Abstract
Purpose
We previously showed that nuclear localization of the actin-binding protein FilaminA (FlnA) corresponded to hormone-dependence in prostate cancer (Oncogene, 2007, 26:6061-6070). Intact FlnA (280kDa, cytoplasmic) cleaved to a 90kDa fragment which translocated to the nucleus in hormone-naïve cells, whereas in hormone-refractory cells, FlnA was phosphorylated, preventing its cleavage and nuclear translocation. We now examined whether FlnA localization determines a propensity to metastasis in advanced androgen independent prostate cancer.
Experimental Design
We examined, by immunohistochemistry, FlnA localization in paraffin-embedded human prostate tissue representing different stages of progression. Results were correlated with in vitro studies in a cell model of prostate cancer.
Results
Nuclear FlnA was significantly higher in benign prostate (0.6612±0.5888), PIN (0.6024±0.4620) and clinically localized cancers (0.69134±0.5686), compared to metastatic prostate cancers (0.3719±0.4992, p=0.0007). Cytoplasmic FlnA increased from benign prostate (0.0833±0.2677), PIN (0.1409±0.2293), localized cancers (0.3008±0.3762, p=0.0150), to metastases (0.7632±0.4414, p<0.00001). Logistic regression of metastatic vs non-metastatic tissue yielded the area-under-ROC curve as 0.67 for nuclear-FlnA, 0.79 for cytoplasmic-FlnA and 0.82 for both, indicating that metastasis correlates with cytoplasmic-to-nuclear translocation. In vitro studies showed that cytoplasmic localization of FlnA induced cell invasion whereas nuclear translocation of the protein inhibited it. FlnA dephosphorylation with the PKA inhibitor H-89 facilitated FlnA nuclear translocation, resulting in decreased invasiveness and AR transcriptional activity, and induced sensitivity to androgen withdrawal in hormone-refractory cells.
Conclusions
The data presented in this study indicate that in prostate cancer, metastasis correlates with cytoplasmic localization of FlnA and may be prevented by cleavage and subsequent nuclear translocation of this protein.
Keywords: Filamin A, immunohistochemistry, hormone refractory, metastasis, prostate cancer
Introduction
Filamins are a family of cytoskeletal proteins that organize filamentous actin into networks and stress fibers (1). Filamin A (FlnA) is a 280 kDa non-muscle actin binding protein whose appropriate function is essential for development (2, 3). FlnA dimerization forms a V-shaped flexible structure which can induce high angle orthogonal branching and efficiently gather actin filaments into a 3-dimensional gel in vitro by cross-linking actin filaments at the leading edge of migrating cells. Hence, filamins are essential for mammalian cell locomotion, anchoring of transmembrane proteins including integrins and also act as interfaces for protein-protein interaction (4). Over 30 proteins of great functional diversity are known to interact with filamins which function as a signaling scaffold by connecting and coordinating a large variety of cellular processes (4).
In prostate cancer, a role for FlnA was identified in prostate-specific membrane antigen (PSMA) enzymatic activity (5). PSMA internalization and recycling was shown to require FlnA and may be related to increased metastatic capacity (6, 7). FlnA has also been identified as an androgen receptor coregulator (8, 9). We have recently shown in an in vitro model that nuclear expression of Filamin A correlated with androgen dependence (10). We demonstrated that in androgen-dependent LNCaP prostate cancer cells, the cleaved 90kDa fragment is localized to the nucleus, whereas in its androgen independent (AI) subline C4-2, FlnA failed to cleave and remained cytoplasmic. Transfection of FlnA16-24 cDNA in C4-2 cells restored expression and nuclear localization of 90kDa FlnA. Unlike LNCaP, C4-2 cells proliferate in androgen-reduced medium and in the presence of the AR-antagonist Casodex. Nuclear expression of 90kDa FlnA in C4-2 cells prevented proliferation in androgen-reduced medium and restored Casodex-sensitivity. These results indicated the importance of FlnA localization in prostate cancer cells. However, the relationship between Filamin A localization and the metastatic capacity of the cell has not been identified.
FlnA is highly susceptible to proteolysis at the two hinge regions H1 and H2 (1, 11), which cleaves the protein to a 170 kDa fragment consisting of the ABD and repeats 1-15 (FlnA1-15) and a 110 kDa protein consisting of repeats 16-24 (FlnA 16-24) further cleaved to yield a 90 kDa fragment (1, 4). Proteolysis of FlnA is regulated by its phosphorylation on Ser 2152 in repeat 20, which renders the protein stable and resistant to cleavage (1). This site is a known substrate for a number of kinases – including, protein kinase A (PKA) (12, 13), p90RSK (14) and PKCα (15). We previously showed that in C4-2 cells, FlnA is phosphorylated at Ser 2152 (10). This resulted in a failure of this molecule to cleave in this model of androgen independence, causing cytoplasmic retention of the protein. Here we show that these effects are reversed by the PKA inhibitor H-89.
In this study, we sought to determine Filamin A expression across prostate cancer progression and whether localization of Filamin A in human prostate cancer corresponded with metastatic potential. We show by immunohistochemical examination in paraffin-embedded human prostate tissues, that benign prostate, PIN and localized prostate cancer had predominantly nuclear FlnA expression whereas in metastatic prostate cancer, FlnA was found to be primarily in the cytoplasm. In vitro studies confirmed that cytoplasmic expression of FlnA is required for the ability of cells to invade and migrate. Since FlnA proteolysis is regulated by protein kinase A (PKA), we also show that inhibition of PKA activity prevented FlnA regulated cell invasion. Our data demonstrate that FlnA cytoplasmic localization correlated with increased metastatic potential and a hormone refractory phenotype in prostate cancer.
Materials and Methods
Patients and tissues used
Tissue microarrays (TMAs) were constructed from benign prostate (n=32), PIN (n=26), localized (n=67) and androgen independent metastatic (n=69) prostate cancer tissues from the University of Michigan Prostate Cancer Specialized Program of Research Excellence (SPORE) (16, 17). Metastatic tissue were obtained from “rapid” or “warm” autopsies (mean of 3 hours lapsed from death to commencement of autopsy) of 26 patients identified with hormone refractory prostate cancer (HRPC). The localized tissues consisted of Grade 3 (n=10), Grade 4 (n=8), Grade 6 (3+3, n=10), Grade 7 (3+4, n=10), Grade 7 (4+3, n=9), Grade 8 (4+4, n=10) and Grade 9 (4+5, n=10) tumors. The metastatic deposits consisted of tissues procured from the following locations: Liver, Lung, Lymph Node, Seminal Vesicle, Bone, Dura and Bladder. The tissues were formalin fixed and paraffin embedded, then the TMAs were constructed with three cores (0.6 mm in diameter) taken from each representative block.
Immunohistochemistry and Tissue microarray evaluation
We used a mouse monoclonal anti-Filamin1(A) C-terminal antibody, clone TI10, (Chemicon, Tamecula,CA). which we validated earlier by Western blotting and immunohistochemistry (10). For details, see supplementary materials. The degree of staining was evaluated blindly in a semi-quantitative fashion by a pathologist taking into account both the intensity of staining as well as the percent of cells exhibiting that intensity. Only the epithelial cells were scored: this is because the stroma, which also stained for FlnA (18), showed uniform staining in all tissues examined. FlnA staining in the epithelial cells was scored separately in the nucleus and the cytoplasm/cytoplasmic membrane and was recorded as a product of the extent and the intensity of the staining. The extent of staining was recorded as positive (score = +1) or negative (score = 0). A specimen was scored +1 in the nucleus if the majority (≥80%) of the cells had positive nuclei, whereas a specimen was scored as 0 nuclear FlnA if <20% of the cells had positive nuclei. Intermediate scores (e.g. 0.5 for 50% of cells staining positively) were also provided. The intensity of the staining was scored 0-3, where 0 represented no staining, +1 represented weak, +2 represented intermediate and +3 represented strong staining. A similar system of scoring was established for cytoplasmic staining and was described earlier (19, 20).
Statistical Analysis
The subcellular staining expression of FlnA as positive (+1) or negative (0) was tabulated separately for nuclear or cytoplasmic staining by groups (benign prostate, PIN, localized prostate cancer and metastatic prostate cancer). Contingency tables with Fisher's exact test were used to compare and assess the differences in the expression of FlnA in the four groups according to the subcellular localization (nuclear or cytoplasmic). The interval estimation is given as a 95% Confidence Interval. The means of staining intensity in the same groups were compared with a two sample t-test with Welch approximation for unequal variances when appropriate. The subcellular localization of Filamin was further explored with a sensitivity-specificity analysis, using the main effects and a stepwise model including the interaction of the main effects.
Protein Extraction from Frozen Tissue
Protein lysates were prepared from flash frozen tissue obtained from the Cancer Center Specimen Repository of the University of California Davis under an IRB approved protocol. Two normal prostate, 9 localized tumors and 2 lymph node metastases were used for these studies. The tissues are described in Table 2. For method of extraction, please see Supplemental Materials.
Table 2.
Description of Frozen Prostatic Tissue Used in Figure 3C.
| SPECIMEN | DESCRIPTION | GLEASON SCORE | TUMOR INVOLVEMENT | PRE-PROSTATECTOMY PSA (ng/ml) |
|---|---|---|---|---|
| 1 | Normal Prostate | 2.5 | ||
| 2 | Normal Prostate | 6.3 | ||
| 3 | Localized Tumor | 6 | 80% | 17.3 |
| 4 | Localized Tumor | 7 | 40% | 10.3 |
| 5 | Localized Tumor | 6 | 30% | 6.3 |
| 6 | Localized Tumor | 7 | 50% | 20.6 |
| 7 | Localized Tumor | 7 | 40% | 11.3 |
| 8 | Localized Tumor | 9 | 30% | 14.0 |
| 9 | Localized Tumor | 6 | 40% | 9.5 |
| 10 | Localized Tumor | 7 | 50% | 7.4 |
| 11 | Localized Tumor | 7 | 80% | 11.9 |
| 12 | Lymph node Metastasis | 16.0 | ||
| 13 | Lymph node Metastasis | 13.0 |
Cell Culture and Transfection
LNCaP cells were purchased from American Type Culture Collection, Manassas, VA, while C4-2 cells were from UroCor, (Oklahoma City, OK). Casodex was kindly provided by Dr. Barry Furr, AstraZeneca, UK. 4,5α-Dihydrotestosterone (DHT) was obtained from Sigma-Aldrich, St. Louis, MO. pCMV-FlnA(16-24) and pCMV-FlnA(1-15) plasmids were kindly provided by Dr. E.W. Yong, National University of Singapore, Singapore. A human PSA reporter plasmid consisting of the human PSA 5′-flanking region (-631/-1) containing androgen response elements I and II (ARE I and ARE II) tagged to a luciferase construct (hPSA-luc) was kindly provided by Dr. Bandana Chatterjee, University of Texas Health Science Center at San Antonio, San Antonio, TX. Filamin A siRNA (siRNA1P, Santa Cruz Biotechnology, Santa Cruz, CA) with the following sequences: Strand #1: 5′-CCAUCACUGACAACAAAGA-3′, Strand #2: 5′-CUGCAGAGUUUAU-CAUUGA-3′, Strand #3: 5′-GCUACCUCAUCUCCAUCAA-3′. Rabbit polyclonal anti- Lamin A/C, were from Cell Signaling Technology, Beverly, MA. Mouse monoclonal anti-C-terminal FlnA (MAB1680) was from Chemicon, Temecula, CA). For in vitro assays, please see Supplementary Materials.
Results
Differential localization of FlnA across human prostate cancer progression
To investigate the expression of FlnA in different human prostate tissues, we stained tissue microarrays (TMA) representing benign prostates, PIN, localized prostate cancer and metastatic prostate cancer with an anti-FlnA (C-terminal) antibody that recognized both the full-length and the 90 kDa FlnA, and counterstained with hematoxylin. Negative controls were stained with a Universal Mouse negative control (Figure 1A). Normal (non-tumor) prostate tissues stained strongly for FlnA in the nucleus and weakly in the cytoplasm (Figure 1B), as did prostate tissue obtained from radical prostatectomies displaying high grade PIN (Figure 1C) and well-differentiated prostate cancer (Figure 1D). Significantly, the stromal cells also stained strongly for FlnA in the nuclei, but not in the cytoplasm. Epithelial cells from normal prostate, PIN or localized prostate cancer displayed a stronger staining in the nucleus compared to the cytoplasm. On the other hand, metastatic prostate tumor tissues had strong cytoplasmic staining of FlnA but lacked FlnA expression in the nucleus (Figure 1E,F).
Figure 1. Differential localization of FlnA in human prostate tissue.
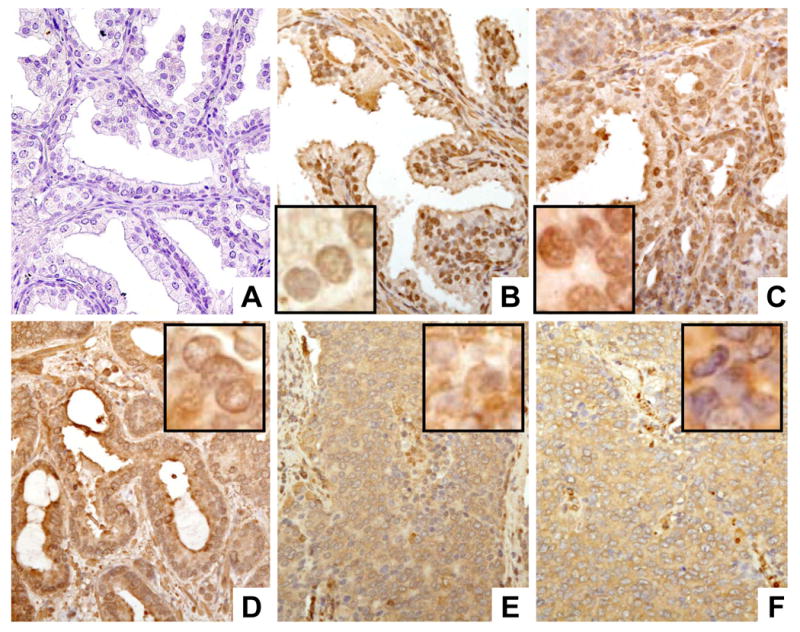
Tissue microarrays were stained with mouse monoclonal anti-FlnA (C-terminal) antibody (brown staining) and counterstained with hematoxylin (blue staining). (A) Negative control showing lack of staining in the absence of antibody. (B) Expression of FlnA in normal prostate tissue. This tissue was obtained during a warm autopsy from a patient who died of other causes. Note that FlnA is expressed in both in both epithelial and stromal cells. (C) Expression of FlnA in high grade PIN. Note the strong nuclear localization in the epithelial cells. (D) Expression of FlnA in a Gleason 3 localized tumor obtained by prostatectomy. (E, F) Expression of FlnA in prostate cancer metastasis in patients who died of HRPC. Note the strong cytoplasmic staining and the absence of FlnA nuclear localization mixed with cells that had FlnA nuclear localization (E) or were completely negative for FlnA in the nucleus (F). magnification: 20×. Inset to B-F: Enlarged photomicrographs (40×) demonstrating decreased nuclear FlnA localization with prostrate cancer progression. In normal (B), PIN (C) and localized tumors (D), the nuclei are sharply outlined and brown staining is seen inside the nuclei, whereas in the metastatic deposits (E, F) FlnA staining did not outline the nuclei and was seen only in the cytoplasm. Magnification: 100×.
Decreased nuclear and increased cytoplasmic expression of Filamin A in metastatic prostate tumor tissue from patients with HRPC
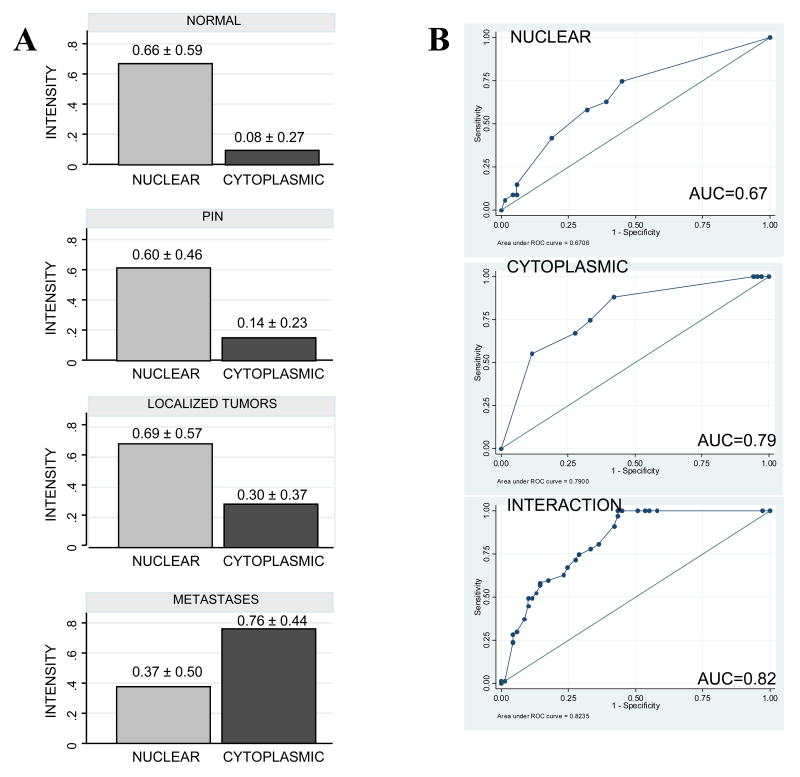
Statistical analysis was performed to compare the localization and expression of FlnA in prostate epithelial cells from benign prostate (n=32), PIN (n=26), localized (n=67) and metastatic prostate cancer (n=69) (Figure 2A). The mean score for normal prostate, PIN and localized prostate tissues remained at 0.65 whereas that for metastatic prostate tissues decreased to 0.37, a decline of about 43%. In contrast, the corresponding nuclear staining scores for metastatic prostate tissues was significantly lower (p = 0.0007) (Table 1), irrespective of the localization of the metastatic deposit. Univariate logistic regression analysis for nuclear FlnA gives an Odds Ratio=3.07 (p= 0.001), indicating that loss of nuclear FlnA is a significant risk factor for the development of metastasis. The area under the ROC curve (AUC) for nuclear FlnA alone, which estimates its discriminatory capacity, was 0.67, indicating good discrimination between non-metastatic and metastatic tissue (Figure 2B, upper panel).
Figure 2. FlnA expression in the cytoplasm, but not the nucleus, corresponds to metastasis.
(A) Mean intensity of tissues staining positively for FlnA in the nucleus (left) and cytoplasm (right) of (upper panel) normal prostate tissue (n=32), (2nd panel) PIN (n=26), (3rd panel) localized prostate tumors (n=67) and (lower panel) distant metastases (n=69). (B) Area under the ROC curve to determine the discriminatory capacity of (upper panel) nuclear, (middle panel) cytoplasmic and (lower panel) both nuclear and cytoplasmic FlnA between metastatic and non-metastatic tissue.
Table 1.
Analysis of nuclear and cytoplasmic FlnA staining.
| NUCLEAR STAINING | # SPECIMEN STAINING | Two sample t-test (p-value) | ||||
|---|---|---|---|---|---|---|
| n | NEGATIVE | POSITIVE | Normal vs PIN | PIN vs LOC. | LOC. vs Mets | |
| NORMAL | 32 | 8 (25.00%) | 24 (75.00%) | 0.6716 | ||
| PIN | 26 | 6 (23.08%) | 20 (76.92%) | 0.4391 | ||
| LOCALIZED PCa | 67 | 17 (25.37%) | 50 (74.63%) | 0.0007** | ||
| METASTATIC PCa | 69 | 38 (55.07%) | 31 (44.93%) | |||
| CYTOPLASM STAINING | # SPECIMEN STAINING | Two sample t-test (p-value) | ||||
| n | NEGATIVE | POSITIVE | Normal vs PIN | PIN vs LOC. | LOC PCa vs Mets | |
| NORMAL | 32 | 29 (90.63%) | 3 (9.37%) | 0.3811 | ||
| PIN | 26 | 18 (69.23%) | 8 (30.77%) | 0.0150** | ||
| LOCALIZED PCa | 67 | 37 (55.22%) | 30 (44.78%) | <0.00001** | ||
| METASTATIC PCa | 69 | 8 (11.59%) | 61 (88.41%) | |||
In contrast to nuclear staining, there was a steady increase in the fraction of prostatic epithelial cells staining positively for FlnA in the cytoplasm from normal tissue (<10%), to PIN (30%), to localized prostate cancer (45%) to metastatic tissue (almost 90%) (Figure 2A). There was a significant increase in cytoplasmic FlnA from PIN to localized tumors (p=0.0150), although there was no significant correlation between Gleason scores and the degree of staining within the “localized tumor” group (p>0.05). The increase in cytoplasmic staining from localized to metastasized tumors was highly significant (p<0.00001) (Table 1), and did not depend on the localization of the metastatic deposit. Logistic regression analysis for cytoplasmic FlnA gives an Odds Ratio=0.06 (p = 0.0001), indicating that decreased cytoplasmic FlnA is a significant protective factor against the development of metastasis. The AUC for cytoplasmic FlnA was 0.79, indicating very good discrimination between non-metastatic and metastatic tissue (Figure 2B, middle panel). Multivariate logistical analysis was used to study the joint effect of both nuclear and cytoplasmic FlnA. The AUC for the interaction of both is 0.82, and the Odd's ratio increases to 26, indicating excellent discrimination (Figure 2B, lower panel). Taken together, these results indicate that increased FlnA expression in the cytoplasm and decreased expression in the nucleus of prostate epithelial cells correlated with metastatic potential of the cell.
Prostate tumors express both full-length and cleaved fragments of Filamin A
Previous studies had shown that full length (280 kDa) FlnA cleaved to a 90 kDa fragment which then translocated to the nucleus (8) (Figure 3A). The relevance of the 90 kDa FlnA in human tumor is demonstrated in Figure 3B, which shows differential expression of this fragment in tissues from different patients. Fresh frozen tissue obtained from patients with prostate cancer were lysed and analyzed by Western blotting. All but one metastatic tissue expressed FlnA as determined by the expression of the 280 kDa band, whereas they showed differential expression of the 90 kDa fragment. Significantly, both non-tumor tissues and all but two primary tumors showed significant FlnA cleavage, while one of two lymph node metastases lacked Filamin A staining altogether (Figure 3B). There was no correlation between Filamin A cleavage and pre-surgery PSA, Gleason grade, or the percentage of tumor involvement, suggesting that Filamin A was an indicator of response to hormone therapy alone.
Figure 3.
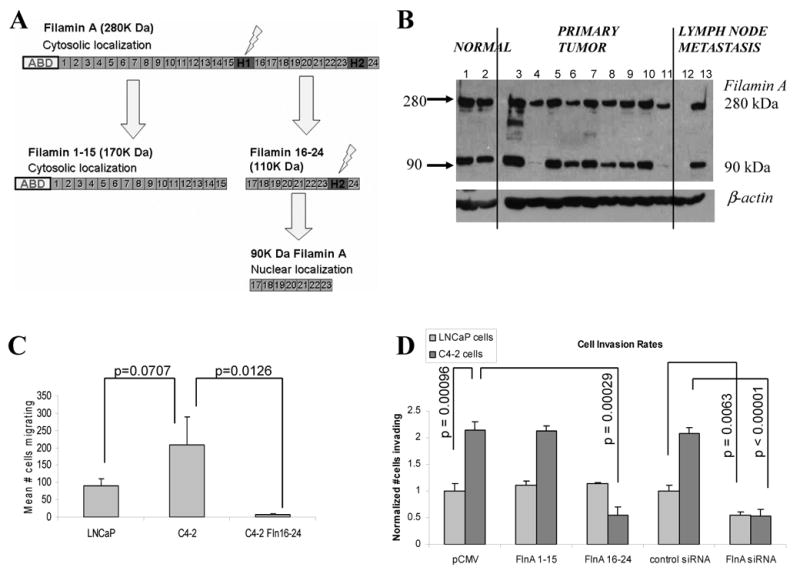
(A) Proteolysis of Filamin A. (B) To detect FlnA cleavage in human tumors, fresh frozen sections from 2 normal prostate (1,2) and 11 tumors (primary: 3-11, metastases: 12-13) obtained by laser capture of epithelial cells selectively were examined by Western blotting. All but one sample showed expression of the 280 kDa full-length FlnA, whereas three lacked expression of the 90 kDa band. (C) Quantification of cell migration assay. LNCaP cells were less migratory compared to C4-2 cells but transfection of the latter with FlnA 16-24 completely inhibited cell migration. For expression of transfected FlnA, see Supplementary Figure 2A. (D) Invasion assay in LNCaP and C4-2 cells. Transfection of FlnA 1-15 (170 kDa) did not affect the ability of these cells to invade whereas FlnA 16-24 inhibited invasiveness in C4-2 cells which lack nuclear FlnA but not in LNCaP cells which express nuclear FlnA. Depletion of cells with a FlnA siRNA prevented invasiveness in both LNCaP and C4-2 cells. For expression of FlnA downregulated with siRNA see Supplementary Figure 2B.
Effect of nuclear and cytoplasmic FlnA on cell migration and invasion
Since metastasis correlated with both increased cytoplasmic localization and decreased nuclear localization of FlnA, we investigated whether the presence of FlnA in the cytoplasm or the absence of FlnA from the nucleus promoted metastasis. Androgen-dependent LNCaP cells expressed FlnA in both the nucleus and the cytoplasm whereas its androgen-independent subline C4-2 expressed FlnA only in the cytoplasm (10) (see also Supplementary Figure 1). Hence, these cells would allow us to distinguish between the effects of cytoplasmic and nuclear FlnA. Androgen-independent C4-2 cells, which express cytoplasmic (full-length) FlnA only (10), and no nuclear (90 kDa) FlnA, expressed >2-fold increased migration rates compared to parental androgen-dependent LNCaP cells (Figure 3C). Hence, we investigated whether the increased migration in C4-2 cells was due to the lack of nuclear FlnA. We previously showed that expression of FlnA 16-24 in C4-2 cells caused the transfected fragment to localize to the nucleus (10). Expression of FlnA 16-24 significantly suppressed cell migration in C4-2 cells (p=0.0126) (Figure 3C). Similarly, the rate of cell invasion also increased >2-fold in C4-2 cells compared to LNCaP (p<0.001), and this effect was significantly inhibited by the expression of FlnA 16-24 (p<0.001) (Figure 3D). However, in LNCaP cells, which spontaneously cleaved FlnA and expressed it in the nucleus, overexpression of FlnA 16-24 did not affect the rate of cell migration (Figure 3D), likely indicating that the presence of nuclear FlnA in LNCaP cells was sufficient for suppressing invasion in the first place. In addition, we downregulated FlnA expression with a pool of 3 FlnA siRNA described earlier (10), which in C4-2 cells decreased cytoplasmic FlnA only. Transfection with FlnA siRNA, but not control siRNA, resulted in decreased FlnA expression and decreased invasiveness of both LNCaP and C4-2 cells (Figure 3D). These results indicated that the expression of FlnA in the cytoplasm promotes cell invasion whereas its expression in the nucleus suppresses it.
Increased FlnA cleavage and cytoplasmic to nuclear translocation prevented cell invasion
The above indicated that FlnA translocation from the cytoplasm to the nucleus inhibits cell invasion. Hence we designed methods to induce FlnA nuclear translocation. Since in C4-2 cells, FlnA was cytoplasmic due to a failure to cleave and introduction of the cleaved FlnA fragment induced nuclear translocation (10), we reasoned that if FlnA can be persuaded to undergo cleavage in these cells, then the 90 kDa would translocate to the nucleus and inhibit invasion and migration. Studies had shown that phosphorylation of FlnA at Ser 2152 rendered the protein resistant to cleavage (1), and we showed that FlnA was phosphorylated at Ser 2152 in C4-2 but not LNCaP cells (10). Hence, we investigated whether dephosphorylation of FlnA at this site induced FlnA cleavage and nuclear translocation. Ser 2152 on FlnA is a consensus site for cyclic AMP (cAMP) dependent protein kinase A (PKA) activity (12, 13), hence we examined if stimulation of PKA activity by the cAMP analog chlorophenylthio-cAMP (CPT-cAMP, 100 μM) or its inhibition by the PKA inhibitor H-89 (1 μM) altered FlnA localization. Figure 4A, upper panels shows in LNCaP cells, that increased cAMP levels by CPT-cAMP treatment decreased FlnA cleavage to the 90 kDa fragment, whereas H-89 increased the levels of the cleaved fragments. H-89, but not CPT-cAMP, induced nuclear localization of the 90 kDa FlnA fragment, suggesting FlnA cleavage and nuclear translocation (Figure 4A, lower panels) (see also Supplementary Figure 3). These results indicate that C4-2 cells, which lack FlnA nuclear localization due to increased cAMP levels and PKA activation, can be induced to undergo FlnA cleavage and consequent nuclear translocation by the inhibition of PKA activation. Treatment with H-89, but not CPT-cAMP or Casodex, prevented the invasive nature of C4-2 cells (Figure 4B). In addition, we had previously shown that cytoplasm to nuclear translocation of the 90 kDa FlnA fragment promoted androgen sensitivity in androgen independent C4-2 cells (10). In support of this observation, 8 days of culture in androgen-reduced medium induced growth arrest in LNCaP, but not in C4-2 cells, an effect reversed by treatment with the PKA inhibitor H-89 (1 μM) (Figure 4C). H-89 also prevented AR transcriptional activity (Figure 4D). Taken together, these results point to an important role for FlnA in cell migration and invasion in prostate cancer cells, which is regulated by its phosphorylation by a PKA-dependent mechanism.
Figure 4.
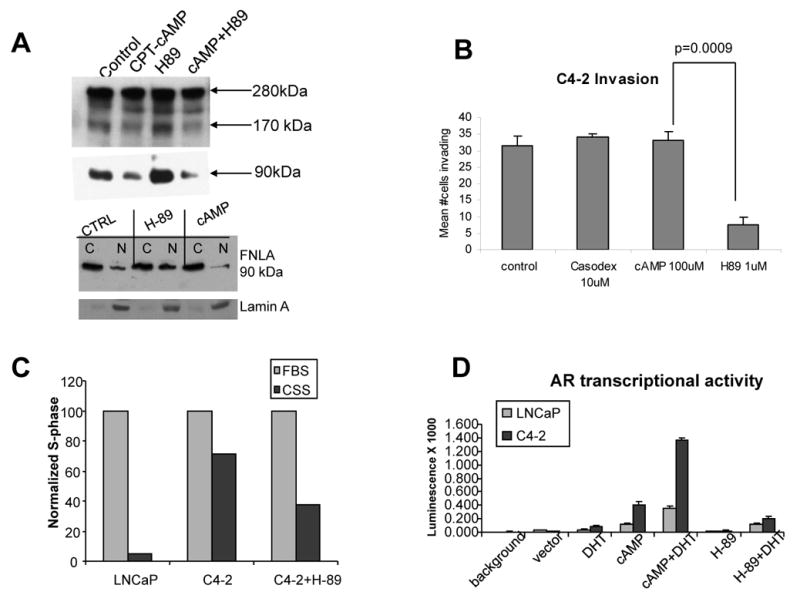
(A) (upper panel) Effect of CPT-cAMP (100 μM) or H-89 (1 μM) on FlnA expression. The cleavage of the full-length protein (280 kDa) increased with H-89 and decreased with cAMP treatment. This is apparent from the levels of both 170 kDa and 90 kDa fragments. (lower panel) C4-2 cells were treated with vehicle, cAMP or H-89 for 48 hours, and fractionated into nuclear and cytoplasmic fractions. In untreated C4-2 cells, the 90 kDa FlnA was mostly nuclear whereas it translocated to the nucleus with H-89 but not cAMP treatment. Lamin A staining determined the efficiency of the nuclear extract. (B-D) Functional effects of H-89 and cAMP treatment in prostate cancer cells. (B) In C4-2 cells, treatment with H-89 prevented invasiveness. (C) and sensitized C4-2 cells to growth inhibition in low-androgen medium (charcoal stripped FBS containing medium). (D) cAMP promoted AR transcriptional activity whereas H-89 inhibited it.
Discussion
The salient feature of the data presented here is that Filamin A translocation from the cytoplasm to the nucleus prevents metastasis in prostate cancer. We show by immunohistochemistry in paraffin-embedded human tissue that in normal prostate, PIN and localized prostate cancer, FlnA is mostly nuclear, whereas in metastatic tissue, it is mostly cytoplasmic. In vitro localization of nuclear FlnA as well as inhibition of FlnA in the cytoplasm prevented cell migration and invasion. Induction of FlnA cleavage by PKA inhibition promoted cytoplasm-to-nucleus translocation and prevented invasion. This is the first time that alterations in the distribution of Filamin A with progression of prostate cancer in humans have been defined.
Filamins are a family of cytoskeletal proteins consisting of three members – Filamins A and B are expressed in non-muscle cells while Filamin C is expressed in muscles (1). Filamin A (280 kDa) is highly susceptible to cleavage by calpains and caspases (1, 11) resulting in two fragments – an N-terminal 170 kDa fragment and a C-terminal 110 kDa fragment which is further cleaved to yield a 90 kDa fragment (1, 4). We previously demonstrated that in androgen-dependent LNCaP prostate cancer cells, the cleaved 90kDa fragment is localized to the nucleus, whereas in its androgen-independent subline C4-2, FlnA failed to cleave and remained cytoplasmic (10).
In the current study we sought to examine the localization of FlnA in human prostate tissue. FlnA is expressed in both the stroma and in the epithelia, thus demonstrating the importance of this protein in prostate development. Nuclear localization of FlnA appears to be the normal configuration in the prostatic epithelium. The mean cytoplasmic expression of FlnA was significantly higher in localized prostate cancer, but there was no direct correlation in this cohort with Gleason scores. However, there is a substantial increase in cytoplasmic staining for FlnA in metastatic tissue, suggesting a role for FlnA in prostate cancer metastases, likely involving cell migration.
Significantly, nuclear translocation of the 90 kDa fragment inhibited the invasiveness of the cell. Further studies are needed to determine how the cell transmits signals to prevent invasion by this cytoplasm-to-nucleus translocation. Our previous studies had shown that in androgen-dependent LNCaP cells, FlnA is cleaved and translocated to the nucleus whereas in its androgen independent subline C4-2, FlnA was phosphorylated at Ser 2152, rendering the protein resistant to cleavage and preventing this translocation. Since Ser 2152 is a PKA consensus site (12, 13), we investigated whether inhibition of PKA activity affected the ability of cells to invade. The PKA inhibitor H-89 induced cleavage of FlnA to the 90 kDa fragment, increased the ability of this fragment to translocate to the nucleus in C4-2 cells, and prevented the invasive quality of C4-2 cells. In addition, it also inhibited AR transcriptional activity and the ability of these cells to grow in low-androgen medium.
These results show that FlnA has multiple roles in a cell. In the cytoplasm, FlnA acts as an actin-binding protein, and helps increase cell motility. The role of Filamin A in cell motility is well known and has been documented in numerous cell models, although not in prostate cancer. Filamin A was shown to be essential in PAK1-mediated cytoskeletal assembly and regulate ruffle-forming which is part of the response of cells to external stimuli resulting in alterations in cell shape, adhesiveness and locomotion (21). Filamin A has been shown to play an essential role in neuronal migration (22), and has also been shown to mediate cell adhesion and migration stimulated by tissue factor, the protease receptor initiating the coagulation system, functions in vascular development, angiogenesis, and tumor cell metastasis (23). Recent studies also indicate a role for Filamin A in heregulin-stimulated cell migration and cell growth in an ovarian cancer cell line (24). Hence a role for FlnA in prostate cancer metastasis and migration are understandable and are to be expected.
Once its role in cell motility and migration is complete, Filamin A undergoes proteolysis and is cleaved to the 90 kDa fragment, which then translocates to the nucleus, where it helps maintain androgen dependence (10). Hence, in the normal adult prostate, which does not require cell motility, FlnA is quickly cleaved and translocated to the nucleus. This is probably the cause for the lack of cytoplasmic Filamin A in normal prostate and its low level of expression in PIN. However, in prostate cancer, FlnA is retained in the cytoplasm which promotes motility in the cell. Since approximately half the localized tumors examined express cytoplasmic Filamin A, our results suggest, and it remains to be proven whether, many of the localized tissues have the potential to undergo metastasis if stimulated to do so by other means, such as growth factor stimulation as shown before in other cell systems (24).
In conclusion, our studies showed that metastatic prostate cancer tissues express high levels of cytoplasmic FlnA and low levels of nuclear FlnA compared to localized cancers. Further, we show that cell invasion and motility is regulated by the localization of this protein in in vitro studies and also shows that inhibition of PKA is likely an important way of regulating the activity of FlnA. This is an important point because, if FlnA cytoplasmic localization is a cause of metastasis, then the presence of FlnA in the cytoplasm may be used as a predictive marker of future metastasis, whereas inhibition of PKA may be used as a therapeutic tool to prevent this effect.
Supplementary Material
Acknowledgments
We are grateful to Dr. Regina Gandour-Edwards and Ms. Stephanie Soares of the Cancer Center Specimen Repository and Dr. XuBao Shi, Department of Urology, University of California Davis for the use of the frozen prostate tumor specimen used in this study, and to Dr. Naveen K. Krishnegowda, Department of Surgery, UT Health Science Center, San Antonio, TX, for technical help. This work was supported by a Merit award from the Department of Veterans Affairs and award CA109057 from the National Cancer Institute.
Footnotes
Translational Relevance: The data presented in this study indicate that cytoplasmic localization of Filamin A (FlnA) correlates to androgen independent metastatic prostate cancer. A major cause of cytoplasmic retention of FlnA appeared to be failure to cleave due to its phosphorylation. Since protein kinase A (PKA) is a putative kinase for FlnA, our results suggest that in patients with metastatic prostate cancer, FlnA nuclear localization may be restored by PKA inhibitors, which could potentially prevent metastasis. These observations indicate that induction of Filamin A cleavage may be of significant clinical relevance in the treatment of patients with advanced prostate cancer.
References
- 1.Gorlin JB, Yamin R, Egan S, et al. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111:1089–105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–8. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 3.Robertson SP, Twigg SR, Sutherland-Smith AJ, et al. Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat Genet. 2003;33:487–91. doi: 10.1038/ng1119. [DOI] [PubMed] [Google Scholar]
- 4.van der Flier A, Sonnenberg A. Structural and functional aspects of filamins. Biochim Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- 5.Anilkumar G, Rajasekaran SA, Wang S, Hankinson O, Bander NH, Rajasekaran AK. Prostate-specific membrane antigen association with filamin A modulates its internalization and NAALADase activity. Cancer research. 2003;63:2645–8. [PubMed] [Google Scholar]
- 6.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. Journal of cellular biochemistry. 2004;91:528–39. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 7.Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? American journal of physiology. 2005;288:C975–81. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 8.Loy CJ, Sim KS, Yong EL. Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc Natl Acad Sci U S A. 2003;100:4562–7. doi: 10.1073/pnas.0736237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozanne DM, Brady ME, Cook S, Gaughan L, Neal DE, Robson CN. Androgen receptor nuclear translocation is facilitated by the f-actin cross-linking protein filamin. Molecular Endocrinology (Baltimore, MD) 2000;14:1618–26. doi: 10.1210/mend.14.10.0541. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Kreisberg JI, Bedolla RG, Mikhailova M, deVere White RW, Ghosh PM. A 90 kDa fragment of filamin A promotes Casodex-induced growth inhibition in Casodex-resistant androgen receptor positive C4-2 prostate cancer cells. Oncogene. 2007;26:6061–70. doi: 10.1038/sj.onc.1210435. [DOI] [PubMed] [Google Scholar]
- 11.Browne KA, Johnstone RW, Jans DA, Trapani JA. Filamin (280-kDa actin-binding protein) is a caspase substrate and is also cleaved directly by the cytotoxic T lymphocyte protease granzyme B during apoptosis. The Journal of biological chemistry. 2000;275:39262–6. doi: 10.1074/jbc.C000622200. [DOI] [PubMed] [Google Scholar]
- 12.Jay D, Garcia EJ, Lara JE, Medina MA, de la Luz Ibarra M. Determination of a cAMP-dependent protein kinase phosphorylation site in the C-terminal region of human endothelial actin-binding protein. Arch Biochem Biophys. 2000;377:80–4. doi: 10.1006/abbi.2000.1762. [DOI] [PubMed] [Google Scholar]
- 13.Jay D, Garcia EJ, de la Luz Ibarra M. In situ determination of a PKA phosphorylation site in the C-terminal region of filamin. Mol Cell Biochem. 2004;260:49–53. doi: 10.1023/b:mcbi.0000026052.76418.55. [DOI] [PubMed] [Google Scholar]
- 14.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Molecular and cellular biology. 2004;24:3025–35. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tigges U, Koch B, Wissing J, Jockusch BM, Ziegler WH. The F-actin cross-linking and focal adhesion protein filamin A is a ligand and in vivo substrate for protein kinase C alpha. The Journal of biological chemistry. 2003;278:23561–9. doi: 10.1074/jbc.M302302200. [DOI] [PubMed] [Google Scholar]
- 16.Rubin MA, Putzi M, Mucci N, et al. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res. 2000;6:1038–45. [PubMed] [Google Scholar]
- 17.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer research. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 18.Porter RM, Holme TC, Newman EL, Hopwood D, Wilkinson JM, Cuschieri A. Monoclonal antibodies to cytoskeletal proteins: an immunohistochemical investigation of human colon cancer. J Pathol. 1993;170:435–40. doi: 10.1002/path.1711700406. [DOI] [PubMed] [Google Scholar]
- 19.Bedolla R, Prihoda TJ, Kreisberg JI, et al. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin Cancer Res. 2007;13:3860–7. doi: 10.1158/1078-0432.CCR-07-0091. [DOI] [PubMed] [Google Scholar]
- 20.Kreisberg JI, Malik SN, Prihoda TJ, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer research. 2004;64:5232–6. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 21.Vadlamudi RK, Li F, Adam L, et al. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–90. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 22.Stossel TP, Condeelis J, Cooley L, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–45. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 23.Ott I, Fischer EG, Miyagi Y, Mueller BM, Ruf W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin-binding protein 280. J Cell Biol. 1998;140:1241–53. doi: 10.1083/jcb.140.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourguignon LY, Gilad E, Peyrollier K. Heregulin-mediated ErbB2-ERK signaling activates hyaluronan synthases leading to CD44-dependent ovarian tumor cell growth and migration. The Journal of biological chemistry. 2007;282:19426–41. doi: 10.1074/jbc.M610054200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.