Abstract
Both stress-system activation and melancholic depression are characterized by fear, constricted affect, stereotyped thinking, and similar changes in autonomic and neuroendocrine function. Because norepinephrine (NE) and corticotropin-releasing hormone (CRH) can produce these physiological and behavioral changes, we measured the cerebrospinal fluid (CSF) levels each hour for 30 consecutive hours in controls and in patients with melancholic depression. Plasma adrenocorticotropic hormone (ACTH) and cortisol levels were obtained every 30 min. Depressed patients had significantly higher CSF NE and plasma cortisol levels that were increased around the clock. Diurnal variations in CSF NE and plasma cortisol levels were virtually superimposable and positively correlated with each other in both patients and controls. Despite their hypercortisolism, depressed patients had normal levels of plasma ACTH and CSF CRH. However, plasma ACTH and CSF CRH levels in depressed patients were inappropriately high, considering the degree of their hypercortisolism. In contrast to the significant negative correlation between plasma cortisol and CSF CRH levels seen in controls, patients with depression showed no statistical relationship between these parameters. These data indicate that persistent stress-system dysfunction in melancholic depression is independent of the conscious stress of the disorder. These data also suggest mutually reinforcing bidirectional links between a central hypernoradrenergic state and the hyperfunctioning of specific central CRH pathways that each are driven and sustained by hypercortisolism. We postulate that α-noradrenergic blockade, CRH antagonists, and treatment with antiglucocorticoids may act at different loci, alone or in combination, in the treatment of major depression with melancholic features.
Major depression is a complex disorder with an estimated lifetime prevalence of 15% in women and 8% in men (1). Depression is the main cause of suicide: ≈70% of all suicides are attributed to untreated depression. Studies in the United States suggest that, at any given time, ≈2–3% of the population is hospitalized or seriously impaired by affective illness. The World Health Organization has declared major depression as the single largest cause of morbidity for women and the leading cause of disability worldwide.
The current standard diagnostic instrument for psychiatry, the Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV) (2), lists two subtypes of major depression, melancholic and atypical. The features of melancholic depression include insomnia (most often early morning awakening), loss of appetite, weight loss, inappropriate guilt, and lack of pleasure (anhedonia). The second major subtype is major depression with atypical features, characterized in part by hypersomnia, hyperphagia, lethargy, and fatigue. The subclassification of depression provides direction for the appropriate choice of antidepressant medication. Studies in identical twins show a significantly higher concordance for either melancholic or atypical depression in a given set of twins (see ref. 50 for review).
Although the term “depression” implies a suppression of thought and feeling, major depression with melancholic features belies the term depression in that it is a state of hyperarousal and fear, often stemming from a profound sense of personal unworthiness and pessimism about the future (3, 4). Although patients with melancholic depression are often clear about the deficiencies that they attribute to themselves, they find it more difficult than usual to concentrate or to fashion complex plans of action. Patients with severe melancholia also seem to have preferential access to painfully charged memories of past losses and failures (4). Inseparable from their loss of both cognitive and emotional ranges is a loss of pleasure in everyday activities. It is now clear that the hyperarousal of melancholia is matched by indices of physiological hyperarousal, including early morning awakening; sustained hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system activation (5, 6); and inhibition of appetite, libido, and endocrine programs for growth and reproduction (3).
The clinical and biochemical manifestations of melancholic depression that often persist for months closely resemble those that occur reflexively during acutely stressful or threatening situations (3, 4). Both melancholic depression and acute stress are characterized by arousal, sustained focus on the threatening stimulus, fear-related behaviors, and relatively stereotyped states of cognition and affect (3, 4). These symptoms are associated with activation of the HPA axis and the sympathetic nervous system, and with inhibition of neurovegetative functions that might be counterproductive during a life-threatening situation (e.g., feeding, sleeping, growth, sexual behavior, reproduction, etc.) (3, 4).
The close clinical and biochemical resemblance between the symptom complex of melancholic depression and the phenomenology of the stress response led us to postulate that melancholic depression represents activation of the principal effectors of the stress response [the locus-ceruleus–norepinephrine (LC-NE) and the corticotropin-releasing hormone (CRH) systems] (3, 4). Centrally, NE acts as a major alarm-producing neurotransmitter in the brain and inhibits feeding, grooming, and sleeping. In addition, NE plays an important modulatory role by activating the amygdala (4, 7) and, by so doing, enhances the eventual coding of emotionally laden memories of disturbing or stressful events. In contrast, NE seems to inhibit medial-prefrontal cortex components that function to shift mood from one state to another, to promote novel over well-rehearsed behaviors, and to inhibit HPA axis and brainstem autonomic activity (4, 8). NE also directly serves as a stimulus to the HPA axis and, during chronic stress, mediates direct sympathetic nervous system input to the adrenal gland, which helps in sustaining hypercortisolism (9). Thus, activation of noradrenergic neurons increases fear-related behaviors, promotes relatively stereotyped behaviors and affects, and may both activate hypothalamic and brainstem neuroendocrine and arousal centers and release them from cortical inhibition (4).
CRH was first isolated as a principal hypothalamic releasing factor for the HPA axis. CRH has also been shown to play a significant role in the acquisition and storage of classically conditioned fear responses in the amygdala. Descending hypothalamic CRH pathways to brainstem noradrenergic nuclei also contribute to the arousal and activation of the sympathetic nervous system. Thus, the central administration of CRH to rodents and primates not only promotes activation of the HPA axis, but also sets into motion a sequence of behavioral and physiological events that closely resemble those in melancholic depression and stress (3, 4, 10–14).
The purpose of the present study was to evaluate centrally directed NE and CRH secretion in medication-free patients with melancholic depression by measuring the NE and CRH levels in lumbar cerebrospinal fluid (CSF) sampled continuously for 30-h by an indwelling lumbar catheter. We also measured the 30-h pattern of plasma adrenocorticotropic hormone (ACTH) and cortisol secretion in the same subjects. Cortisol is not only hypersecreted in melancholic depression but also significantly influences behavior and affects the LC-NE and CRH systems, as well as the activities of the reproductive and growth hormone axes. By evaluating CSF NE and CRH levels at night while patients slept, as well as during the daytime, we also hoped to determine whether the activation of stress mediators in major depression represented a potential contributor to its clinical and biochemical manifestations, or whether the stress mediators were merely artifacts of the conscious distress of the illness.
Specifically, this study asked four major questions: (i) Is either CSF NE or CRH hypersecreted in melancholic depression? (ii) If so, do abnormalities persist while patients sleep? (iii) Are abnormalities in CSF NE and CRH secretion related to one another? and (iv) Does hypercortisolism seem to influence or to reinforce potential abnormalities in CSF NE or CSF CRH secretion?
Methods
Clinical Research Protocol.
Under an Institutional Review Board-approved clinical protocol (National Institute of Mental Health Protocol 90-M-199), eight female and two male patients with major depressive episodes with melancholic features [age (mean ± SD) 40.9 ± 2.7 yr; range, 28.2–53.7 yr], who were medication-free for at least 2 weeks, and six female and eight male healthy volunteers [age 37.7 ± 2.2 yr; range, 27.4–53.0 yr] participated in this study. Patients' discontinuation of medication did not take place for the purpose of participation in this study. Patients were either medication-free when they were referred to us or taken off medications that were not clinically effective. All depressed patients were hospitalized on the clinical research units of the National Institute of Mental Health. The presence of a current major depressive episode was diagnosed by both the Diagnostic and Statistical Manual of Mental Disorders, 3rd Ed., Revised (DSM-III-R) (15) and the World Health Organization's Research Diagnostic Criteria (RDC) (16). A minimum Hamilton Rating Scale for Depression score (17) of 15 was required for inclusion in this study. Healthy volunteers were free of ongoing physical illness, determined by history and by physical examination, and showed no evidence of major psychiatric illness, determined by both clinical and structured interviews. Female subjects were studied during days 1–7 of the menstrual cycle. Before the actual sampling of CSF and plasma, all subjects were adapted to the hospital setting for at least 1 night.
We conducted continuous CSF and plasma sampling in healthy volunteers and in patients with major depression as previously described (18). All patients and volunteers were on a low-monoamine diet for at least 3 days before CSF sampling. CSF sampling began at 09:00–10:00 a.m. with the introduction of a standard 20-gauge epidural catheter through an 18-gauge Touhy spinal needle into the subarachnoid space at the L3/4 or L4/5 level. The catheter was attached to a miniroller pump and 6 ml/h was exfused at a constant rate that represents 25–33% of the normal CSF production rate (19). Sampling lasted for 30 h, at which time the catheter was removed; during CSF sampling, the subject remained flat in bed, but was free to lie in any position (i.e., prone, supine, or lateral).
Six 1-ml aliquots initially were placed in the refrigerator for 30 min and then placed on dry ice. The 1-ml aliquot taken between 30 and 40 min was assayed for CSF CRH and the 1-ml aliquot taken from 40 to 50 min was measured for CSF NE. This procedure previously has been demonstrated to be a safe and reliable method for sampling CSF continuously (18, 20). Previous studies have shown human CRH to be stable in CSF under these conditions (20). Blood was drawn through an indwelling i.v. catheter in the arm, on the half-hour and at half-hour intervals for 30 h, for measurement of plasma ACTH and cortisol. Subjects remained on bed rest during the study and at least until the morning after the withdrawal of the catheters.
Assays.
CSF CRH levels were measured in Sep-Pak C18 column-extracted samples in a pool of two consecutive 1-ml aliquots per hour, representing a total of 20 min of continuous sampling beginning at 30 min past the hour. Extracted samples were reconstituted in buffer at a concentration 3-fold higher than the original sample and assayed in duplicate. The CSF CRH concentration was measured by an RIA as previously described (20, 21). The detection limit ranged from 2 to 2.5 pg per tube. Plasma ACTH was measured by RIA with prior extraction by using a standard method (22). Plasma cortisol was measured by direct RIA using a standard kit (Diagnostic Products, Los Angeles). CSF NE levels were measured by reversed-phase high-performance liquid chromatography with electrochemical detection in the aliquot consecutive to those used for CRH levels, according to a standard method (23). All samples from the same subject were analyzed together, and assay runs included both patients and control subjects. Interassay coefficients of variation were <15% for all assays.
Statistical Analysis.
For each patient, each series of measurements was averaged, and the average values were compared. Analyses were performed by using the entire series of hormone measurements or by cropping data from the initial hour and the last 2 h of each series. Cropping was done to minimize the effects of hormonal changes related to the stress of beginning and terminating the CSF-sampling procedure in our analysis. Differences between control and depressed groups were examined by using an unpaired, two-tailed Student's t test, using statistica software (Statsoft, Tulsa, OK) on a Macintosh computer.
Correlation Analysis.
Crosscorrelation analyses with and without detrending were performed on series of measurements of the four substances as previously described (24). Crosscorrelation analysis was computed between the series of measurements for two informational substances at various time lags covering the 30-h period of study. If the release of a substance Y is regulated by a substance X (X, releasing substance; Y, effector substance), then one might expect the concentration time series of substance Y to follow (lag) quantitatively in time the concentration time series of substance X. Crosscorrelation was computed after lagging (shifting) the concentration time series of substance X relative to the concentration time series of substance Y. If we call rk the coefficient of correlation between two informational-substance time series at a lag time k (any lag time from 0 to ±1440 min) for one person, then the mean rk of all persons in each group was considered significant when it exceeded 0 by more than 2 standard errors (SE). The SE was calculated from the individual rk values for all persons in that group at a lag time k. We mitigated the effect of baseline shifts and of the circadian component (24) by performing correlation analysis that removed the impact of the inherent circadian component by detrending analysis (using a five-point moving average). As the relationship between plasma ACTH and plasma cortisol is well characterized, the short-term, circadian-independent correlation between plasma ACTH and cortisol around lag 0 was used as positive control for our correlation analysis.
Analysis of 24-h Rhythm.
Each series of measurements (CSF NE, CSF CRH, plasma cortisol, and plasma ACTH) was analyzed for circadian rhythmicity. The presence of sinusoidally varying diurnal trends was tested by cosinor analysis, which represents a linear reduction of sinusoidal regression (25, 26). Cosinor analysis was done using chronolab for the Macintosh computer (Universidade de Vigo, Spain, Bioengineering and Chronobiology Laboratory, http://www.tsc.uvigo.es/BIO/). Each series of measurements was individually input into chronolab, and parameters of the sinusoidal regression such as MESOR (midline-estimating statistic of rhythm or rhythm-adjusted mean), acrophase [a measure of time, the lag from a defined reference time point (midnight of the first day of measurement in our analysis) of the crest time in the cosine-curve-fitted curve to the data], and amplitude (half the extent of rhythmic change, or the difference between the maximum concentration and the MESOR of the fitted curve) were obtained for a 24-h period. Rhythm detection is sought by testing the null hypothesis of zero amplitude with an F test by the chronolab program.
Results
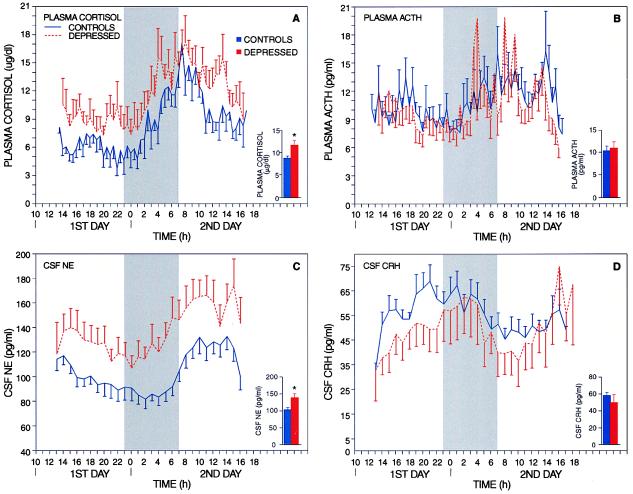
Fig. 1 displays the average of the curves of plasma cortisol, plasma ACTH, CSF NE, and CSF CRH concentrations (mean ± SE) for control and major depression (melancholic type) groups of subjects. Unless otherwise specified, the results presented are from the analysis of the cropped series of measurements. Mean 30-h plasma cortisol levels were significantly elevated in patients with melancholic depression when compared with controls [11.6 ± 1.2 (mean ± SE) and 8.7 ± 0.6 μg/dl, respectively; P < 0.02] (Fig. 1A). Despite significant increases in basal cortisol levels, plasma ACTH levels were similar in patients with depression and in controls (10.8 ± 1.6 and 10.2 ± 1.1 pg/ml, respectively; P = not significant) (Fig. 1B). Compared with the ratio in controls, the plasma cortisol-to-ACTH ratio was significantly higher in patients with melancholic depression, indicating that patients with major depression showed a relatively greater plasma cortisol response to a given simultaneous level of plasma ACTH (1,572,000 ± 164,000 and 4,071,000 ± 778,000, respectively; P < 0.001). Mean 30-h CSF NE levels were significantly increased in depressed patients when compared with controls (137.4 ± 12.7 and 102.3 ± 7.0 pg/ml, respectively; P < 0.02) (Fig. 1C). CSF NE levels were elevated both at night (including during sleep) and during the day to the same proportional extent. Mean 30-h CSF CRH levels, despite significant increases in basal cortisol levels, were also similar in patients with depression and in controls (48.9 ± 9.8 and 57.1 ± 4.1 pg/ml, respectively; P = not significant) (Fig. 1D). Compared with the ratio in controls, the plasma cortisol to CSF CRH ratio was significantly higher in patients with melancholic depression (1,558,000 ± 102,000 and 3,032,000 ± 606,000, respectively; P < 0.01).
Figure 1.
Diurnal curves of plasma cortisol (A), plasma ACTH (B), CSF NE (C), and CSF CRH (D) levels (mean ± SE) in 14 healthy volunteers and 10 patients with major depression, melancholic type. Curves result from the averaged measurement per time point across a group of subjects by using the cropped hormonal series. The shaded area represents data recorded with the lights off (23:00–07:00 h). In the right corner Insets under each pair of curves, the bar graphs represent the average of the mean value for each series of hormonal measurements (mean ± SE). *, P < 0.02.
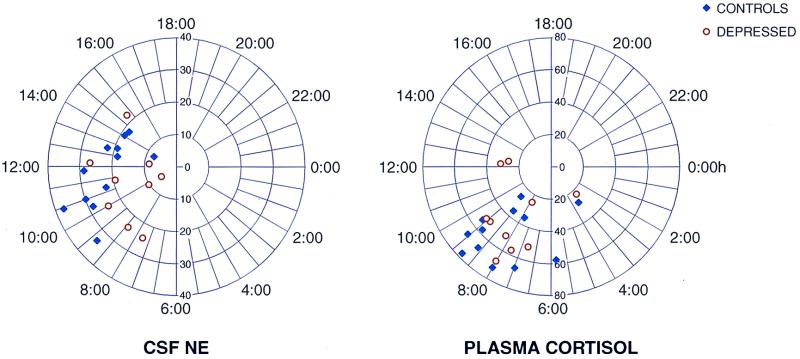
Analysis of the 24-h pattern in plasma cortisol, plasma ACTH, and CSF CRH showed significant diurnal variations. Polar chronograms of CSF NE and plasma cortisol are shown in Fig. 2. CSF NE showed a 24-h pattern very similar to that of plasma cortisol in both patients and controls; the highest levels for both substances occurred in the morning. It is important to note that the detection of rhythm in CSF NE was virtually as consistent as that for plasma cortisol. Plasma cortisol levels showed the expected diurnal variation in both patients and controls, with the mean peak occurring in the morning around 8:00 a.m. (Fig. 2) and the mean nadir occurring between 1:00 and 2:00 a.m. (Fig. 1A) (27).
Figure 2.
Polar representations of the biologic rhythm's percentage amplitude/MESOR (radius) and acrophase (angle) relations obtained by a single cosinor analysis of CSF NE and plasma cortisol 24-h circadian rhythm. In these graphs, a statistical summary is shown with a bivariate statistical confidence region computed for a 24-h period to detect a rhythm by a confidence region not overlapping the pole by using the cropped hormonal series. Note that in both polar graphs all the acrophases are within a 12-h time span, and most of them are within a 6-h time span. These characteristics indicate that the rhythm of CSF NE seems to be as tightly regulated as plasma cortisol is by the circadian clock. The circadian representation for plasma cortisol is shown as a reference of our analysis and population.
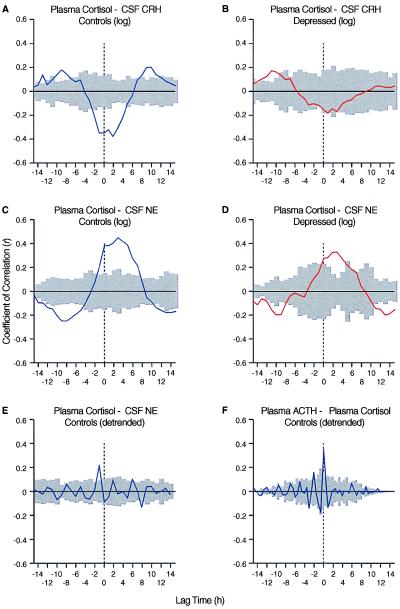
The results of our crosscorrelation analyses are summarized in Fig. 3. Crosscorrelation for CSF CRH and plasma cortisol showed a significant negative correlation in controls (Fig. 3A) for lag 0 (P ≤ 0.05); the correlation between CSF CRH and plasma cortisol was not found to be significant in depressed patients (Fig. 3B). Crosscorrelation analysis revealed a significant positive correlation at lag 0 between CSF NE and plasma cortisol in both patients and controls (Fig. 3 C and D; P ≤ 0.05). Even after detrending the hormonal series, a significant correlation (P ≤ 0.05) between CSF NE and plasma cortisol was observed, and this correlation was nearly as robust as that seen for plasma ACTH and plasma cortisol (Fig. 3 E and F).
Figure 3.
Graphs depicting the crosscorrelation analyses of the mean coefficients of correlation between CRH and cortisol (A and B), NE and cortisol (C–E), and ACTH and cortisol (F), by using the cropped hormonal series. Note that CRH and cortisol display a negative correlation in controls, but that correlation is lost in patients. A positive correlation exists between NE and cortisol. The shaded area includes the two SEs calculated from the individual values of rk for all the subjects in a certain group at the time k and indicates the limits of significance (P = 0.05). Crosscorrelation between ACTH and cortisol (F) is shown as a reference of our analysis and population.
Discussion
This study shows that around the clock, patients with melancholic depression have elevated levels of CSF NE and plasma cortisol, but not of CSF CRH or plasma ACTH. The CSF NE and plasma cortisol changes are tightly linked, but the normal inverse relation between CSF CRH and plasma cortisol is lost. This pattern of a central hypernoradrenergic state and adrenocortical secretion not only may characterize melancholic depression but also produce it.
The syndrome of melancholic depression consists of intense anxiety and arousal, constriction of affect and cognition, activation of the HPA axis, and inhibition of neurovegetative functions, including the programs for growth and reproduction (3, 4). Both NE and CRH each can contribute to these clinical and biochemical manifestations. Previous studies regarding levels of CSF NE and CSF CRH have reported normal, reduced, and increased levels (5, 21, 28–30). The differences between these prior results and those of the current study could reflect greater resolution with continuous sampling, acute stress-related changes during a single-time-point study, or differences in the populations of depressed patients under study.
CSF NE.
The continuous elevation in CSF NE levels that persists during sleep indicates that the activation of the central noradrenergic system in depression is not related to the conscious distress of the disorder.
NE serves globally as an emergency or alarm system that leads to decreases in neurovegetative functions, such as eating and sleeping, and contributes to accompanying increases in autonomic and neuroendocrine responses to stress, including HPA axis activation. NE also activates the amygdala, a principal brain locus for fear-related behaviors. In addition, NE release during stress inhibits the medial prefrontal cortex (8) and, by so doing, could interfere with two of its key functions (i.e., the shifting of mood from one state to the other based on internal and external cues and the generation of novel, complex behaviors) (4). Thus, hyperfunctioning of the central noradrenergic system could contribute to the full spectrum of the clinical and biochemical manifestations of melancholic depression (3, 4). Moreover, by enhancing the long-term storage of aversively charged emotional memories in sites such as the hippocampus and striatum, NE could also contribute to subsequent episodes of depression by facilitating the recall of previous ones (4).
Bilateral lesioning of the LC in the rat causes decreases in NE content in the CSF and brain (31, 32). An important blood–brain barrier to peripheral levels of NE has been described (33), and despite a positive correlation between plasma and CSF levels of NE (33), CSF levels are not markedly affected by acute alterations in the plasma levels. Thus, the majority of CSF NE is likely to derive from brainstem noradrenergic nuclei.
Crosscorrelation analysis showed a significant positive correlation between CSF NE and plasma cortisol in depressed patients and in controls. In controls, the crosscorrelations between CSF NE and plasma cortisol were nearly as robust as those between plasma ACTH and plasma cortisol levels. Thus, the positive relationship between CSF NE and plasma cortisol is virtually as great as one of the most classic relationships in clinical endocrinology.
Several factors have been identified that potentially contribute to the strong positive correlation between CSF NE and plasma cortisol concentrations. First, NE activates hypothalamic CRH neurons directly (34). Second, NE activates the amygdala, which in turn activates the HPA axis (7). Third, NE inhibits the medial prefrontal cortex, which normally restrains the HPA axis (8). Fourth, it now clearly is established that the sympathetic nervous system exerts extrapituitary modulation of adrenocortical corticosteroid secretion, especially during chronic stress (8, 35–39).
In addition to noradrenergic mediation of hypercortisolism, hypercortisolism per se activates brainstem noradrenergic neurons by CRH. Thus, glucocorticoids accentuate a descending hypothalamic CRH pathway that activates brainstem noradrenergic nuclei. Moreover, in a postmortem study of brains taken from depressed patients who had committed suicide, the significant increase in hypothalamic neurons expressing CRH was greatest in those neurons sending descending projections to brainstem noradrenergic nuclei (40). We postulate that hyperactivity of this pathway plays a role in the pronounced hyperactivity of central noradrenergic neurons and serves to help maintain this condition for prolonged periods of time. We further postulate that either α-noradrenergic antagonists or glucocorticoid antagonists will prove to exert therapeutic effects in patients with major depression associated with significant hypercortisolism, including not only patients with melancholic depression but also those with psychotic depression.
CSF CRH.
Several populations of CRH-containing neurons have been identified that are of potential relevance to the symptom complex of melancholic depression. The largest of these is a glucocorticoid-suppressible hypothalamic system that sends CRH nerve terminals to the median eminence for activation of the pituitary–adrenal axis. As we noted previously, a separate, smaller descending hypothalamic pathway that responds positively to glucocorticoids conveys CRH for the activation of brainstem noradrenergic neurons. We have previously shown that glucocorticoids also significantly increase the levels of CRH mRNA in an extrahypothalamic CRH pathway that is located in the amygdala that plays an important role in fear-related behaviors (41).
Glucocorticoids not only activate the descending hypothalamic and amygdala CRH systems, but also accentuate CRH-induced fear-related behaviors and can produce them in their own right. These findings further accentuate the potentially important role of glucocorticoid excess in contributing to and sustaining the pathological hyperarousal of melancholic depression and support the use of glucocorticoid antagonists in major depression with melancholic and/or psychotic features.
Despite several lines of data suggesting that CRH is involved in the hypercortisolism of depression, we found normal 30-h CSF CRH levels in hypercortisolemic patients with melancholic depression, analogous to our previous finding based on a single data point (21). This does not mean, however, that the CRH component of the HPA axis is not abnormal. In a previous study of patients with Cushing's disease (a rare form of hypercortisolism caused by a pituitary rather than a central nervous system defect), we found profound suppression of CSF CRH levels in the patients, indicating that in the absence of a central nervous system defect, CSF CRH levels are suppressed by hypercortisolism (42). Indeed, in a comparison of CSF CRH levels in groups of patients with major depression or Cushing's disease, individually matched for the severity of hypercortisolism, we found that depressed patients showed significantly higher CSF CRH levels than did Cushing's disease patients. Thus, CSF CRH levels in patients with melancholic depression, though quantitatively “normal,” are inappropriately elevated for their degree of hypercortisolism.
We found a significant negative correlation between CSF CRH and plasma cortisol levels in controls that was similar to that which we had previously reported (20). However, this significant negative correlation was not found in patients with depression. This loss of the normal relationship between CSF CRH and plasma cortisol could mean either a disruption in the integrity of glucocorticoid negative feedback on hypothalamic CRH-containing neurons that project to the median eminence or an overriding of glucocorticoid-negative feedback by excitatory stimuli, such as NE from the LC or other brainstem loci. In addition, disproportionate glucocorticoid-mediated activation of descending hypothalamic and amygdala ACTH pathways in depression could also contribute to the loss of the negative correlation between plasma cortisol and CSF CRH seen in healthy volunteers.
In clinical studies exploring hormonal responses to CRH, we advanced data indicating that the CRH system in major depression was activated as compared with controls (43). Holsboer et al. (44) subsequently replicated this finding. Our findings in experimental animals (45, 46) and human subjects (47) that antidepressants consistently down-regulate the CRH system are compatible with this formulation. Thus, even though overall CSF CRH levels in melancholic depression were “normal” in depressed patients, CRH is likely to play an important role in the symptom complex of melancholic depression, including hypercortisolism, inhibition of vegetative function, and behavioral arousal and anxiety.
As we noted, hypercortisolism fails to suppress adequately CSF CRH levels in depressed patients. Moreover, compared with controls, CRH function in depression is likely to be relatively more active in areas in which CRH neurons are activated by glucocorticoids. These areas (the amygdala and descending parvocellular paraventricular nucleus projections) promote fear-related behaviors, as well as autonomic and behavioral arousal. Data also suggest that CRH and glucocorticoid excess could exert additive or synergistic effects on fear-related behaviors (48, 49). Glucocorticoids serve to heighten behavioral responses to CRH, such as acoustic startle (49), and can themselves mimic the effects of CRH on acoustic-startle responses (49).
Conclusions
The most striking findings of this study were the pronounced activation of the central noradrenergic system in patients with melancholic depression and the data indicating that cortisol is likely to play several roles in producing and sustaining the depressive syndrome. Several implications for future therapeutic intervention in major depression with melancholic features or in psychotic depression emerge from these findings. First, the potential efficacy of an α-noradrenergic blocker should be investigated; α-noradrenergic blockers given to patients with pheochromocytoma are known to reduce the anxiety associated with this disorder. Second, given the positive correlation between plasma cortisol and NE levels and the capacity of glucocorticoids to also potentiate the activity of the descending hypothalamic and amygdala CRH pathways, further trials of glucocorticoid antagonism should be conducted in patients with major depression with melancholic features or with psychotic depression, including newly available, relatively pure glucocorticoid antagonists. Third, the application of the new nonpeptide, orally absorbed CRH type 1 receptor antagonists that cross the blood–brain barrier could mitigate the hypercortisolism of depression and interfere with the CRH-mediated transduction of fear-related behaviors and the activation of brainstem noradrenergic neurons.
Acknowledgments
This work was partially supported by the National Alliance for Research on Schizophrenia and Depression (M.-L.W.), the University of Virginia National Center for Research Resources Grant M01 RR-00847 (J.D.V.), and the National Institutes of Health Grant MH 51853 (S.M.M.).
Abbreviations
- NE
norepinephrine
- CRH
corticotropin-releasing hormone
- CSF
cerebrospinal fluid
- ACTH
adrenocorticotropic hormone
- HPA
hypothalamic–pituitary–adrenal
- LC
locus ceruleus
References
- 1.Kessler R C, McGonagle K A, Zhao S, Nelson C B, Hughes M, Eshleman S, Wittchen H U, Kendler K S. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: Am. Psychiatr. Assoc.; 1994. [Google Scholar]
- 3.Gold P W, Goodwin F K, Chrousos G P. N Engl J Med. 1988;319:348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- 4.Gold P W, Chrousos G P. Proc Assoc Am Phys. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- 5.Post R M, Ballenger J C. Neurobiology of Mood Disorders. Baltimore: Williams and Wilkins; 1984. [Google Scholar]
- 6.Carroll B J, Curtis G C, Davies B M, Mendels J, Sugerman A A. Psychol Med. 1976;6:43–50. doi: 10.1017/s0033291700007480. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein L E, Rasmusson A M, Bunney B S, Roth R H. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnsten A F, Mathew R, Ubriani R, Taylor J R, Li B M. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 9.Bornstein S R, Chrousos G P. J Clin Endocrinol Metab. 1999;84:1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- 10.Brown M, Fisher L, Spiess J, Rivier C, Rivier J, Vale W. Endocrinology. 1982;111:928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- 11.Sutton R E, Koob G F, LeMoal M, Rivier J, Vale W. Nature (London) 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 12.Britton D R, Koob G F, Rivier J, Vale W. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- 13.Sirinathsinghji D J S, Rees L H, Rivier J, Vale W. Nature (London) 1983;305:232–235. doi: 10.1038/305232a0. [DOI] [PubMed] [Google Scholar]
- 14.Rivier C, Vale W. Endocrinology. 1984;114:914–921. doi: 10.1210/endo-114-3-914. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Ed. Washington, DC: Am. Psychiatr. Assoc.; 1987. , Revised. [Google Scholar]
- 16.World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. Geneva: W.H.O.; 1993. [Google Scholar]
- 17.Hamilton M. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruce J N, Oldfield E H. Neurosurgery. 1988;23:788–790. doi: 10.1227/00006123-198812000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Wood J H. In: Neurobiology of Cerebrospinal Fluid. Wood J H, editor. New York: Plenum; 1980. pp. 1–16. [Google Scholar]
- 20.Kling M A, DeBellis M D, O'Rourke D K, Listwak S J, Geracioti T D, Jr, McCutcheon I E, Kalogeras K T, Oldfield E H, Gold P W. J Clin Endocrinol Metab. 1994;79:233–239. doi: 10.1210/jcem.79.1.8027234. [DOI] [PubMed] [Google Scholar]
- 21.Roy A, Pickar D, Paul S, Doran A, Chrousos G P, Gold P W. Am J Psychiat. 1987;144:641–645. doi: 10.1176/ajp.144.5.641. [DOI] [PubMed] [Google Scholar]
- 22.Gold P W, Loriaux D L, Roy A, Kling M A, Calabrese J R, Kellner C H, Nieman L K, Post R M, Pickar D, Gallucci W. N Engl J Med. 1986;314:1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- 23.Kaler S G, Goldstein D S, Holmes C, Salerno J A, Gahl W A. Ann Neurol. 1993;33:171–175. doi: 10.1002/ana.410330206. [DOI] [PubMed] [Google Scholar]
- 24.Magiakou M A, Mastorakos G, Rabin D, Margioris A N, Dubbert B, Calogero A E, Tsigos C, Munson P J, Chrousos G P. Clin Endocrinol (Oxford) 1996;44:419–428. doi: 10.1046/j.1365-2265.1996.683505.x. [DOI] [PubMed] [Google Scholar]
- 25.Bingham C, Arbogast B, Guillaume G C, Lee J K, Halberg F. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 26.Fernandez J R, Hermida R C. Chronobiol Int. 1998;15:191–204. doi: 10.3109/07420529808998683. [DOI] [PubMed] [Google Scholar]
- 27.Krieger D T. Med Clin N Am. 1978;62:251–259. doi: 10.1016/s0025-7125(16)31802-8. [DOI] [PubMed] [Google Scholar]
- 28.Potter W Z, Manji H K. Clin Chem. 1994;40:279–287. [PubMed] [Google Scholar]
- 29.Nemeroff C B, Wilderlov E, Bisette G. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 30.Geracioti T D, Jr, Loosen P T, Orth D N. Biol Psychiatry. 1997;42:165–174. doi: 10.1016/S0006-3223(96)00312-5. [DOI] [PubMed] [Google Scholar]
- 31.Anselmo F J, Franci C R, Krulich L, Antunes-Rodrigues J, McCann S M. Brain Res. 1997;767:289–296. doi: 10.1016/s0006-8993(97)00613-6. [DOI] [PubMed] [Google Scholar]
- 32.Fornai F, Alessandri M G, Torracca M T, Bassi L, Corsini G U. J Pharmacol Exp Ther. 1997;283:100–107. [PubMed] [Google Scholar]
- 33.Ziegler M G, Wood J H, Lake R, Kopin I J. Am J Psychiatry. 1977;134:565–568. doi: 10.1176/ajp.134.5.565. [DOI] [PubMed] [Google Scholar]
- 34.Calogero A E, Gallucci W T, Chrousos G P, Gold P W. J Clin Invest. 1988;82:839–846. doi: 10.1172/JCI113687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein D S, Zimlichman R, Kelly G D, Stull R, Bacher J D, Keiser H R. J Neurochem. 1987;49:1484–1490. doi: 10.1111/j.1471-4159.1987.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 36.Holst J J, Ehrhart B M, Messell T, Poulsen S S, Harling H. Am J Physiol. 1991;261:E31–E40. doi: 10.1152/ajpendo.1991.261.1.E31. [DOI] [PubMed] [Google Scholar]
- 37.Ehrhart B M, Bornstein S R, Guse B H, Stromeyer H G, Rasmussen T N, Scherbaum W A, Adler G, Holst J J. Neuroendocrinology. 1994;59:406–412. doi: 10.1159/000126685. [DOI] [PubMed] [Google Scholar]
- 38.Ehrhart B M, Hinson J P, Bornstein S R, Scherbaum W A, Vinson G P. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 39.Dallman M F, Engeland W C, McBride M H. Ann NY Acad Sci. 1977;297:373–392. doi: 10.1111/j.1749-6632.1977.tb41869.x. [DOI] [PubMed] [Google Scholar]
- 40.Raadsheer F C, Hoogendijk W J G, Stam F C, Tilders F J H, Swaab D F. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 41.Makino S, Gold P W, Schulkin J. Brain Res. 1994;64:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 42.Kling M A, Roy A, Doran A R, Calabrese J R, Rubinow D R, Whitfield H J, Jr, May C, Post R M, Chrousos G P, Gold P W. J Clin Endocrinol Metab. 1991;72:260–271. doi: 10.1210/jcem-72-2-260. [DOI] [PubMed] [Google Scholar]
- 43.Gold P W, Chrousos G P, Kellner C H, Post R M, Roy A, Avgerinos P C, Schulte H M, Oldfield E H, Loriaux D L. Am J Psychiatry. 1981;141:622–626. doi: 10.1176/ajp.141.5.619. [DOI] [PubMed] [Google Scholar]
- 44.Holsboer F, Gerken A, Steiger A. N Engl J Med. 1984;311:1127. doi: 10.1056/NEJM198410253111718. (lett.). [DOI] [PubMed] [Google Scholar]
- 45.Brady L S, Whitfield H J, Fox R J, Gold P W, Herkenham M. J Clin Invest. 1988;87:831–837. doi: 10.1172/JCI115086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brady L S, Gold P W, Herkenham M, Lynn A, Whitfield H. Brain Res. 1992;572:117–125. doi: 10.1016/0006-8993(92)90459-m. [DOI] [PubMed] [Google Scholar]
- 47.Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chousos G P, Gold P W. N Engl J Med. 1996;335:1176–1181. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 48.Corodimas K P, LeDoux J E, Gold P W, Schulkin J. Ann NY Acad Sci. 1994;746:392–393. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee Y, Schulkin J, Davis M. Brain Res. 1994;666:93–98. doi: 10.1016/0006-8993(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 50.Kendler K S, Eaves L J, Walters E E, Neale M C, Heath A C, Kessler R C. Arch Gen Psychiatry. 1996;53:391–399. doi: 10.1001/archpsyc.1996.01830050025004. [DOI] [PubMed] [Google Scholar]