrating such a thyromimetic to prevent the development of atherosclerosis, underlining the importance of further development of this drug class. In summary, these studies suggest liver-selective thyromimetic compounds to have great potential as agents to treat hyperlipidemia and to protect from atherosclerosis and its clinical sequelae.
Acknowledgments
We thank Professor Hermann Dietrich and his assistants Anton Hanni, Bruno Sailer, and Hermann Hoeller from the Central Laboratory Animal Facilities Innsbruck.
Abbreviations
ABCA1, adenosine 5′-triphosphate-binding cassette transporter 1
AcLDL, acetylated low density lipoprotein
ALT, alanine-aminotransferase
apoB, apolipoprotein B
AST, aspartate-aminotransferase
CETP, cholesteryl ester transfer protein
CYP7A1, cholesterol 7 α-hydroxylase
FPLC, fast-protein liquid chromatography
HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA
LDL-C, low density lipoprotein cholesterol
LDLr, low density lipoprotein receptor
NZW, New Zealand White
RCT, reverse cholesterol transport
SR-BI, scavenger receptor class B, type I
T3, 3,3′,5′-triiodo-L-thyronine
T4, 3,5,3′,5′-tetraiodothyronine
TH, thyroid hormone
VLDL-C, very low density lipoprotein cholesterol
This work was supported by the Hans and Blanca Moser Stiftung (No. 61-1994/95 to I.T.), by the Medizinische Forschungsfoerderung Innsbruck (No. 4316 to I.T.), by the Jubiläumsfond der Oesterreichischen Nationalbank (No. 12156 to I.T. and A.R.), and by the Fonds zur Foerderung der wissenschaftlichen Forschung (P19999-B05 to A.R.).
Published, JLR Papers in Press, December 22, 2008.
References
- 1.Duntas L. H. 2002. Thyroid disease and lipids. Thyroid. 12 287–293. [DOI] [PubMed] [Google Scholar]
- 2.Staels B., A. Van Tol, L. Chan, H. Will, G. Verhoeven, and J. Auwerx. 1990. Alterations in thyroid status modulate apolipoprotein, hepatic triglyceride lipase, and low density lipoprotein receptor in rats. Endocrinology. 127 1144–1152. [DOI] [PubMed] [Google Scholar]
- 3.Bakker O., F. Hudig, S. Meijssen, and W. M. Wiersinga. 1998. Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem. Biophys. Res. Commun. 249 517–521. [DOI] [PubMed] [Google Scholar]
- 4.Scarabottolo L., E. Trezzi, P. Roma, and A. L. Catapano. 1986. Experimental hypothyroidism modulates the expression of the low density lipoprotein receptor by the liver. Atherosclerosis. 59 329–333. [DOI] [PubMed] [Google Scholar]
- 5.Packard C. J., J. Shepherd, G. M. Lindsay, A. Gaw, and M. R. Taskinen. 1993. Thyroid replacement therapy and its influence on postheparin plasma lipases and apolipoprotein-B metabolism in hypothyroidism. J. Clin. Endocrinol. Metab. 76 1209–1216. [DOI] [PubMed] [Google Scholar]
- 6.Johansson L., M. Rudling, T. S. Scanlan, T. Lundasen, P. Webb, J. Baxter, B. Angelin, and P. Parini. 2005. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc. Natl. Acad. Sci. USA. 102 10297–10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morkin E., P. Ladenson, S. Goldman, and C. Adamson. 2004. Thyroid hormone analogs for treatment of hypercholesterolemia and heart failure: past, present and future prospects. J. Mol. Cell. Cardiol. 37 1137–1146. [DOI] [PubMed] [Google Scholar]
- 8.Gullberg H., M. Rudling, C. Salto, D. Forrest, B. Angelin, and B. Vennstrom. 2002. Requirement for thyroid hormone receptor beta in T3 regulation of cholesterol metabolism in mice. Mol. Endocrinol. 16 1767–1777. [DOI] [PubMed] [Google Scholar]
- 9.Grover G. J., K. Mellstrom, L. Ye, J. Malm, Y. L. Li, L. G. Bladh, P. G. Sleph, M. A. Smith, R. George, B. Vennstrom, et al. 2003. Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc. Natl. Acad. Sci. USA. 100 10067–10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erion M. D., E. E. Cable, B. R. Ito, H. Jiang, J. M. Fujitaki, P. D. Finn, B. H. Zhang, J. Hou, S. H. Boyer, P. D. van Poelje, et al. 2007. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc. Natl. Acad. Sci. USA. 104 15490–15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkenstam A., J. Kristensen, K. Mellstrom, B. Carlsson, J. Malm, S. Rehnmark, N. Garg, C. M. Andersson, M. Rudling, F. Sjoberg, et al. 2008. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc. Natl. Acad. Sci. USA. 105 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi M., H. Ohnota, T. Tamura, J. Kuroda, N. Shibata, M. Akahane, H. Moriwaki, N. Machida, and K. Mitsumori. 2004. Inhibitory effects of KAT-681, a liver-selective thyromimetic, on development of hepatocellular proliferative lesions in rats induced by 2-acetylaminofluorene and partial hepatectomy after diethylnitrosamine initiation. Arch. Toxicol. 78 460–466. [DOI] [PubMed] [Google Scholar]
- 13.Ritsch A., I. Tancevski, W. Schgoer, C. Pfeifhofer, R. Gander, P. Eller, B. Foeger, U. Stanzl, and J. R. Patsch. 2004. Molecular characterization of rabbit scavenger receptor class B types I and II: portal to central vein gradient of expression in the liver. J. Lipid Res. 45 214–222. [DOI] [PubMed] [Google Scholar]
- 14.Ritsch A., and J. R. Patsch. 2003. Cholesteryl ester transfer protein: gathering momentum as a genetic marker and as drug target. Curr. Opin. Lipidol. 14 173–179. [DOI] [PubMed] [Google Scholar]
- 15.Tancevski I., S. Frank, P. Massoner, U. Stanzl, W. Schgoer, A. Wehinger, C. Fievet, P. Eller, J. R. Patsch, and A. Ritsch. 2005. Increased plasma levels of LDL cholesterol in rabbits after adenoviral overexpression of human scavenger receptor class B type I. J. Mol. Med. 83 927–932. [DOI] [PubMed] [Google Scholar]
- 16.Singaraja R. R., C. Fievet, G. Castro, E. R. James, N. Hennuyer, S. M. Clee, N. Bissada, J. C. Choy, J. C. Fruchart, B. M. McManus, et al. 2002. Increased ABCA1 activity protects against atherosclerosis. J. Clin. Invest. 110 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miesenbock G., B. Holzl, B. Foger, E. Brandstatter, B. Paulweber, F. Sandhofer, and J. R. Patsch. 1993. Heterozygous lipoprotein lipase deficiency due to a missense mutation as the cause of impaired triglyceride tolerance with multiple lipoprotein abnormalities. J. Clin. Invest. 91 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones C., R. E. Hammer, W. P. Li, J. C. Cohen, H. H. Hobbs, and J. Herz. 2003. Normal sorting but defective endocytosis of the low density lipoprotein receptor in mice with autosomal recessive hypercholesterolemia. J. Biol. Chem. 278 29024–29030. [DOI] [PubMed] [Google Scholar]
- 19.Tancevski I., A. Wehinger, W. Schgoer, P. Eller, S. Cuzzocrea, B. Foeger, J. R. Patsch, and A. Ritsch. 2006. Aspirin regulates expression and function of scavenger receptor-BI in macrophages: studies in primary human macrophages and in mice. FASEB J. 20 1328–1335. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S. P., and S. Z. Dong. 2008. Effect of tumor necrosis factor alpha on cholesterol efflux in adipocytes. Clin. Chim. Acta. 389 67–71. [DOI] [PubMed] [Google Scholar]
- 21.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. In Bioinformatics Methods and Protocols: Methods in Molecular Biology. S. Krawetz and S. Misener, editors. Humana Press, Totowa, NJ. 365–386. [DOI] [PubMed]
- 22.Alexandersen P., C. Hassager, and C. Christiansen. 2001. Influence of female and male sex steroids on body composition in the rabbit model. Climacteric. 4 219–227. [PubMed] [Google Scholar]
- 23.Schaefer A., G. P. Meyer, B. Brand, D. Hilfiker-Kleiner, H. Drexler, and G. Klein. 2005. Effects of anesthesia on diastolic function in mice assessed by echocardiography. Echocardiography. 22 665–670. [DOI] [PubMed] [Google Scholar]
- 24.Tangirala R. K., E. M. Rubin, and W. Palinski. 1995. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid Res. 36 2320–2328. [PubMed] [Google Scholar]
- 25.Wang N., W. Weng, J. L. Breslow, and A. R. Tall. 1996. Scavenger receptor BI (SR-BI) is up-regulated in adrenal gland in apolipoprotein A-I and hepatic lipase knock-out mice as a response to depletion of cholesterol stores. In vivo evidence that SR-BI is a functional high density lipoprotein receptor under feedback control. J. Biol. Chem. 271 21001–21004. [DOI] [PubMed] [Google Scholar]
- 26.Han J., A. C. Nicholson, X. Zhou, J. Feng, A. M. Gotto, Jr., and D. P. Hajjar. 2001. Oxidized low density lipoprotein decreases macrophage expression of scavenger receptor B-I. J. Biol. Chem. 276 16567–16572. [DOI] [PubMed] [Google Scholar]
- 27.Niemeier A., W. J. Kovacs, W. Strobl, and H. Stangl. 2008. Atherogenic diet leads to posttranslational down-regulation of murine hepatocyte SR-BI expression. Atherosclerosis. 202 169–175. [DOI] [PubMed] [Google Scholar]
- 28.Tancevski I., A. Wehinger, E. Demetz, P. Eller, K. Duwensee, J. Huber, K. Hochegger, W. Schgoer, C. Fievet, F. Stellaard, et al. 2008. Reduced plasma high-density lipoprotein cholesterol in hyperthyroid mice coincides with decreased hepatic adenosine 5′-triphosphate-binding cassette transporter 1 expression. Endocrinology. 149 3708–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanigawa H., J. T. Billheimer, J. Tohyama, Y. Zhang, G. Rothblat, and D. J. Rader. 2007. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 116 1267–1273. [DOI] [PubMed] [Google Scholar]
- 30.Mason R. L., H. M. Hunt, and L. Hurxthal. 1930. Blood cholesterol values in hyperthyroidism and hypothyroidism. N. Engl. J. Med. 203 1273–1278. [Google Scholar]
- 31.The Coronary Drug Project Group. 1972. The coronary drug project. Findings leading to further modifications of its protocol with respect to dextrothyroxine. The coronary drug project research group. JAMA. 220: 996–1008. [PubMed]
- 32.Moreno M., P. de Lange, A. Lombardi, E. Silvestri, A. Lanni, and F. Goglia. 2008. Metabolic effects of thyroid hormone derivatives. Thyroid. 18 239–253. [DOI] [PubMed] [Google Scholar]