Abstract
Quantitative trait mapping identified a locus colocalizing with L-Fabp, encoding liver fatty acid binding protein, as a positional candidate for murine gallstone susceptibility. When fed a lithogenic diet (LD) for 2 weeks, L-Fabp−/− mice became hypercholesterolemic with increased hepatic VLDL cholesterol secretion. Seventy-five percent of L-Fabp−/− mice developed solid gallstones compared with 6% of wild-type mice with an increased gallstone score (3.29 versus 0.62, respectively; P < 0.01). Hepatic free cholesterol content, biliary cholesterol secretion, and the cholesterol saturation index of hepatic bile were increased in LD-fed L-Fabp−/− mice. Chow-fed L-Fabp−/− mice demonstrated increased fecal bile acid (BA) excretion accompanied by decreased ileal Asbt expression. By contrast, there was an increased BA pool and decreased fecal BA excretion in LD-fed L-Fabp−/− mice, associated with increased proximal intestinal Asbt mRNA expression, suggesting that intestinal BA absorption was enhanced in LD-fed L-Fabp−/− mice. The increase in biliary BA secretion and enterohepatic pool size in LD-fed L-Fabp−/− mice was accompanied by downregulation of Cyp7a1 mRNA and increased intestinal mRNA abundance of Fgf-15, Fxr, and Fabp6. These findings suggest that changes in hepatic cholesterol metabolism and biliary lipid secretion as well as changes in enterohepatic BA metabolism increase gallstone susceptibility in LD fed L-Fabp−/− mice.
Keywords: genetic susceptibility, quantitative trait locus, bile acid metabolism, enterohepatic lipid flux, hepatic steatosis, cholesterol absorption
Gallstone disease is a major health problem in western society, and its attendant complications and comorbidities impose a substantial financial burden on the health care economy (1). Among the factors that predispose to cholesterol cholelithiasis, considerable attention has focused on environmental modifiers, such as obesity, because even in populations at increased genetic risk there appears to be a strong link between gallstone susceptibility and body habitus that may be further modified in a gender-specific manner (2). However, despite recent advances in the mechanisms that link hepatic insulin resistance and gallstone susceptibility (3), there remain many unanswered questions concerning the pathways by which alterations in hepatic lipid metabolism result in biliary cholesterol supersaturation, leading to cholesterol monohydrate crystal formation and eventually the emergence of gallstones (4).
Our understanding of the genetic factors that predispose to gallstone formation has been advanced through study of inbred murine crosses in which susceptibility loci have been mapped using diet-induced gallstone formation as a quantitative trait. Using this approach, >23 quantitative trait loci have been mapped, allowing formal evaluation of potential candidate genes [reviewed in (2)]. Among these loci, Liver fatty acid binding protein (L-Fabp) emerged as a positional candidate from a quantitative trait loci near D6Mit123 on chromosome 6 (71.5 Mb, 30 centimorgans) (2, 5). L-Fabp is an abundant cytosolic lipid binding protein expressed in mammalian enterocytes and hepatocytes with a flexible lipid binding pocket that accommodates FAs and other hydrophobic ligands, including cholesterol, acyl-CoA, and bile acids (BAs) (6, 7). More recent studies in L-Fabp−/− mice imply an important role for this gene in modulating FA trafficking and decreasing hepatic steatosis following fasting (8) and in attenuating diet-induced hepatic steatosis and obesity in response to prolonged high-fat feeding (9).
A role for L-Fabp was proposed in modifying the response to cholesterol feeding based on a striking gender-specific phenotype that included enhanced obesity and hepatic steatosis in female L-Fabp−/− mice (10). Those findings, however, are at odds with more recent studies in which cholesterol feeding alone (in the absence of high fat feeding) produced generally subtle alterations in hepatic lipid metabolism and no effect on weight gain in L-Fabp−/− mice in a congenic C57BL/6 background (11). However, other studies demonstrated an increase in BA pool size in male L-Fabp−/− mice fed a zero-cholesterol, low-fat diet and a >2-fold increase in gallbladder cholesterol content along with a 4-fold decrease in BA pool size following dietary cholesterol supplementation (12). Differences in the obesity phenotypes notwithstanding, these studies together suggest that gender-specific effects play an important role in modulating the response to cholesterol feeding in L-Fabp−/− mice but left unanswered whether an informative phenotype might emerge when these animals were challenged with a diet known to produce cholesterol cholelithiasis (13).
The current studies were undertaken to examine the importance of L-Fabp as a genetic modifier of diet-induced cholesterol gallstone formation in congenic lines of mice. In particular, we were intrigued by the possibility that the protection against high-fat-induced obesity and hepatic steatosis (9) might protect L-Fabp−/− mice from cholesterol gallstone disease. However, our findings revealed that male L-Fabp−/− mice manifest a striking increase in gallstone susceptibility when fed a lithogenic diet, a phenotype coupled to changes in cholesterol metabolism and to alterations in both hepatic and intestinal BA metabolism.
MATERIALS AND METHODS
Animals and diets
Male L-Fabp−/− mice (backcrossed onto a C57BL/6 background) and C57BL/6 wild-type mice (Jackson Laboratories, Bar Harbor, ME) were maintained on a 12 h light-dark cycle, in a full-barrier facility. Eight- to ten-week-old mice were fed either a standard rodent chow (PicoLab Rodent Diet 20) or a lithogenic diet (LD) (Research Diet 960393; 18.8% fat, 1.2% cholesterol, and 0.5% cholic acid) for 2 to 4 weeks, as indicated. All animal protocols were approved by the Washington University Animal Studies Committee and conformed to criteria outlined in the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.”
Hepatic lipid and serum analyses
Animals were sacrificed after a 4 h fast. Liver and serum were collected and frozen at −80°C until analyzed. Hepatic triglyceride (TG), cholesterol, FFA, phospholipids, and BAs were assayed enzymatically with Wako reagent kits (Neuss, Germany): L-Type Triglyceride H kit (Cat. No. 993-37592, 993-37492), cholesterol E kit (Cat. No.439-17501), Free cholesterol E kit (Cat. No. 435-35801), HR series NRFA-HR2 kit (Cat. No. 995-34691, 995-34791), phospholipid B kit (Cat. No. 990-54009), and total bile acids kit (Cat. No. 431-15001). Serum glucose and alanine aminotransferase were analyzed by Wako Autokit Glucose (Cat. No.439-90901) and Teco ALT set (Cat. No. A526-120). Lipoprotein distribution was examined by fast-protein liquid chromatography (FPLC) (14).
Gallbladder bile and gallstone analysis
Mice were fasted for 4 h and anesthetized, and gallbladders ligated and punctured at the fundus for collection of gallbladder bile. Bile samples were collected and immediately analyzed by polarizing light microscopy using a visual scale (high power field = 400× magnification) with the following criteria: 0 = absence of cholesterol monohydrate crystals (ChMCs), 1 = small number of ChMCs (<3/high-power field); 2 = many ChMCs (≥3/high power field); 3 = aggregated ChMCs; and 4 = presence of “sandy” light-translucent stones or “solid” light-opaque stones (13).
Biliary lipid and BA species determination
An external bile fistula was established surgically via the gallbladder fundus. Bile samples collected during the first 15 min were discarded, and bile samples collected for another 60 min were used for further analysis. Hepatic bile volume was determined gravimetrically assuming a density of 1 g·ml−1. Biliary phospholipids, cholesterol, and total BA content were determined enzymatically. Cholesterol saturation indices in hepatic bile were calculated using published parameters (15). Individual bile salt concentrations were measured via high-pressure liquid chromatography (16). BA standards were purchased from Steraloids (Newport, RI) or Calbiochem (Madison, WI). The hydrophobic index of hepatic bile sample was calculated according to published methods (17).
BA pool size and fecal BA excretion determination
BA pool size was determined as the sum total BA content of the entire small intestine, gallbladder, and liver, which were homogenized and extracted together in ethanol with [24-14C]taurocholic acid (0.025 μCi) added as an internal recovery standard (18). Total BA mass was determined enzymatically. In addition, fecal BA excretion was determined from stool quantitatively collected from individually housed mice for 72 h. For each determination, 200 mg triplicate aliquots of dried feces were extracted into ethanol as described (18, 19).
Cholesterol and fat absorption
Cholesterol absorption was measured by a fecal dual-isotope ratio method as described previously (14, 20, 21). Mice were housed individually in metabolic cages and food intake and fecal output measured daily. Fecal fat excretion (percentage) was determined gravimetrically after lipid extraction (9).
Gene expression analysis
For microarray analysis, total liver RNA was pooled from groups of LD-fed (2 weeks) wild-type or L-Fabp−/− mice (n = 4 animals per group). Pooled RNA was hybridized to an Agilent Technologies Mouse Whole Genome microarray (G4122A; Agilent, Palo Alto, CA). Microarray results were confirmed by real-time quantitative RT-PCR (qRT-PCR) using individual cDNA from four or five animals per group. qRT-PCR assays were performed in triplicate on an ABI Prism 7000 Sequence Detection System using SYBR Green PCR Master Mix (Applied Biosystems) and primer pairs designed by Primer Express software (Applied Biosystems) (primer sequences are available upon request). Relative mRNA abundance is expressed as fold change compared with mRNA levels in wild-type mice consuming the same diet, normalized to 18S rRNA. Western blotting was performed using anti-L-Fabp antisera (1:2,000) as previously detailed (8). Asbt expression was determined on membrane fractions (50 μg protein) prepared from scraped distal intestinal mucosa as detailed using anti-Asbt IgG (1:1,000) (22).
Statistical analysis
Statistical significance was determined with an unpaired, two-tailed Student's t-test. Data are expressed as the mean ± SE unless otherwise noted.
RESULTS
Metabolic alterations following LD feeding in wild-type and L-Fabp−/− mice
Both genotypes exhibited weight loss following consumption of the LD, with L-Fabp−/− mice demonstrating significantly greater weight loss (Table 1). Serum cholesterol was significantly increased in L-Fabp−/− mice, with a shift into less dense lipoprotein fractions revealed by FPLC (Fig. 1A). This pattern was augmented after 4 weeks of LD feeding (Fig. 1B) and was associated with increased hepatic secretion of cholesterol-rich lipoproteins in L-Fabp−/− mice (Fig. 1C), although VLDL-TG secretion was comparable in L-Fabp−/− and wild-type mice (data not shown). In addition, L-Fabp−/− mice manifested decreased hepatic TG content and an increase in hepatic free cholesterol and phospholipid content (Table 1). L-Fabp expression in wild-type mice showed a tissue-specific divergence in response to LD feeding. There was an ∼3-fold decrease in hepatic L-Fabp mRNA and protein expression with an ∼3-fold increase in intestinal L-Fabp expression (Fig. 1D). These findings collectively point to a range of adaptive alterations in hepatic and serum cholesterol metabolism following 2 weeks of LD feeding in L-Fabp−/− mice, whose functional consequences were further explored.
TABLE 1.
Body weight, liver weight, food consumption, fat absorption, and serum and hepatic lipid profile of LD-fed wild-type and L-Fabp−/− mice
| Parameters | Wild Type | L-Fabp−/− | P |
|---|---|---|---|
| Body weight (g) | 22.7 ± 0.3 (22) | 20.1 ± 0.5 (21) | ** |
| Weight gain (g) | −0.9 ± 0.2 (22) | −1.9 ± 0.3 (21) | ** |
| Liver weight (g) | 1.4 ± 0.04 (22) | 1.3 ± 0.06 (21) | * |
| Liver weight (% body weight) | 6.3 ± 0.2 (22) | 6.3 ± 0.2 (21) | NS |
| Fat absorption (%) | 93.9 ± 0.3 (4) | 93.6 ± 0.4 (4) | NS |
| Feed efficiency | −23.7 ± 9.2 (4) | −50.6 ± 8.6 (4) | NS |
| Serum cholesterol (mg/dl) | 167.2 ± 8.0 (13) | 241.3 ± 29.5 (13) | * |
| Serum TG (mg/dl) | 10.1 ± 1.3 (8) | 12.2 ± 2.2 (8) | NS |
| Serum phospholipids (mg/dl) | 204.3 ± 7.8 (8) | 208.2 ± 13 (8) | NS |
| Serum FFAs (mmol/l) | 0.24 ± 0.04 (8) | 0.26 ± 0.09 (8) | NS |
| Serum glucose (mg/dl) | 174.5 ± 8.0 (13) | 128.7 ± 8.3 (13) | ** |
| Serum BA (μmol/L) | 15.9 ± 2.0 (8) | 33.8 ± 2.0 (8) | * |
| Serum ALT (IU/L) | 74.4 ± 16.4 (13) | 117.7 ± 13.0 (13) | * |
| Hepatic total cholesterol (μg/mg protein) | 210.2 ± 10.3 (8) | 232.7 ± 20.3 (8) | NS |
| Hepatic free cholesterol (μmol/mg protein) | 31.96 ± 1.73 (8) | 45.20 ± 3.1 (8) | * |
| Hepatic cholesterol ester (μmol/mg protein) | 178.2 ± 11.1 (8) | 187.5 ± 21.4 (8) | NS |
| Hepatic TG (μg/mg protein) | 120.7 ± 10.8 (8) | 83.9 ± 6.6 (8) | ** |
| Hepatic phospholipid (μg/mg protein) | 98.5 ± 1.7 (8) | 123.4 ± 4.9 (8) | ** |
| Hepatic FFA (nmol/mg protein) | 33.9 ± 1.6 (8) | 37.0 ± 3.7 (8) | NS |
Values represent the mean ± SE (n). ALT, alanine aminotransferase; NS, not significant. Feed efficiency is milligrams of weight change per gram of food consumed. *P < 0.05; **P < 0.01.
Fig. 1.
Serum cholesterol distribution and VLDL cholesterol secretion in L-Fabp−/−and wild-type mice. A and B: Increased serum cholesterol (predominantly non-HDL cholesterol) in L-Fabp−/− mice fed LD either for 2 weeks (A) or 4 weeks (B) compared with wild-type controls. Serum (200 μl) from four mice per genotype was separated by FPLC with two Superose 6 columns connected in series (Pharmacia Biotech). The 40 × 0.8-ml fractions were collected on ice and cholesterol concentrations measured enzymatically. The figure insert indicates serum total cholesterol content (mg/dl). C: Hepatic VLDL cholesterol secretion in L-Fabp−/− mice and wild-type controls. Mice of both genotypes were fed a LD for 2 weeks and fasted for 4 h. Pluronic F127 (1 g/kg body weight; Invitrogen) (36) was intravenously injected and blood collected at 0, 30, 60, 90, 120, and 180 min and 18 h. Serum cholesterol was then measured enzymatically. D: LD feeding downregulates hepatic L-Fabp expression and upregulates intestinal L-Fabp expression in wild-type mice at both mRNA and protein level. mRNA abundance of L-Fabp was measured by qRT-PCR. Bar graph (left) is mean ± SE, data from four mice per group. L-Fabp protein (right) analyzed by Western blotting. * Indicates P < 0.05; ** indicates P < 0.01.
Increased gallstone susceptibility in LD-fed L-Fabp−/− mice
L-Fabp−/− mice demonstrated increased numbers of aggregated cholesterol monohydrate crystals in gallbladder bile at 2 weeks of LD feeding (Fig. 2A) and a naked-eye appearance of a thickened, opaque gallbladder that contrasted with the findings in wild-type mice (Fig. 2B). The gross appearance of a thickened gallbladder was confirmed by histological examination (Fig. 2C). Gallstones were found in 6/8 L-Fabp−/− mice compared with 1/17 wild-type mice, and the gallstone score (Fig. 2D) was correspondingly higher (3.29 ± 0.53 versus 0.62 ± 0.28 in L-Fabp−/− and wild-type mice, respectively; P < 0.01), demonstrating conclusively that gallstone susceptibility is increased in L-Fabp−/− mice.
Fig. 2.
Biliary cholesterol crystallization, gallbladder inflammation, and gallstone score in LD-fed mice. Wild-type and L-Fabp−/− mice were fed a LD for 2 weeks. A: Fresh gallbladder bile samples were collected and analyzed by polarizing microscopy. A few single ChMCs were present in wild-type gallbladder bile, while aggregated ChMCs were observed in gallbladder bile from L-Fabp−/− mice. B: Gallbladders of wild-type mice appeared transparent, while gallbladders from L-Fabp−/− mice appeared opaque. C: Histological examination of gallbladder mucosal epithelium by hematoxylin and eosin staining. Gallbladder walls of L-Fabp−/− mice were thicker with increased stromal granulocyte infiltration (magnification: 400×). D: Gallstone score (left) and gallbladder volume (right) as described in Materials and Methods. There is an increase in the gallstone score and a trend toward increased gallbladder volume in L-Fabp−/− mice. Data derived from eight animals per genotype; ** indicates P < 0.01.
Biliary lipid secretion is altered in LD-fed but not chow-fed L-Fabp−/− mice
Biliary lipid secretion was unaltered in chow-fed L-Fabp−/− mice compared with wild-type controls (Fig. 3A). However, after LD feeding, there was a 2-fold increase in biliary cholesterol secretion in L-Fabp−/− mice (0.27 ± 0.04) versus the wild type (0.13 ± 0.01 μmol/min/kg; P < 0.05) and a significant increase in secretion of phospholipid as well as a trend to increased BA secretion (Fig. 3A). The net effects of these alterations in biliary lipid secretion increased the cholesterol saturation indices of bile (Fig. 3B). There were no changes in the BA species distribution or hydrophobicity index in chow-fed L-Fabp−/− mice (Fig. 4A, C), but the proportion of taurocholate increased in LD-fed L-Fabp−/− mice, which tended to increase the hydrophobicity index (Fig. 4B, D). We also examined the profile of biliary lipid secretion in mice of both genotypes following dietary cholesterol supplementation alone, as other workers reported a 2-fold increase in gallbladder cholesterol and a 4-fold decrease in BA content in cholesterol-fed male L-Fabp−/− mice (12). Our findings show increased biliary cholesterol secretion following dietary cholesterol supplementation (see supplementary Tables I and II) but revealed no differences between the genotypes. In addition and again in contrast with those earlier results, our findings demonstrated no effect of cholesterol supplementation alone on BA secretion in either genotype (see Supplementary Table I). These findings demonstrate that LD feeding (but not cholesterol supplementation alone) in male L-Fabp−/− mice alters hepatobiliary cholesterol and BA metabolism and results in increased gallstone formation.
Fig. 3.
Biliary lipid secretion and hepatic bile cholesterol saturation indices on chow or LD. A: Hepatic bile was collected for 60 min and biliary lipid assayed enzymatically. Biliary cholesterol and phospholipid secretion (μmol/min/kg body weight) were similar on chow but increased on LD in L-Fabp−/− mice compared with wild-type controls. There were no differences in BA secretion on either diet between the two genotypes. B: Hepatic biliary cholesterol saturation indices were calculated as described in Materials and Methods. L-Fabp−/− mice demonstrated increased cholesterol saturation indices on LD (right) but not on chow (left) compared with wild-type mice. Bar graphs indicate the mean ± SE. Data were derived from five mice per genotype on chow diet and eight mice per genotype on LD; * indicates P < 0.05.
Fig. 4.
BA species distribution and hydrophobicity indices on chow or LD. Individual BA species were examined in hepatic bile from mice fed chow (A) (n = 5 per genotype) or LD (B) (n = 8 per genotype) by HPLC. Bar graphs represent the mean ± SE. BA profiles were similar on chow, but altered on LD, specifically with increased taurocholate, decreased glycocholate, and taurochenodeoxycholate in L-Fabp−/− mice compared with the wild type. TβMC, tauro-β-muricholate; TUDC, tauroursodeoxycholate; TC, taurocholate; GC, glycocholate; TCDC, taurochenodeoxycholate; TDC, taurodeoxycholate. Bile salt hydrophobicity indices (HI) were comparable in chow-fed animals (C) but showed a trend to increase in L-Fabp−/− mice versus the wild type when fed the LD (D); * indicates P < 0.05.
We also examined the possibility that changes in intestinal cholesterol absorption might contribute to the alterations noted in biliary cholesterol secretion in LD-fed L-Fabp−/− mice. Cholesterol absorption was significantly decreased in chow-fed L-Fabp−/− mice compared with wild-type controls (42 ± 4% versus 55 ± 2%; P < 0.05), but there were no differences observed in LD-fed mice between the genotypes (Fig. 5). In addition, there were no differences in mRNA expression of Npc1L1, Cd36, Fatp4, Abcg5/8 or Srb-1, genes implicated in regulating cholesterol absorption (data not shown).
Fig. 5.
Decreased cholesterol absorption in chow-fed L-Fabp−/− mice, but comparable cholesterol absorption in mice fed LD for 4 or 8 weeks. Cholesterol absorption was measured by the fecal dual isotope ratio method. Bar graphs are mean ± SE, with data from four mice per genotype on chow, nine mice per genotype on LD for 4 weeks, and four wild-type mice and six L-Fabp−/− mice on LD for 8 weeks; * indicates P < 0.05.
Changes in gene expression associated with LD feeding in L-Fabp−/− mice
To pursue the mechanisms by which LD feeding results in such a striking phenotype in L-Fabp−/− mice, we turned to microarray analysis of hepatic RNA. Among the most informative changes (Table 2), we discovered increased mRNA expression of the canalicular phospholipid transporter Abcb4, which is likely associated with the increased biliary phospholipid secretion noted above. Although not tested directly, the increased free cholesterol content may reflect increased lipogenesis as a result of increased mRNA abundance of fatty acid synthase, HMG-CoA reductase, and Srebp-1 (Table 2), coupled with decreased mRNA abundance of acyl-CoA cholesterol acyltransferase 1. However, there was no change in the cholesterol transporter Abcg5/g8 mRNA; thus, the mechanisms accounting for increased biliary cholesterol secretion in LD-fed L-Fabp−/− mice remain incompletely resolved. In addition, there was a significant reduction in Cyp7a1 mRNA in LD-fed L-Fabp−/− mice (Table 2), an observation we will return to below.
TABLE 2.
Liver microarray data: LD
| Gene Name | Gene Description | Accession Number | Ratioa | qRT-PCR | P |
|---|---|---|---|---|---|
| BA synthesis, transport, and metabolism | |||||
| Cyp7a1 | Cholesterol 7α-hydroxylase | NM_007824 | 0.21 | 0.43 | * |
| Cyp27a1 | Sterol 27-hydroxylase | NM_024264 | 0.97 | 1.03 | |
| Cyp8b1 | Sterol 12α-hydroxylase | NM_010012 | 0.27 | 0.14 | |
| Bsep/Abcb11 | Bile salt export pump | NM_021022 | 1.80 | 1.01 | |
| Ntcp | Na+-taurocholate cotransporting protein | NM_011387 | 1.20 | 0.85 | |
| Oatp4 | Organic anion transporter, 1b2 | NM_178235 | 1.60 | 1.14 | |
| Oatp2 | Organic anion transporter, 1a4 | NM_030687 | 2.00 | 1.51 | * |
| Ostb | Organic solute transporter β | NM_178933 | 2.00 | 2.49 | ** |
| Mdr3/Abcb4 | ATP binding cassette, B4 | NM_008830 | 2.80 | 1.41 | * |
| Mrp2/Abcc2 | ATP binding cassette, C2 | NM_013806 | 1.30 | 0.92 | |
| Cyp2b10 | Cytochrome P450, 2b10 | NM_009998 | 0.82 | ||
| Cyp3a11 | Cytochrome P450, 3a11 | NM_007818 | 3.00 | ||
| Cholesterol synthesis, esterification, and transport | |||||
| Hmgcr | HMG-CoA reductase | NM_008255 | 1.60 | 1.53 | * |
| Abca1 | ATP binding cassette, A1 | NM_013454 | 1.80 | 1.28 | * |
| Abcg5 | ATP binding cassette, G5 | NM_031884 | 1.90 | 1.07 | |
| Abcg8 | ATP binding cassette, G8 | NM_026180 | 1.20 | 1.31 | |
| Abcg1 | ATP binding cassette, G1 | NM_009593 | 0.70 | 0.50 | ** |
| Acat1 | Acetyl-CoA acetyltransferase 1 | NM_009230 | 0.43 | 0.29 | ** |
| Acat2 | Acetyl-CoA acetyltransferase 2 | NM_146064 | 1.25 | 1.42 | |
| Cd36 | FA translocase | NM_007643 | 0.85 | 0.84 | |
| Srb1 | Scavenger receptor class, B1 | NM_016741 | 1.20 | 0.83 | |
| Scp2 | Sterol carrier protein 2, liver | NM_011327 | 1.29 | 0.93 | |
| CAV1 | Caveolin 1 | NM_007616 | 1.00 | ||
| NPC1 | Niemann pick type C1 | NM_008720 | 1.27 | ||
| StarD4 | StAR-related lipid transfer domain containing 4 | NM_133774 | 2.00 | 1.48 | |
| Transcription factors or nuclear receptors | |||||
| Lxrα | Liver X receptor | NM_013839 | 1.00 | 0.77 | |
| Fxr | Farnesoid X receptor | NM_009108 | 1.50 | 0.95 | |
| Shp | Small heterodimer partner | NM_011850 | 1.60 | 1.28 | |
| Pparα | Peroxisome proliferator activated receptor α | NM_011144 | 1.60 | 1.54 | * |
| Srebp1 | Sterol regulatory element binding protein 1 | NM_011480 | 2.50 | 2.36 | * |
| Srebp2 | Sterol regulatory element binding protein 2 | NM_033218 | 0.90 | 0.86 | |
| Rxr | Retinoid X receptor, α | NM_011305 | 1.60 | 1.14 | |
| FgfR4 | Fibroblast growth factor receptor 4 | AF127140 | 1.10 | 1.11 | |
| Hnf4a | Hepatic nuclear factor 4, α | NM_008261 | 1.50 | 1.03 | |
| FA synthesis and oxidation | |||||
| Fasn | Fatty acid synthase | NM_007988 | 5.40 | 3.80 | * |
| Ucp2 | Uncoupling protein 2 | NM_011671 | 0.83 | ||
| Scd1 | Stearoyl-CoA desaturase 1 | NM_009127 | 3.40 | 0.93 | |
| Scd2 | Stearoyl-CoA desaturase 2 | NM_009128 | 0.80 | ||
| Acaa1 | Acetyl-CoAacyltransferase 1 | NM_130864 | 2.19 | 5.62 | * |
| Acadl | Acyl-CoA dehydrogenase, long chain | NM_007381 | 2.13 | 1.61 | |
| Acot1 | Acyl-CoA thioesterase 1 | NM_012006 | 9.33 | 4.85 | ** |
| Lipoprotein metabolism | |||||
| apoB | Apolipoprotein B | XM_137955 | 1.13 | ||
| apoA4 | Apolipoprotein A-IV | NM_007468 | 2.00 | 1.94 | * |
| apoC3 | Apolipoprotein C-III | NM_023114 | 1.80 | ||
| apoC2 | Apolipoprotein C-II | NM_009695 | 1.90 | ||
| Ldlr | LDL receptor | NM_010700 | 1.40 | 1.70 | |
| Vldlr | VLDL receptor | NM_013703 | 1.60 | 1.30 | |
| ApoA1 | Apolipoprotein A | NM_009692 | 1.70 | 1.12 | |
| apoE | Apolipoprotein E | NM_009696 | 1.70 | 0.92 | |
| apoA5 | Apolipoprotein A-V | NM_080434 | 1.30 | 1.93 | |
| Mttp | Microsomal TG transfer protein | NM_008624 | 1.40 | 2.06 | ** |
| others | |||||
| Cyp4a14 | Cytochrome P450, 4A14 | NM_007822 | 34.40 | 26.55 | ** |
Relative expression of some genes was confirmed by real time qRT-PCR by individual samples (four mice per group). qRT-PCR results were expressed as a fold change normalized to the wild-type control. *P < 0.05; **P < 0.01.
Microarray data are expressed as an expression ratio of L-Fabp−/− versus the wild type.
Alterations in enterohepatic bile salt metabolism in L-Fabp−/− mice
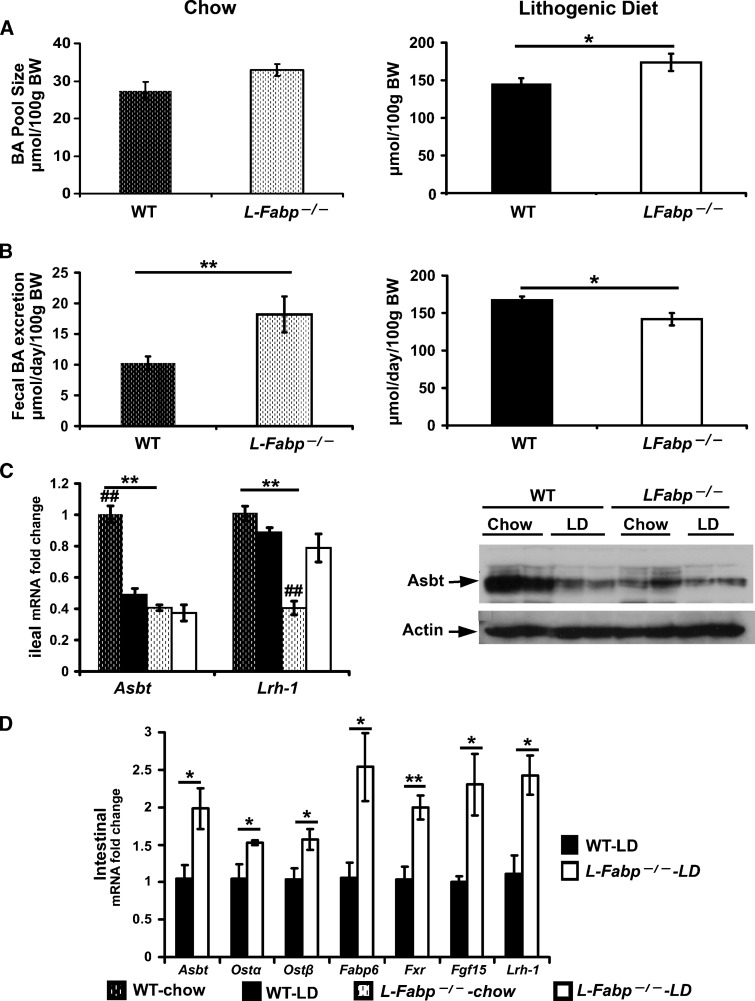
Previous studies suggested that (low-fat, zero-cholesterol) chow-fed male L-Fabp−/− mice manifest alterations in biliary lipid metabolism, specifically a 1.5-fold increase in BA pool size (12). Our analyses were again at odds with these findings, demonstrating no significant difference in BA pool size in chow-fed mice but a significant increase in fecal BA excretion (Fig. 6A, B). These findings raised the possibility that ileal absorption of BA might be impaired in chow-fed L-Fabp−/− mice. To address this possibility, we examined the expression of Asbt in ileal membranes prepared from wild-type and L-Fabp−/− mice. As shown in Fig. 6C, our findings revealed a significant decrease in ileal Asbt mRNA and protein expression in chow-fed L-Fabp−/− mice and decreased mRNA abundance of the transcription factor Lrh-1. These findings demonstrate that ileal Asbt expression and enterohepatic BA metabolism are altered in chow-fed L-Fabp−/− mice, a finding amplified below.
Fig. 6.
BA pool size, fecal BA excretion, and expression of intestinal genes related to BA signaling pathways. A: BA pool size (μmol/100 g body weight) was comparable in wild-type and L-Fabp−/− mice on a chow diet (left), but increased in L-Fabp−/− mice fed a LD for 2 weeks (right). Liver, gallbladder, and intestine were pooled and subjected to ethanolic BA extraction. Total BA mass was measured enzymatically. B: Fecal BA excretion is increased in chow-fed L-Fabp−/− mice, but LD-fed L-Fabp−/− mice excrete less BA than wild-type controls. Mice were individually housed, and feces were collected up to 72 h. Fecal BAs were extracted and quantitated enzymatically. C: Effects of LD on ileal Asbt and Lrh-1 gene expression. mRNA abudance (left) of ileal Asbt and Lrh-1 was determined by qRT-PCR. Ileal microvillus membrane Asbt protein (right) was evaluated by Western blotting with anti-Asbt IgG. The same blot was stripped and reprobed with anti-Actin antibody. D: Adaptive changes in intestinal mRNA expression of genes related to BA signaling in LD-fed L-Fabp−/− mice, including Asbt, Ostα, Ostβ, Fabp6, Fxr, Lrh-1, and Fgf15. mRNA abundance was measured in scraped intestinal mucosa using qRT-PCR. Bar graphs represent the mean ± SE from four mice per genotype on chow and four mice per genotype on LD; * indicates P < 0.05; ** indicates P < 0.01, wild type versus L-Fabp−/− mice on the same diet; ## indicates P < 0.01, chow-fed versus LD-fed mice of the same genotype.
There were further adaptations in regard to enterohepatic BA metabolism in LD-fed L-Fabp−/− mice, including an increased BA pool (Fig. 6A) and a decrease in fecal BA excretion, compared with wild-type controls (Fig. 6B). To understand the pathways by which these changes may be mediated, we turned to an examination of candidate genes involved in BA uptake and metabolism in intestinal mucosa of LD-fed L-Fabp−/− mice. We found comparable expression of ileal Asbt mRNA and protein in LD-fed mice of both genotypes (Fig. 6C), suggesting that the differences noted above in chow-fed mice are eliminated with LD feeding. However, turning to the expression profiles of candidate genes from the entire pooled intestinal mucosa (i.e., duodenum, jejunum, and ileal mucosa), we found increased mRNA expression of Fxr and its downstream targets Fabp6 (ileal BA binding protein) and Fgf15 as well as the basolateral BA transporters Ostα and Ostβ (Fig. 6D) in LD-fed L-Fabp−/− mice. These findings suggest that in the absence of L-Fabp (whose expression is generally regulated in a proximal to distal gradient), alterations in intestinal BA flux induce Fxr expression, which results in transcriptional induction of downstream targets, including Fgf15 (23). Increased intestinal Fgf15 expression would in turn be anticipated to suppress hepatic Cyp7a1 expression (24, 25), which are findings in accord with the microarray and qRT-PCR findings alluded to above. In addition, we found that while ileal expression of Asbt was decreased in chow-fed L-Fabp−/− mice and no different from wild-type mice with LD feeding (Fig. 6C), there was a significant (1.9-fold) increase in Asbt mRNA abundance in whole intestinal mucosa with LD feeding (Fig. 6D). This response was associated with a corresponding (2.4-fold) increase in mRNA abundance of the nuclear hormone receptor Lrh-1 (Fig. 6D), suggesting the possibility that transcriptional induction of Asbt in the proximal intestine of LD-fed L-Fabp−/− mice may contribute to the increased BA pool size and decreased fecal BA output noted above.
DISCUSSION
The central finding of this report demonstrates a dramatic diet-gene interaction with regard to gallstone susceptibility in male L-Fabp−/− mice. Furthermore, this phenotype is dependent not just on dietary cholesterol supplementation but requires a high-fat, cholic-acid-supplemented diet for complete manifestation of the trait, an important distinction in view of the observations that a LD is known to promote both increased intestinal cholesterol absorption and increased biliary cholesterol secretion (26) [reviewed in (27)]. The current findings demonstrate that LD-fed male L-Fabp−/− mice are more susceptible to gallstone formation as a result of alterations in hepatic cholesterol metabolism that promote the accumulation of hepatic-free cholesterol and its secretion into both plasma and bile, coupled with alterations in intestinal BA metabolism. The net result of these complex adaptations is a striking increase in gallstone susceptibility. Several of these conclusions and their underlying mechanisms bear further examination.
Prior observations in L-Fabp−/− mice have revealed evidence for diet-gene interactions that in turn modulate elements of hepatic lipid metabolism, including protection against high-fat-induced weight gain and hepatic steatosis (9), a response restricted to diets containing saturated but not polyunsaturated fatty acids (11). In considering the response of L-Fabp−/− mice to LD feeding, we reasoned that the protection against high-fat-induced obesity in this strain might actually attenuate gallstone formation because an abundant literature points to the role of obesity as an environmental modifier of gallstone susceptibility (2). Indeed, L-Fabp−/− mice lost more weight when consuming the LD and manifested lower fasting glucose levels and lower hepatic TG content than did congenic wild-type controls (Table 1), suggesting that the increased gallstone susceptibility observed in L-Fabp−/− mice cannot be attributed to augmented obesity, steatosis, or impaired glucose tolerance. The changes observed in hepatic cholesterol metabolism in LD-fed L-Fabp−/− mice, by contrast, suggest a plausible mechanism for the increased susceptibility to gallstone formation. In particular, we observed a 50% increase in hepatic free cholesterol content, coupled with higher plasma cholesterol levels in LD-fed L-Fabp−/− mice. These observations, coupled with increased hepatic Mttp mRNA expression and increased hepatic secretion of cholesterol-rich lipoproteins in LD-fed L-Fabp−/− mice, suggest that the assembly and secretion of hepatic lipoproteins is substantially modified. Furthermore, these findings provide additional insight into the importance of candidate genes, i.e., Mttp and L-Fabp, shown to undergo coordinate regulation in the context of VLDL secretion (28) in modifying biliary lipid secretion. In this regard, the current findings extend studies showing that mice with liver-specific inactivation of Mttp are protected against diet-induced gallstones (29), while increased hepatic Mttp activity was found in humans with cholesterol gallstone disease (30).
The underlying mechanisms accounting for the metabolic shifts in hepatic cholesterol use in LD-fed L-Fabp−/− mice may be inferred from earlier studies in mouse L-cells in which cells transfected with L-Fabp contained twice as much cholesterol in the inner leaflet of the plasma membrane and demonstrated enhanced sterol transfer to the microsomal membrane and enhanced esterification (31, 32). That said, the integrated metabolic adaptations following dietary cholesterol supplementation in L-Fabp−/− mice in vivo remain incompletely resolved. By way of example, earlier findings in female L-Fabp−/− mice suggested a dramatic obesity phenotype with increased hepatic TG following dietary cholesterol supplementation (10). Our own results using female L-Fabp−/− mice, however, revealed diametrically opposite results to those studies, with cholesterol-fed L-Fabp−/− mice showing if anything reduced weight gain and attenuated hepatic TG accumulation (11). Other studies from those workers using cholesterol-fed male L-Fabp−/− mice revealed no differences in weight gain, but demonstrated a 4-fold decrease in BA pool size and 1.9-fold increased gallbladder cholesterol content (12). Our findings reveal important distinctions from that report. We find that cholesterol feeding alone had no effect on biliary BA secretion in either genotype, and although biliary cholesterol secretion increased following dietary cholesterol supplementation, there were no differences between wild-type and L-Fabp−/− mice. The source of the discrepancy in this phenotype remains unclear, but it is possible that the genetic background (our animals were congenic C57BL/6) or other environmental modifiers, such as bacterial colonization, may play a role. These distinctive outcomes are of relevance to the interpretation of our findings in LD-fed L-Fabp−/− mice, where complex interactions between dietary and genetic modifiers of a complex trait like gallstone susceptibility are likely to be challenging to resolve. It is certainly possible, for example, that other modifiers may have been introduced in the backcrossing of L-Fabp−/− mice into the C57BL/6 background.
Another consideration in terms of the response to LD feeding is the possibility that alterations in intestinal lipid trafficking might play a role. This possibility seems reasonable in view of our earlier findings that intestinal FA trafficking is less efficient in L-Fabp−/− mice (9). Our findings suggest that cholesterol absorption is indeed reduced in chow-fed male L-Fabp−/− mice, but this effect was eliminated after the animals consumed a cholic-acid-containing LD. It is worth emphasizing that while the data presented in Fig. 5 suggest that the percentage of absorption of cholesterol tended to decrease in LD-fed animals of both genotypes, net intestinal cholesterol mass absorbed was vastly increased with the cholate-supplemented diet as evidenced by the increase in hepatic cholesterol content [Table 1 compared with data in chow-fed mice from (8)]. Nevertheless, we conclude that differences in intestinal cholesterol absorption are unlikely to account for the increased gallstone susceptibility in L-Fabp−/− mice.
We also considered the possibility that alterations in intestinal BA metabolism might play a role in this phenotype, as we demonstrated increased intestinal mRNA expression of Fxr and its downstream target Fabp6 in LD-fed L-Fabp−/− mice. Further evidence of compensatory alterations in intestinal BA metabolism in LD-fed L-Fabp−/− mice is the finding that intestinal Fgf15 mRNA was increased, which might plausibly account for the observed decrease in hepatic Cyp7a1 expression in LD-fed L-Fabp−/− mice. The observation that fecal BA output was decreased in LD-fed L-Fabp−/− mice, coupled to the increased BA pool size, leads us to speculate that intestinal BA absorption and enterohepatic cycling might be increased. In support of this possibility is the observation that expression of the apical bile salt transporter Asbt was increased in LD-fed L-Fabp−/− mice in concert with increased Lrh-1 expression (33). The role of altered ileal Fgf15 gene expression in modulating gallbladder filling (34) is also worth consideration in regard to the phenotype in LD-fed L-Fabp−/− mice, and future studies will address the possibility that changes in gallbladder volume might precede gallstone formation in LD-fed L-Fabp−/− mice. This becomes an important consideration in light of earlier findings from Wang et al. (35) that targeted disruption of the cholecystokinin-1 receptor promotes gallbladder stasis and is associated with altered nucleation and growth of cholesterol monohydrate crystals in bile.
Finally, the findings place in context some of the earlier reports concerning quantitative trait loci for murine cholesterol cholelithiasis (2, 5, 13). In particular, the close positional linkage of the L-Fabp gene within the locus near D6Mit123 on mouse chromosome 6 identified as a possible Lith locus in mouse crosses now appears to have a plausible mechanistic basis since LD-fed male L-Fabp−/− mice exhibit dramatically increased susceptibility to gallstones. The implications for these observations in terms of human gallstone susceptibility will require further study.
Acknowledgments
The authors are most grateful to Dr. Paul Dawson for gifts of antisera and for useful insights, to Dr. Elizabeth Brunt for helpful discussions, and to May Fu for technical assistance with some of the studies.
Abbreviations
BA, bile acid
ChMC, cholesterol monohydrate crystal
FPLC, fast-protein liquid chromatography
LD, lithogenic diet
L-Fabp, Liver fatty acid binding protein
qRT-PCR, quantitative RT-PCR
TG, triglyceride
This work was supported in part by grants from the National Institutes of Health (R01 DK-056260 and R37 HL-038180) to N.O.D. and the Washington University Digestive Diseases Research Core Center (P30 DK052574) to N.O.D., in particular the Morphology and Functional Genomics Cores.
Published, JLR Papers in Press, January 9, 2009.
Footnotes
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
References
- 1.Sandler R. S., J. E. Everhart, M. Donowitz, E. Adams, K. Cronin, C. Goodman, E. Gemmen, S. Shah, A. Avdic, and R. Rubin. 2002. The burden of selected digestive diseases in the United States. Gastroenterology. 122 1500–1511. [DOI] [PubMed] [Google Scholar]
- 2.Lyons M. A., and H. Wittenburg. 2006. Cholesterol gallstone susceptibility loci: a mouse map, candidate gene evaluation, and guide to human LITH genes. Gastroenterology. 131 1943–1970. [DOI] [PubMed] [Google Scholar]
- 3.Biddinger S. B., J. T. Haas, B. B. Yu, O. Bezy, E. Jing, W. Zhang, T. G. Unterman, M. C. Carey, and C. R. Kahn. 2008. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 14 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanlungo S., A. Rigotti, and F. Nervi. 2004. Hepatic cholesterol transport from plasma into bile: implications for gallstone disease. Curr. Opin. Lipidol. 15 279–286. [DOI] [PubMed] [Google Scholar]
- 5.Lyons M. A., H. Wittenburg, R. Li, K. A. Walsh, M. R. Leonard, R. Korstanje, G. A. Churchill, M. C. Carey, and B. Paigen. 2003. Lith6: a new QTL for cholesterol gallstones from an intercross of CAST/Ei and DBA/2J inbred mouse strains. J. Lipid Res. 44 1763–1771. [DOI] [PubMed] [Google Scholar]
- 6.Cistola D. P., J. C. Sacchettini, L. J. Banaszak, M. T. Walsh, and J. I. Gordon. 1989. Fatty acid interactions with rat intestinal and liver fatty acid-binding proteins expressed in Escherichia coli. A comparative 13C NMR study. J. Biol. Chem. 264 2700–2710. [PubMed] [Google Scholar]
- 7.Cistola D. P., J. C. Sacchettini, and J. I. Gordon. 1990. 13C NMR studies of fatty acid-protein interactions: comparison of homologous fatty acid-binding proteins produced in the intestinal epithelium. Mol. Cell. Biochem. 98 101–110. [DOI] [PubMed] [Google Scholar]
- 8.Newberry E. P., Y. Xie, S. Kennedy, X. Han, K. K. Buhman, J. Luo, R. W. Gross, and N. O. Davidson. 2003. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 278 51664–51672. [DOI] [PubMed] [Google Scholar]
- 9.Newberry E. P., Y. Xie, S. M. Kennedy, J. Luo, and N. O. Davidson. 2006. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 44 1191–1205. [DOI] [PubMed] [Google Scholar]
- 10.Martin G. G., B. P. Atshaves, A. L. McIntosh, J. T. Mackie, A. B. Kier, and F. Schroeder. 2006. Liver fatty acid binding protein gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am. J. Physiol. Gastrointest. Liver Physiol. 290 G36–G48. [DOI] [PubMed] [Google Scholar]
- 11.Newberry E. P., S. M. Kennedy, Y. Xie, B. T. Sternard, J. Luo, and N. O. Davidson. 2008. Diet-induced obesity and hepatic steatosis in L-Fabp/mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am. J. Physiol. Gastrointest. Liver Physiol. 294 G307–G314. [DOI] [PubMed] [Google Scholar]
- 12.Martin G. G., B. P. Atshaves, A. L. McIntosh, J. T. Mackie, A. B. Kier, and F. Schroeder. 2005. Liver fatty-acid-binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem. J. 391 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyons M. A., H. Wittenburg, R. Li, K. A. Walsh, G. A. Churchill, M. C. Carey, and B. Paigen. 2003. Quantitative trait loci that determine lipoprotein cholesterol levels in DBA/2J and CAST/Ei inbred mice. J. Lipid Res. 44 953–967. [DOI] [PubMed] [Google Scholar]
- 14.Xie Y., E. P. Newberry, S. G. Young, S. Robine, R. L. Hamilton, J. S. Wong, J. Luo, S. Kennedy, and N. O. Davidson. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281 4075–4086. [DOI] [PubMed] [Google Scholar]
- 15.Carey M. C. 1978. Critical tables for calculating the cholesterol saturation of native bile. J. Lipid Res. 19 945–955. [PubMed] [Google Scholar]
- 16.Rossi S. S., J. L. Converse, and A. F. Hofmann. 1987. High pressure liquid chromatographic analysis of conjugated bile acids in human bile: simultaneous resolution of sulfated and unsulfated lithocholyl amidates and the common conjugated bile acids. J. Lipid Res. 28 589–595. [PubMed] [Google Scholar]
- 17.Heuman D. M. 1989. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 30 719–730. [PubMed] [Google Scholar]
- 18.Schwarz M., D. W. Russell, J. M. Dietschy, and S. D. Turley. 1998. Marked reduction in bile acid synthesis in cholesterol 7alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 39 1833–1843. [PubMed] [Google Scholar]
- 19.Setchell K. D., A. M. Lawson, N. Tanida, and J. Sjovall. 1983. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J. Lipid Res. 24 1085–1100. [PubMed] [Google Scholar]
- 20.Wang D. Q., and M. C. Carey. 2003. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct versus indirect methodologies. J. Lipid Res. 44 1042–1059. [DOI] [PubMed] [Google Scholar]
- 21.Wang D. Q., B. Paigen, and M. C. Carey. 2001. Genetic factors at the enterocyte level account for variations in intestinal cholesterol absorption efficiency among inbred strains of mice. J. Lipid Res. 42 1820–1830. [PubMed] [Google Scholar]
- 22.Dawson P. A., M. Hubbert, J. Haywood, A. L. Craddock, N. Zerangue, W. V. Christian, and N. Ballatori. 2005. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 280 6960–6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houten S. M., D. H. Volle, C. L. Cummins, D. J. Mangelsdorf, and J. Auwerx. 2007. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol. Endocrinol. 21 1312–1323. [DOI] [PubMed] [Google Scholar]
- 24.Houten S. M., M. Watanabe, and J. Auwerx. 2006. Endocrine functions of bile acids. EMBO J. 25 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukumoto S. 2008. Actions and mode of actions of FGF19 subfamily members. Endocr. J. 55 23–31. [DOI] [PubMed] [Google Scholar]
- 26.Wang D. Q., F. Lammert, D. E. Cohen, B. Paigen, and M. C. Carey. 1999. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. Am. J. Physiol. 276 G751–G760. [DOI] [PubMed] [Google Scholar]
- 27.Lammert F., and T. Sauerbruch. 2005. Mechanisms of disease: the genetic epidemiology of gallbladder stones. Nat. Clin. Pract. Gastroenterol. Hepatol. 2 423–433. [DOI] [PubMed] [Google Scholar]
- 28.Spann N. J., S. Kang, A. C. Li, A. Z. Chen, E. P. Newberry, N. O. Davidson, S. T. Hui, and R. A. Davis. 2006. Coordinate transcriptional repression of liver fatty acid-binding protein and microsomal triglyceride transfer protein blocks hepatic very low density lipoprotein secretion without hepatosteatosis. J. Biol. Chem. 281 33066–33077. [DOI] [PubMed] [Google Scholar]
- 29.Amigo L., J. Castro, J. F. Miquel, S. Zanlungo, S. Young, and F. Nervi. 2006. Inactivation of hepatic microsomal triglyceride transfer protein protects mice from diet-induced gallstones. Gastroenterology. 131 1870–1878. [DOI] [PubMed] [Google Scholar]
- 30.Castro J., L. Amigo, J. F. Miquel, C. Galman, F. Crovari, A. Raddatz, S. Zanlungo, R. Jalil, M. Rudling, and F. Nervi. 2007. Increased activity of hepatic microsomal triglyceride transfer protein and bile acid synthesis in gallstone disease. Hepatology. 45 1261–1266. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson J. R., J. P. Slotte, G. Nemecz, A. Pastuszyn, T. J. Scallen, and F. Schroeder. 1991. Intracellular sterol distribution in transfected mouse L-cell fibroblasts expressing rat liver fatty acid-binding protein. J. Biol. Chem. 266 5486–5496. [PubMed] [Google Scholar]
- 32.Woodford J. K., W. D. Behnke, and F. Schroeder. 1995. Liver fatty acid binding protein enhances sterol transfer by membrane interaction. Mol. Cell. Biochem. 152 51–62. [DOI] [PubMed] [Google Scholar]
- 33.Chen F., L. Ma, P. A. Dawson, C. J. Sinal, E. Sehayek, F. J. Gonzalez, J. Breslow, M. Ananthanarayanan, and B. L. Shneider. 2003. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J. Biol. Chem. 278 19909–19916. [DOI] [PubMed] [Google Scholar]
- 34.Choi M., A. Moschetta, A. L. Bookout, L. Peng, M. Umetani, S. R. Holmstrom, K. Suino-Powell, H. E. Xu, J. A. Richardson, R. D. Gerard, et al. 2006. Identification of a hormonal basis for gallbladder filling. Nat. Med. 12 1253–1255. [DOI] [PubMed] [Google Scholar]
- 35.Wang D. Q., F. Schmitz, A. S. Kopin, and M. C. Carey. 2004. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J. Clin. Invest. 114 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millar J. S., D. A. Cromley, M. G. McCoy, D. J. Rader, and J. T. Billheimer. 2005. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 46 2023–2028. [DOI] [PubMed] [Google Scholar]