Abstract
In order to develop a model for screening the agonists of human β2-adrenoceptor from Chinese medicinal herbs extracts, we used a cell-based functional assay based on a common G protein-coupled receptor (GPCR) regulation mechanism and destabilized enhanced green fluorescent protein (d2EGFP) reporter gene technique. The positive cell clone was confirmed by real-time polymerase chain reaction (PCR) and imaging analysis. To assess the value of this model, we screened over 2000 high performance liquid chromatography (HPLC)-fractionated samples from the ethanol extracts of Chinese medicinal herbs. Six fractions (isolated from Panax japonicus, Veratrum nigrum, Phellodendron amurense, Fructus Aurantii Immaturus, Chaenomeles speciosa, and Dictamnus dasycarpus) showed significant effects on active reporter gene expression, three of which (isolated from Phellodendron amurense, Fructus Aurantii Immaturus, and Chaenomeles speciosa) were selected for further concentration response analysis and the half maximal effective concentration (EC1/2 max) values were 4.2, 2.7, and 4.8 µg/ml, respectively. Therefore, this reporter gene assay was suitable for screening β2-adrenoceptor agonists. The results suggest that the six herbal extracts are the possible agonists of β2-adrenoceptor.
Keywords: β2-adrenoceptor, Enhanced green fluorescent protein (EGFP), Chinese medicinal herbs, Screening
INTRODUCTION
β2-adrenoceptor is the first G protein-coupled receptor (GPCR) to be cloned (Kobilka et al., 1987), and belongs to the superfamily of GPCRs, which contain a conserved structure of seven transmembrane helices linked by three alternating intracellular and extracellular loops (Barki-Harrington et al., 2004). Once the ligand binds to the receptor, the receptor will change the conformation and couple to a heterotrimeric G protein that consists of an α subunit binding a guanosine triphosphate (GTP), a β subunit and a γ subunit. The G protein undergoes an activation-inactivation cycle to convey a signal from an activated receptor to an effector. The stimulatory G (Gs) protein- or inhibitory G (Gi) protein-coupled receptors activate adenylyl cyclase (AC) that in turn generates the second messenger cyclic adenosine monophosphate (cAMP) and activates cAMP-dependent protein kinase (PKA); the receptors that couple to Gq-protein modulate the activity of phospholipase C to generate diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) and subsequently activate protein kinase C (PKC) (Barki-Harrington et al., 2004). The β2-adrenoceptor couples to Gs and activates AC, resulting in elevated cAMP levels and subsequent activation of PKA. In the respiratory system, there is great therapeutic interest in the β2-adrenoceptor (Abraham et al., 2003). The β2-adrenoceptor agonists are the most widely used agents in the treatment of asthma with their bronchodilator actions (Milic et al., 2006).
In recent years, a cell-based functional assay to directly monitor GPCR activation, based on a common GPCR regulation mechanism and reporter gene techniques, has been used in drug discovery (Goetz et al., 2000). In the cell model, when the agonist activates GPCR, it will couple to Gs-protein and lead to activation of adenylate cyclase cAMP formation, subsequently increasing the activity of PKA. PKA then phosphorylates intracellular cAMP response element binding (CREB) protein. This phosphorylation allows the CREB to recruit the transcriptional adapter CREB protein, and therefore mediates a reporter gene, such as green fluorescent protein (GFP) transcription (Clark et al., 1999). GFP is originally isolated from the bioluminescent jellyfish Aequorea avictoria (Shimomura et al., 1962), and has been used to investigate many properties and behaviors of cells. The chromophore of GFP is produced through an internal posttranslational autocatalytic cyclization that does not require any cofactors or substrates (Zimmer, 2002). GFP as a reporter that is expressed under the control of an interested promoter can be used to monitor the interested gene expression. Measuring the GFP fluorescence can directly indicate the gene expression level in living cells. Enhanced GFP (EGFP) is extremely stable. Its variant form destabilized EGFP (d2EGFP) has a reduced half life of about 2 to 3 h. d2EGFP has been used extensively in cell-based assays as a transcription reporter (Kain, 1999).
Chinese medicinal herbs have been used to treat many diseases (such as asthma and hypertension) and have shown effectiveness for thousands of years. In the search for new candidates for β2-adrenoceptor agonists, the natural compounds from these herbs could provide a rich source. In this article, we describe in detail the process of assay development for β2-adrenoceptor and its screening of the compounds.
MATERIALS AND METHODS
Cells and culture conditions
Human embryonic kidney 293 (HEK293) cells were obtained from the American Type Culture Collection, Manassas, VA (ATCC CRL-1573). Cells were cultured in 75 ml flasks (Orange, Belgium) containing Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (PAA, Austria) in 5% CO2 and at 37 °C according to standard procedures.
Plasmid cloning and preparation
Plasmids pd2EGFP and pCRE-Blax were from BD Clontech, Palo Alto, CA, USA. The plasmid pd2EGFP was promoterless, whereas in the plasmid pCRE-Blax, the β-lactamase gene was controlled by a synthetic promoter consisting of four copies of CRE-binding sequences. The CRE-binding sequences were excised by BamHI and EcoRI from the pCRE-Blax. The plasmid p4*CRE-d2EGFP was constructed by inserting CRE-binding sequences into pd2EGFP. The resulting product was confidential by sequencing. Full length cDNA clone encoding the human β2-adrenoceptor was cloned by polymerase chain reaction (PCR) from human genome DNA. The primers used were 5′-CTAGCTAGCACCATGGGGCAACCCGGGAAC-3′ and 5′-CCCAAGCTTCAGCAGTGAGTCATTTGTAC-3′. The cloned β2-adrenoceptor was inserted into the eukaryotic expression vector pcDNA3.1/Hygro(+), and the construct pcDNA3.1-h β2-adrenoceptor was confirmed by sequencing.
Transfection and cell line generation
HEK293 cells were cotransfected with p4*CRE-d2EGFP and pcDNA3.1-h β2-adrenoceptor by lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Transfected cells were selected by hygromycin (150 μg/ml). After around 15 d, hygromycin-resistant cells were picked and diluted into a 96-well plate at a concentration of 1~2 cells per well and cultivated for 15 d. The resulting colonies were stimulated with 1 μmol/L of β2-adrenoceptor agonist isoproterenol to monitor the d2EGFP expression. The stably transfected cell clone with the best signal/noise (S/N) was selected for further experiments.
Confirmation of the selected cell clone
Cells were homogenized in TRIzol reagent (Life Technologies, Inc., Roskilde, Denmark), and total RNA was extracted following the manufacturer’s protocol. RNA was quantified by measuring absorbency at 260 and 280 nm and the ratio was 1.8 or higher. The integrity of the RNA was checked by visual inspection of the two ribosomal RNAs on an ethidium bromide-stained agarose gel. The first strand cDNA was synthesized from the isolated RNA using reverse transcriptase with an oligo-dT primer. The relative quantities of targets (β2-adrenoceptor and d2EGFP) to reference (glyceraldehyde-3-phosphate dehydrogenase, GAPDH) were performed with an SYBR-green fluorescence (Invitrogen) using ABI PRISM 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The increase in fluorescence was measured in real-time PCR during the extension step. The threshold cycle (Ct) was calculated, and the relative gene expression was calculated as described (Livak and Schmittgen, 2001). All samples were amplified in triplicate. All reaction sets included water blanks as negative controls. Primers used were listed in Table 1.
Table 1.
Primers used in the real-time PCR
| Gene | Primer | Product length (bp) |
| β2-adrenoceptor | Forward: 5′-CCATTGCCAAGTTCGAGCGTCTG-3′ | 292 |
| Reverse: 5′-ATGATCACCCGGGCCTTATTCTTG-3′ | ||
| d2EGFP | Forward: 5′-TCATGGCCGACAAGCAGAAGAACG-3′ | 228 |
| Reverse: 5′-CGGCGGCGGTCACGAACTC-3′ | ||
| GAPDH | Forward: 5′-GACCACAGTCCATGCCATCAC-3′ | 210 |
| Reverse: 5′-AGGTCCACCACTGACACGTTG-3′ |
d2EGFP: destabilized enhanced green fluorescent protein; GAPDH: glyceraldehyde-3-phosphate dehydrogenase
Preparation of Chinese medicinal herb extracts
One hundred and twenty herbal plants with therapeutic indications for asthma treatment based on traditional Chinese medicine (TCM) were collected from a herb market in Anguo, Northern China. A modified method by Zhang et al.(2007) was used. Briefly, the dry Chinese herbs were dissolved in 90% (v/v) ethanol for 12 h and then sonicated for 0.5 h. Then the remains were soaked with 50% (v/v) ethanol for 12 h and extracted for 0.5 h. Then the ethanol extract was filtered and lyophilized. The product after lyophilization was re-dissolved in 90% (v/v) ethanol for high performance liquid chromatography (HPLC) isolation. A total of 16 gradients were obtained by HPLC isolation for each Chinese herb extract. All gradients were lyophilized and dissolved in dimethylsulfoxide (DMSO) and were kept in −80 °C freezer.
Screening and quantification
Stably transfected cells were seeded into 96-well tissue culture plates (Corning, USA) at 2×104 cells per well, and the final volume of each well was 90 μl. The cells were cultured for 24 h before treatment. The testing compounds, 10-fold diluted in DMEM, along with the controls, were added in the medium (10 μl). The cells were cultured for an additional 16 h and then screened for d2EGFP expression using Olympus IX70 fluorescence microscope (Olympus, Japan) equipped with a filter set (U-MWIB2, excitation BP 460~490 nm, emission LP 510 nm) according to the manufacturer’s instruction. The fluorescent intensity of d2EGFP was quantified by measuring the area and intensity of whole image, and the results were expressed as integrated optical density (IOD): IOD=area×intensity of fluorescence. The herbs that possessed activity (hit samples) were extracted on a larger scale and were HPLC-fractionated as described earlier (Zhang et al., 2007). The isolated compounds were subjected to activity verification using the HEK293-PTHR (parathyroid hormone receptor)-d2EGFP cell line as well as parental control cells (HEK293-d2EGFP), and the dose response curve was determined using a receptor assay cell line.
Data analysis
Data were presented as mean±SD. Statistical significance was evaluated with Student’s t-test and the difference was considered statistically significant at P<0.05.
RESULTS
Cell line generation
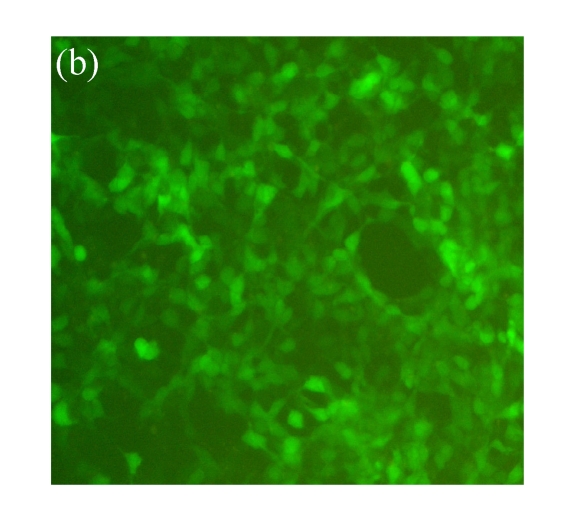
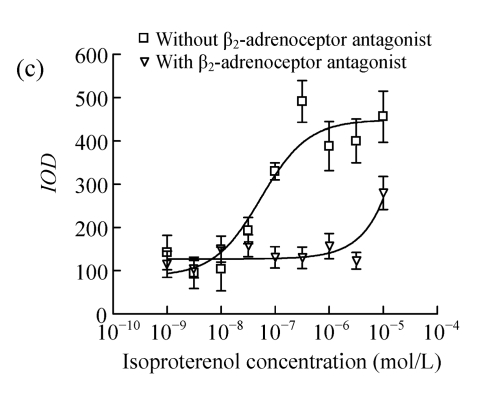
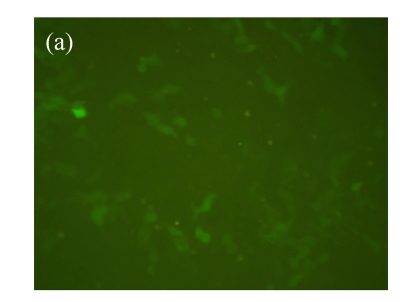
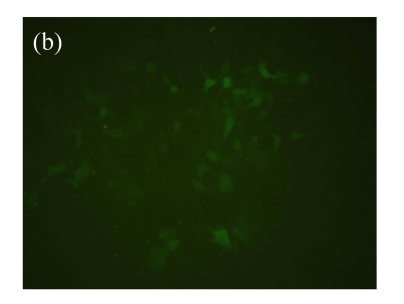
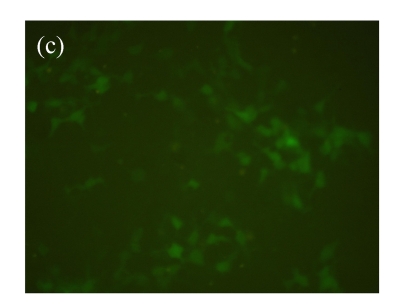
Vector p4*CRE-d2EGFP and human β2-adrenoceptor gene were stably cotransfected into HEK293 cells. As shown in Figs.1a and 1b, from the stably transfected clones, selected were those that showed d2EGFP expression after treatment with the β2-adrenoceptor agonist isoproterenol. Low or high serum (0.1% (v/v) bovine serum albumin (BSA) or 10% (v/v) FBS) concentration had no influence on d2EGFP expression in HEK293 cells induced by isoproterenol (data not shown). The responses to agonist stimulation were in a dose-dependent manner with the half maximal effective concentration (EC1/2 max) value of 87 nmol/L (Fig.1c). Pretreatment of β2-adrenoceptor antagonist propranolol for 15 min blocked the effect.
Fig. 1.
Effect of isoproterenol on HEK293-β2-adrenoceptor-d2EGFP clone. (a) Cell morphology and density under inverted microscopy (magnification 100×); (b) d2EGFP expression after addition of isoproterenol (1 μmol/L) for 16 h (magnification 100×); (c) Dose-response curve for isoproterenol-stimulated d2EGFP signal on positive cell clone in the presence or absence of 1 μmol/L of the β2-adrenoceptor antagonist
Cell clone was incubated for 16 h after addition of isoproterenol. Results are shown as mean±SD (n=3)
Cell clone validation
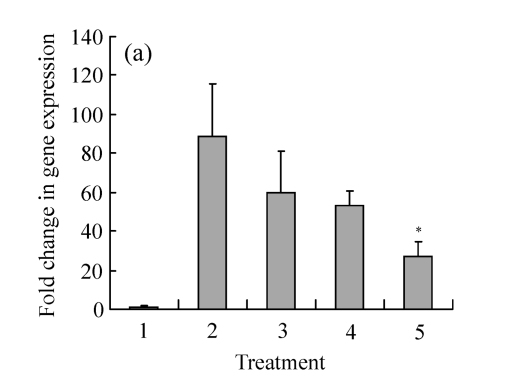
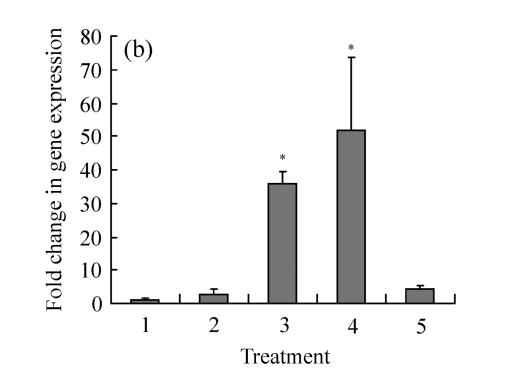
We analyzed β2-adrenoceptor and d2EGFP mRNA expressions in the positive clone that showed ligand-dependent fluorescence. Analysis by real-time PCR showed that this clone expressed significantly more β2-adrenoceptors than the parent HEK293 cell (Fig.2a). Moreover, compared with the clone without treatment, stimulation of different concentrations of isoproterenol had no effect on the receptor expression. The control compound (20 μg/ml cryptotanshinone) induced significant reduction in β2-adrenoceptor mRNA levels; the mechanism was not clear. The same clone was analyzed by real-time PCR for d2EGFP mRNA expression (Fig.2b). A significant increase in d2EGFP mRNA expression was observed in clones treated with isoproterenol. As expected, d2EGFP mRNA expression was not induced by control compound.
Fig. 2.
The mRNA expression on HEK293-β2-adrenoceptor-d2EGFP clone compared with HEK293 cell with or without compound treatment for 16 h. (a) β2-adrenoceptor mRNA expression; (b) d2EGFP mRNA expression
Treatment: 1: HEK293; 2: HEK293-β2-adrenoceptor-d2EGFP clone; 3: HEK293-β2-adrenoceptor-d2EGFP clone treated with isoproterenol 100 nmol/L; 4: HEK293-β2-adrenoceptor-d2EGFP clone treated with isoproterenol 1 μmol/L; 5: HEK293-β2-adrenoceptor-d2EGFP clone treated with compound control. Results are shown as mean±SD (n=3). * P<0.05 vs HEK293-β2-adrenoceptor-d2EGFP clone
Screening of Chinese medicinal herbs extracts
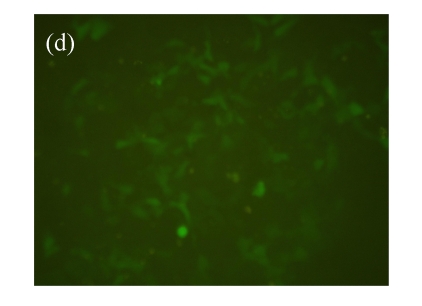
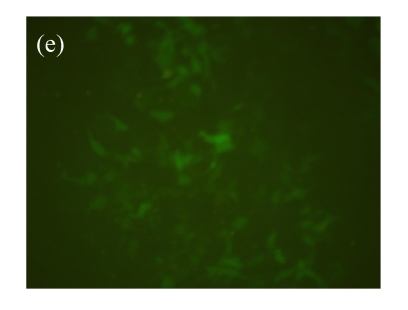
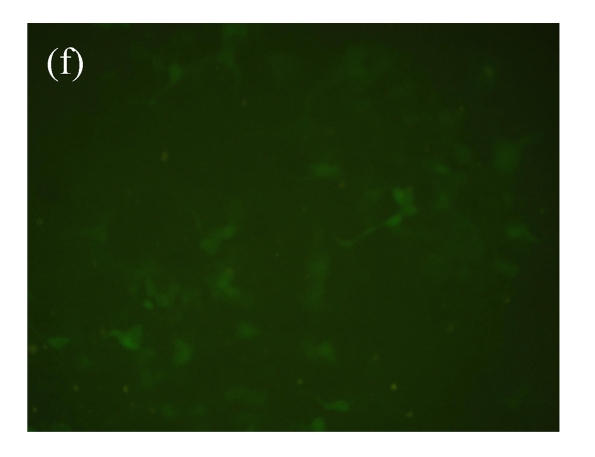
To assess the value of this cell model for searching for the β2-adrenoceptor agonists, over 2000 HPLC-fractionated samples from the ethanol extracts of Chinese medicinal herbs were used. The d2EGFP expression in the cell was recorded. As shown in Fig.3, fractions of six samples, Panax japonicus, Veratrum nigrum, Phellodendron amurense, Fructus Aurantii Immaturus, Chaenomeles speciosa, and Dictamnus dasycarpus, exhibited significant effects on active d2EGFP expression, and were retested for their activities using fresh dilution from the same DMSO compound stocks. The medium control (background) and isoproterenol (1 μmol/L, positive control) were used. DMSO (1%, v/v) and medium control did not induce d2EGFP expression. The test using parental HEK293-CRE-d2EGFP cell line was conducted to ensure that the activation of reporter was not due to the non-specific stimulation of signaling pathways by selected hit compounds (data not shown).
Fig. 3.
Effect of hit compounds on d2EGFP expression. (a) Panax japonicus; (b) Veratrum nigrum; (c) Phellodendron amurense; (d) Fructus Aurantii Immaturus; (e) Chaenomeles speciosa; (f) Dictamnus dasycarpus
Cells stably expressing β2-adrenoceptor and d2EGFP were incubated with test compounds (20 µg/ml each) for 16 h and images were taken by fluorescence microscopy (magnification 100×)
Identification of hit compounds
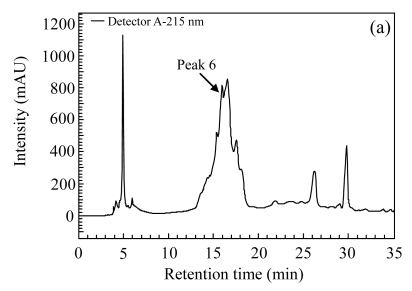
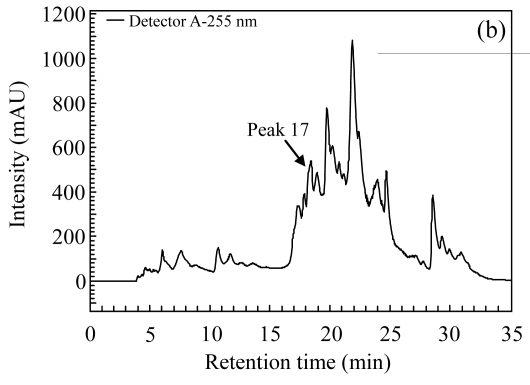
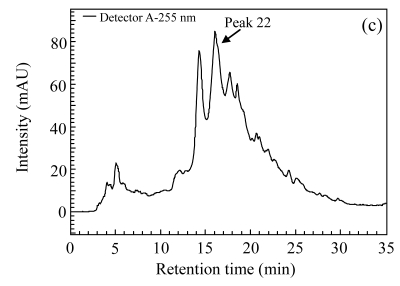
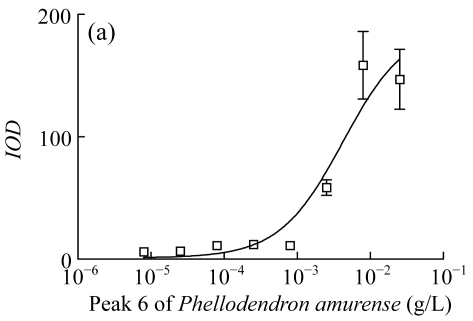
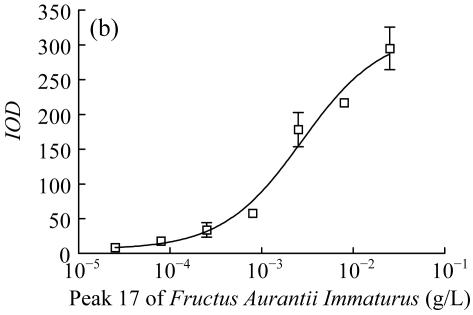
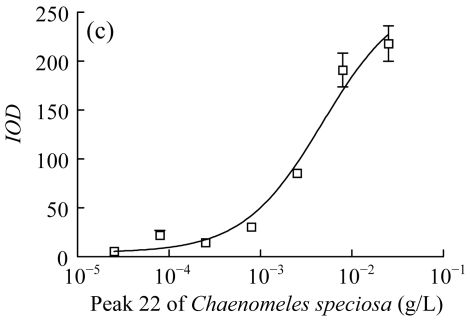
Three herbs, Phellodendron amurense, Fructus Aurantii Immaturus, and Chaenomeles speciosa, which generated fractionated samples and showed the better activities in the screening, were selected for further dose-response study. Fig.4 shows HPLC chromatograms of Phellodendron amurense, Fructus Aurantii Immaturus, and Chaenomeles speciosa. As shown in Fig.5, the three hit compounds stimulated the d2EGFP expression in a dose-dependent manner, with EC1/2 max values of 4.2, 2.7, and 4.8 µg/ml, respectively. In addition, methyl thiazolyl tetrazolium (MTT) test showed that compound treatment did not decrease cell viability (data not shown). We used HEK293 cells cotransfected with parathyroid hormone receptor (PTHR) and p4*CRE-d2EGFP to assess the specificities of these hits. PTHR is a GPCR, also transmits its signals to the Gs protein, subsequently causing an increase of the second messenger cAMP, and thereby mediates reporter-gene transcription. The three hit compounds showed no effects on active d2EGFP expression in the HEK293-PTHR-d2EGFP cell line (data not shown). All the results indicate that the three hit compounds are β2-adrenoceptor-specific agonists.
Fig. 4.
HPLC chromatograms. (a) Phellodendron amurense; (b) Fructus Aurantii Immaturus; (c) Chaenomeles speciosa
The arrows indicate the positions of active fractions on the chromatograms
Fig. 5.
Dose-response curve of induced d2EGFP expression. (a) Peak 6 of Phellodendron amurense; (b) Peak 17 of Fructus Aurantii Immaturus; (c) Peak 22 of Chaenomeles speciosa
Cell clone was incubated for 16 h after addition of compounds. Results are shown as mean±SD (n=3). The peak numbers are shown in Fig.4
DISCUSSION
The human β2-adrenoceptor is a member of the 7-transmembrane family of receptors widely distributed in the respiratory tract (Johnson, 2006). It plays an important role in regulation of vascular and bronchial smooth muscle tone. It also exists in human heart where it can mediate positive inotropic and chronotropic effects (Brodde and Leineweber, 2005). The β2-adrenoceptor agonists, including terbutaline, formoterol, salmeterol, albuterol, levalbuterol, etc., are commonly used drugs to treat asthma. These agents dilate airway by stimulating β2-adrenoceptors that couple to the Gs proteins. Once activated by receptor binding, the α subunit of Gs protein activates AC, resulting in cAMP formation. cAMP, in turn, activates PKA, which results in cell relaxation through effects on K+ channels, Na+/K+ ATPases, Ca2+ sequestration, Ca2+ sensitivity of myosin, IP3 formation, and CREB phosphorylation at serine 133 that permits CREB protein bound to CRE to initiate gene transcription (Guyot et al., 1998).
In this study, the plasmid containing the reporter gene d2EGFP under the control of CRE promoter was constructed. The resulting product p4*CRE-d2EGFP was cotransfected with β2-adrenoceptor to HEK293 cell to construct a cell model for screening β2-adrenoceptor agonists. The d2EGFP is a powerful tool for cell-based assays owing to the intrinsic fluorescence of this protein that allows real-time analysis of molecular events in living cells, while there are some limitations about GFP, including the slow formation of posttranslational chromophore, the requirement of oxygen, and the difficulty in distinguishing GFP from background fluorescence when the GFP expressed weakly. The low sensitivity of GFP is due to the fact that there is no signal amplification, because each GFP has only one chromophore, but the low sensitivity can be overcome by using a sensitive photon counting devices (Zimmer, 2002). In this assay, we used Olympus IX70 fluorescence microscope equipped with a filter set to detect the fluorescent intensity of d2EGFP and quantified by Image-Pro Plus. The results show that the sensitivity and the specificity were sufficient for the primary screening.
In the HEK293-β2-adrenoceptor-d2EGFP cells, the binding of ligands to β2-adrenoceptors resulted in the expression of d2EGFP. The screening of large libraries in order to obtain hits for receptors of interest has been the mainstay of drug research for some time. It is increasingly being recognized that this is a relatively inefficient way to achieve this end, and the screening of libraries either designed or selected to hit particular targets is rapidly becoming the method of choice (Crossley, 2004). Traditional Chinese medicinal herbs are effective for treatment of asthma and hypertension (Qiu and Kao, 2003; Shichinohe et al., 1996; Chang et al., 1979). We tried to develop natural products and natural compounds from various Chinese herbs to screen novel leads for new drugs. This study investigated the assay to screen hits in traditional Chinese medicinal herb library, which could activate reported gene expression by β2-adrenoceptor/cAMP-mediated signaling. We constructed a cell model for the screening of the library and found six hits from over 2000 samples. The six hits all showed the ability to activate d2EGFP expression in HEK293-β2-adrenoceptor-d2EGFP cells, while they did not activate d2EGFP expression in HEK293-PTHR-d2EGFP cells or parental HEK293-CRE-d2EGFP cells (data not shown). Therefore, we speculate that they might activate β2-adrenoceptor/cAMP-mediated signaling. In the previous studies, the extracts from Phellodendron amurense and Chaenomeles speciosa showed anti-inflammatory and other important activities (Cuéllar et al., 2001; Uchiyama et al., 1989; Chen and Wei, 2003). Phellodendron extracts have been investigated for their potential antitumor effect on human prostate cancer cells (Garcia et al., 2006). The ethanol extract of Fructus Aurantii Immaturus was found to activate α-adrenoceptors (Airriess et al., 1997). Our research shows other possible effect of those traditional Chinese medicinal herbs, such as stimulating β2-adrenoceptor, therefore, to dilate airway.
In summary, the assay based on β2-adrenoceptor-mediated cAMP accumulation and CRE-mediated d2EGFP transcription was suitable for drug screening in a low-cost manner.
Acknowledgments
We would like to thank Dr. J.C. Chen and Dr. H.Q. Jiang from HD Biosciences, Zhangjiang East Campus, Pudong, Shanghai, China for giving helpful advice.
Footnotes
Project (No. 30873103) supported by the National Natural Science Foundation of China
References
- 1.Abraham G, Kottke C, Dhein S, Ungemach FR. Pharmacological and biochemical characterization of the beta-adrenergic signal transduction pathway in different segments of the respiratory tract. Biochem Pharmacol. 2003;66(6):1067–1081. doi: 10.1016/S0006-2952(03)00460-X. [DOI] [PubMed] [Google Scholar]
- 2.Airriess CN, Rudling JE, Midgley JM, Evans PD. Selective inhibition of adenylyl cyclase by octopamine via a human cloned alpha 2A-adrenoceptor. Br J Pharmacol. 1997;122(2):191–198. doi: 10.1038/sj.bjp.0701348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barki-Harrington L, Perrino C, Rockman HA. Network integration of the adrenergic system in cardiac hypertrophy. Cardiovasc Res. 2004;63(3):391–402. doi: 10.1016/j.cardiores.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Brodde OE, Leineweber K. Beta2-adrenoceptor gene polymorphisms. Pharmacogenetics and Genomics. 2005;15(5):267–275. doi: 10.1097/01213011-200505000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Chang CC, Tung LH, Chen RR, Chiueh CC. A study on the antihypertensive action of uncarine A, an alkaloid of Uncaria formosana used in Chinese herb medicine. Taiwan Yi Xue Hui Za Zhi. 1979;78(2):61–69. (in Chinese) [PubMed] [Google Scholar]
- 6.Chen Q, Wei W. Effects and mechanisms of glucosides of Chaenomeles speciosa on collagen-induced arthritis in rats. Int Immunopharmacol. 2003;3(4):593–608. doi: 10.1016/S1567-5769(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 7.Clark RB, Knoll BJ, Barber R. Partial agonists and G protein-coupled receptor desensitization. Trends Pharmacol Sci. 1999;20(7):279–286. doi: 10.1016/S0165-6147(99)01351-6. [DOI] [PubMed] [Google Scholar]
- 8.Crossley R. The design of screening libraries targeted at G-protein coupled receptors. Curr Top Med Chem. 2004;4(6):581–588. doi: 10.2174/1568026043451140. [DOI] [PubMed] [Google Scholar]
- 9.Cuéllar MJ, Giner RM, Recio MC, Máñez S, Ríos JL. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia. 2001;72(3):221–229. doi: 10.1016/S0367-326X(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 10.Garcia GE, Nicole A, Bhaskaran S, Gupta A, Kyprianou N, Kumar AP. Akt- and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia. 2006;8(6):523–533. doi: 10.1593/neo.05745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz AS, Andrews JL, Littleton TR, Ignar DM. Development of a facile method for high throughput screening with reporter gene assays. J Biomol Screen. 2000;5(5):377–384. doi: 10.1177/108705710000500510. [DOI] [PubMed] [Google Scholar]
- 12.Guyot DJ, Newbound GC, Lairmore MD. CD2 signalling induces phosphorylation of CREB in primary lymphocytes. Immunology. 1998;95(4):544–552. doi: 10.1046/j.1365-2567.1998.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol. 2006;117(1):18–24. doi: 10.1016/j.jaci.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Kain SR. Green fluorescent protein (GFP): applications in cell-based assays for drug discovery. Drug Discov Today. 1999;4(7):304–312. doi: 10.1016/S1359-6446(99)01330-6. [DOI] [PubMed] [Google Scholar]
- 15.Kobilka BK, Dixon RA, Frielle T, Dohlman HG, Bolanowski MA, Sigal IS, Yang-Feng TL, Francke U, Caron MG, Lefkowitz RJ. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor forplatelet-derived growth factor. Proc Natl Acad Sci USA. 1987;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Milic M, Bao X, Rizos D, Liu F, Ziegler MG. Literature review and pilot studies of the effect of QT correction formulas on reported beta2-agonist-induced QTc prolongation. Clin Ther. 2006;28(4):582–590. doi: 10.1016/j.clinthera.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs in R&D. 2003;4(1):1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 19.Shichinohe K, Shimizu M, Kurokawa K. Effect of M-711 on experimental asthma in rats. J Vet Med Sci. 1996;58(1):55–59. doi: 10.1292/jvms.58.55. [DOI] [PubMed] [Google Scholar]
- 20.Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59(3):223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- 21.Uchiyama T, Kamikawa H, Ogita Z. Anti-ulcer effect of extract from Phellodendri cortex . Yakugaku Zasshi. 1989;109(9):672–676. doi: 10.1248/yakushi1947.109.9_672. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhang H, Liu X, Hua S, Zhou L, Yu J, Tan X. Identification of human dopamine receptors agonists from Chinese herbs. Acta Pharmacologica Sinica. 2007;28(1):132–139. doi: 10.1111/j.1745-7254.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 23.Zimmer M. Green fluorescent protein (GFP): applications, structure, and related photophysical behavior. Chem Rev. 2002;102(3):759–781. doi: 10.1021/cr010142r. [DOI] [PubMed] [Google Scholar]