Abstract
Alopecia (hair loss) is experienced by thousands of cancer patients every year. Substantial-to-severe alopecia is induced by anthracyclines (e.g., adriamycin), taxanes (e.g., taxol), alkylating compounds (e.g., cyclophosphamide), and the topisomerase inhibitor etoposide, agents that are widely used in the treatment of leukemias and breast, lung, ovarian, and bladder cancers. Currently, no treatment appears to be generally effective in reliably preventing this secondary effect of chemotherapy. We observed in experiments using different rodent models that localized administration of heat or subcutaneous/intradermal injection of geldanamycin or 17-(allylamino)-17-demethoxygeldanamycin induced a stress protein response in hair follicles and effectively prevented alopecia from adriamycin, cyclophosphamide, taxol, and etoposide. Model tumor therapy experiments support the presumption that such localized hair-saving treatment does not negatively affect chemotherapy efficacy.
Introduction
Alopecia (hair loss) is arguably the most feared side effect of cancer chemotherapy (Dorr 1998; Munstedt et al. 1997). Despite substantial efforts, no reliable and generally effective preventative treatment has become available (Hesketh et al. 2004; Wang et al. 2006). Scalp tourniquets and cooling devices have been utilized for decades to restrict blood flow to the scalp during chemotherapy treatment. Although such treatments were found to be successful in reducing alopecia in connection with certain chemotherapy regimens, they were difficult to standardize and not generally useful over the wide range of pharmacological regimens used in the clinic. Although more recent studies utilizing improved hypothermia devices reported increased reliability, certain antineoplastic drug combinations, notably combinations comprising a taxane could not be protected against (Katsimbri et al. 2000; Christodoulou et al. 2002). Among the many pharmacological approaches for alopecia prevention that were investigated, vitamin D3 appeared to be the most promising protective compound because it was effective against several different antineoplastic agents in preclinical experiments (e.g., Jimenez et al. 1995; Schilli et al. 1998). However, a clinical trial was ultimately unsuccessful (Hidalgo et al. 1999; Wang et al. 2006).
All cells possess protective mechanisms that increase their resistance to various adverse conditions. Perhaps best known is the ubiquitous stress protein (Hsp) response that involves the enhanced expression of classical stress proteins such as Hsp90, Hsp70, and Hsp25, and of certain other proteins such as P-glycoprotein, in response to physical or chemical stresses (Parsell and Lindquist 1993; Vilaboa et al. 2000). Elevated levels of Hsps are known to result in increased stress tolerance (Li and Werb 1982; Liu et al. 1992; Lavoie et al. 1993). The spectrum of toxicants an activated stress protein response can mitigate against is broad. As shown by previous studies, elevated levels of the cohort of Hsps or of individual Hsps are also protective against cytotoxicity from many antineoplastic agents used in the clinic. Table 1 summarizes selected studies relating to the reduction or prevention of toxicity from adriamycin, cyclophosphamide, taxol, and etoposide. Because the stress protein response is an intracellular protective mechanism, it should be possible to locally activate a stress protein response in noncancerous tissues without affecting the cytotoxic effects of an antineoplastic agent in cancerous tissues. We hypothesized that localized activation of a stress protein response in the hair follicles of a patient’s scalp (and eyebrows) would prevent chemotherapy-induced alopecia and that this protective effect could be achieved without reduction of tumor therapy efficacy.
Table 1.
Stress response-mediated protection against antineoplastic agents in vitro
| Agent | Cell type studied | Experimental approach | Results | References |
|---|---|---|---|---|
| Adriamycin | Dunning rat carcinoma; rat cardiac cells | Heat pretreatment | Increased survival; protection against apoptosis | Roigas et al. 1998; Ito et al. 1999 |
| Rat cardiac cells | Heat pretreatment and Hsp70 antisense | Negation of induced protection against apoptosis | Ito et al. 1999 | |
| ME-180, WEHI-S | Hsp70 overexpression | Protection against apoptosis | Jaattela et al. 1998 | |
| MDA-MB-231, MCF-7, CHO | Hsp27 overexpression | Protection against cell killing | Huot et al. 1991; Oesterreich et al. 1993 | |
| ME-180, WEHI-S | Hsp70 antisense | Enhanced apoptosis | Jaattela et al. 1998 | |
| Etoposide | HeLa S3, HL60, PC-3, LNCaP | Heat pretreatment | Protection against cell killing/apoptosis | Inoue et al. 1999; Kampinga 1995; Gibbons et al. 2000 |
| PC-3, LNCaP | Heat pretreatment and Hsp70 antisense | Negation of induced protection against apoptosis | Gibbons et al. 2000 | |
| U937, WEHI-S | Heat pretreatment or overexpression of Hsp70 or Hsp27 | Reduced apoptosis | Samali and Cotter 1996; Garrido et al. 1999 | |
| Cyclophosphamide | HepG2 | Heat pretreatment or expression of active HSF1 mutant | Protection against cell killing | Salminen et al. 1996; Xia et al. 1999 |
| Taxol | HL60 | Heat pretreatment or Hsp70 overexpression | Prevention of apoptosis | Kwak et al. 1998 |
Materials and methods
Prevention of chemotherapy-induced alopecia in the young rat model
Sprague–Dawley rat mothers with 7-day-old litters were obtained from Charles River Laboratories, Wilmington, MA, USA. One day later, the pups were used in experiments. Heat was applied to the skin with a copper cylinder through which heated water was circulated. The temperatures reported were measured in the precision waterbath that fed the heating cylinder. (Temperature at the working end of the heating cylinder was less than 0.1°C lower.) Before heat treatment, vaseline was applied to the skin to improve heat conductance. At the end of a heat treatment of an animal, the heat-treated area was cooled using a small icepack. This procedure effectively suppressed inflammatory responses as evidenced by the virtual absence of infiltration of the skin with inflammatory cells. Pups were kept with their mothers, except for the brief periods required for injections, heat treatments, and examination. Geldanamycin (GA), obtained from the Developmental Therapeutics Program of NCI/NIH or purchased from Sigma, was suspended and diluted in 0.9% NaCl and 1% DMSO. Aliquots (100 μl) were injected subcutaneously (s.c.) in the nape of the neck. Adriamycin, cyclophosphamide, and taxol were from Sigma. Stock solutions were prepared in water and appropriate dilutions made in phosphate-buffered saline (PBS). Stocks (20 mg/ml) of etoposide (Sigma) were in 30.5% ethanol supplemented with 2 mg/ml citric acid, 30 mg/ml benzyl alcohol, 80 mg/ml Tween-80, and 150 mg/ml PEG-300. Taxol was injected s.c. (100 μl); all other antineoplastic agents were administered intraperitoneally (i.p.; 100 μl).
Immunohistochemistry of skin biopsies
Paraffin-embedded blocks were sectioned at 5 μm, and sections were attached to positively charged slides. After drying, the slides were deparaffinized in xylene, rehydrated, and placed in PBS. Subsequent to incubation in 3% H2O2 in PBS, slides were boiled in antigen unmasking solution (Vector Laboratories, Burlingame, CA, USA). Sections were then sequentially incubated with normal horse serum (Vector Laboratories), mouse monoclonal anti-Hsp70 antibody SPA-810 (Assay Designs, Ann Arbor, MI, USA) at 5 μg/ml, antimouse IgG biotinylated antibody (Vector Laboratories), and horseradish peroxidase avidin complex (Vector Laboratories). Signal (brown color) was developed by incubation with H2O2 and 3,3′-diaminobenzidine. Counterstaining was with Mayer’s hematoxylin.
Model cancer chemotherapy experiments
Eight-day-old rats were randomly assigned to 4 groups of 45 animals each. All groups were injected i.p. with 2.5 × 105 MIA C51 leukemia cells (Moloney et al. 1962; Yunis et al. 1975) grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum (FCS, Invitrogen) at 37°C and 5% CO2. Six hours after the administration of leukemic cells, two groups of animals were subjected to localized heat treatment to the nape of the necks for 20 min at 48°C. Twenty-four hours later, one group with and one group without heat treatment received 35.5 μg/g cyclophosphamide i.p. Animals were followed-up for 30 days (after chemotherapy) and were then killed by CO2 inhalation. The presence of leukemia was estimated from bone marrow aspirates. An observation of >30% nonerythroid blasts was considered as positive evidence for leukemia. One of the femurs was removed under sterile conditions (Jimenez and Yunis 1988). The marrow was flushed from the femur shaft with 2 ml DMEM supplemented with 10% FCS (Invitrogen). Aliquots were prepared at a concentration of 1 × 105 cells/ml and immobilized onto slides using a cytospin (Shandon, Canterbury, UK). Slides were stained with Wright stain (Sigma) for microscopic examination. Differential counts were done on 300 cells by 3 independent examiners. As a confirmatory analysis, aliquots that were not used for cytospin were cultured in DMEM plus 10% FCS. After 10 days of culture, only chloroleukemic cells survive as normal blasts require growth factors not present in the culture medium. An absence of cell proliferation at this time was considered as evidence for the absence of leukemia.
Prevention of chemotherapy-induced alopecia in young and adult mouse models
Twelve-day-old C57BL/6 pups were locally heat-treated by contacting a heating cylinder (see above) with an area on their lower backs. Conducting gel was applied to the contacted area to improve heat conductance. Drugs were administered i.p. 7 and/or 24 h after heat treatment (e.g., 2 × 2.5 μg/g etoposide). Results were scored and recorded 5–8 days after chemotherapy. A first study on an adult model used 8-week-old male C57BL/6 mice (approximately 24 g). Animals were anesthetized with pentobarbital (50 μg/g i.p.), and a 1-cm patch of hair was removed from the lower back using a rosewax depilation procedure. A wider surrounding area was cleared by chemical depilation (Church & Dwight, [Nair], Emeryville, CA). Thirteen to 14 days later, the area from which hair had been pulled was subjected to localized heat treatment. Heat was administered either by means of a heating cylinder or by irradiation using an 812-nm laser (1.0 W; 3–5 min; optical path = 87 cm). A single dose of cyclophosphamide in saline (120 μg/g; 10 μl/g) was administered i.p. 24 h later. Prevention of hair loss was observed and recorded 7–10 days after chemotherapy. A second study used 4- to 6-week-old C57BL6 mice of both sexes (approximately 16–18 g). A wide area on the lower back of animals was shaved. Five to 7 days later, 17-(allylamino)-17-demethoxygeldanamycin (17AAG) (0.3 μg in 50 μl) was injected intradermally in the cleared area. Cyclophosphamide in saline (120 μg/g) was administered i.p. 2 days later. 17AAG (Sigma) was dissolved in DMSO and diluted in PBS. All animal work was performed in accordance with institutional guidelines.
Results and discussion
The young rat is a commonly employed model of human hair growth in studies of chemotherapy-induced alopecia (Hussein et al. 1990). For many antineoplastic agents that cause significant alopecia in patients, dose regimens were identified that resulted in essentially complete loss of body hair in this model. In most of our experiments, heat was used to induce a stress protein response. A simple device for administering a defined heat dose was built, consisting of a hollow copper cylinder heated internally by circulating water provided by a precision waterbath. For heat treatment, an animal was restrained by gauze wrap and placed in a holding device, and the base of the heating cylinder was brought into contact with the nape of the neck. Although in vitro experiments suggested that a strong stress protein response should be elicited by exposure to 43–44°C for 15–30 min, we tested an expanded range of heating conditions, expecting the nascent fur coat of the animals to have an insulating effect of unknown magnitude. Groups of four 8-day-old Sprague–Dawley rats were spot-heated for 20°min at 42–50°C. Etoposide (2.5 μg/g) was injected i.p. in two doses, 7 h after heat treatment and 24 h later. Animals were scored visually for retention of hair in the heat-treated area about 1 week (6–9 days) after chemotherapy, at which time they normally had lost essentially all body hair. A patch of protected fur in the nape of the neck could be seen in animals heat-treated at 45°C and higher. At and above 46°C, a patch was observed typically on all animals in the group. Example results are shown in Fig. 1a (see Fig. 1b for an untreated animal). Protection of hair in heat-treated areas appeared to be nearly complete. An experiment in which we heat-treated animals for increasing periods of time at a suboptimal temperature (44°C) suggested that the effectiveness of the preventative treatment was a function of the heat dose delivered. The fraction of animals having a patch of protected hair subsequent to etoposide chemotherapy rose from 0 to 0.12 and 0.75 for heat treatments of 20, 60, and 120 min, respectively. Hair-saving effects were observed in animals that had been heat-treated as little as 2 h or as much as 24 h before etoposide administration. To demonstrate that the observed protective effects correlated with increased levels of Hsps, we took biopsies 1 or 2 days after heat treatment and estimated levels of inducible Hsp70 in hair follicles immunohistochemically (Fig. 1c). Hsp70 was not detectably elevated after heat treatment at 42°C or 44°C for 20 min (not shown). However, subsequent to a 46°C/20 min treatment, increased Hsp70 staining was observed in an upper portion of hair follicles, and after treatment at 48°C or 48.5°C for 20 min, hair follicles were stained over their entire length.
Fig. 1.
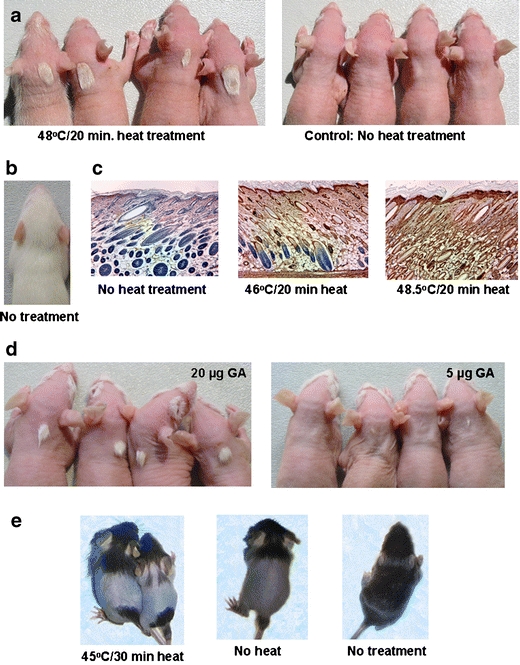
Localized protection of hair from the toxicity of antineoplastic agents in young rodent models. a Heat-induced protection from etoposide toxicity in the young rat model. Groups of animals are shown that were heat-treated at the nape of the neck or not heat-treated before drug administration. b Untreated young rat. c Skin sections from the nape of the neck of untreated or heat-treated young rats immunostained for Hsp70 (brown color). d Protection from etoposide toxicity in the young rat model induced by an activator of the stress protein response. Groups of young rats are shown that were injected s.c. with GA in the nape of the neck before etoposide administration. e Heat-induced protection of hair (on the lower back) from cyclophosphamide toxicity in a young mouse model. GA geldanamycin (for experimental detail see the text)
We obtained independent evidence that the protective effects were due to an activated stress protein response from experiments in which we injected animals s.c. with the benzoquinone ansamycin antibiotic geldanamycin (GA) 24 h before etoposide chemotherapy. Because benzoquinone ansamycins are selective inhibitors of Hsp90 function (Whitesell et al. 1994; Pratt and Toft 1997), and Hsp90 is a key repressor of heat shock factor 1, which mediates enhanced Hsp expression (Ali et al. 1998; Zou et al. 1998); these compounds can be considered to be the most specific activators of a stress protein response available at present. Dose-dependent, localized prevention of hair loss was observed (Fig. 1d).
Next, we tested whether the same protective mechanism could also mitigate or suppress the alopecic effects of anthracyclines, alkylating agents, and taxanes. Groups of (typically four) animals were subjected to heat treatment to the nape of the neck or were not heat-treated. After a delay of 7 h, the groups were administered alopecia-causing doses of cyclophosphamide or a combination of cyclophosphamide and adriamycin. Because no alopecia-causing, sublethal i.p. dose of taxol could be identified, amounts sufficient to cause local hair loss were injected s.c. in the region that had previously been subjected to heat treatment. Results summarized in Table 2 indicated that hair in heat-treated areas was effectively protected against the latter antineoplastic agents. No protection was observed in unheated, drug-exposed animals. Subcutaneous injection of GA also prevented hair loss caused by cyclophosphamide and taxol (data not shown). Following standard practice in the field, we had observed and recorded protective effects at the time animals had lost most of their body hair, which occurred about 1 week after exposure to chemotherapeutic agents. Anticipating a potential future use of the same preventative method in human patients, we investigated whether the observed protective effects were long-lasting. We followed-up heat-preconditioned, chemotherapeutic drug-treated (etoposide, cyclophosphamide, or cyclophosphamide/adriamycin) animals for longer periods, in some cases, until they had acquired a new fur coat about 3 weeks after chemotherapy. We observed that a majority of animals, and in some groups, all animals exposed to 48–48.5°C heat for 20 min retained their patches of protected hair. However, at lower heat doses, animals tended to gradually lose their patches.
Table 2.
Localized, heat-induced protection against chemotherapy-induced alopecia in young rats
| Chemotherapy agent(s) | Range(s) of concentration(s) | Route of administration | Animals with patches of protected fur | No. of animals exposed | Frequency of protective effect |
|---|---|---|---|---|---|
| Etoposide | 2.5 μg/g, twice | Intraperitoneal | 45 | 48 | 0.94 |
| Cyclophosphamide | 35.5 μg/g, once | Intraperitoneal | 29 | 30 | 0.97 |
| Cyclophosphamide/adriamycin | 20–30 μg/g, once/2.5–4.5 μg/g, twice | Intraperitoneal | 56 | 56 | 1.0 |
| Taxola | 5 μg/animal, twice | Subcutaneous | 7 | 7 | 1.0 |
Heat dose = 48–48.5°C/20 min.
aIncludes data from an experiment using GA to induce a stress protein response.
To generalize our findings, we carried out confirmatory experiments using other models of induced alopecia. We developed a young mouse model by adaptation of conditions previously established for young rats. We observed clear-cut protective effects in experiments in which 12-day-old C57BL/6 mice received a 43–45°C/30 min heat dose to the lower back before etoposide. Example results are shown in Fig. 1e. Experiments using adult mouse models (Paus et al. 1990, 1994) produced similarly unambiguous effects. In one such model, an area of fur on the lower back of adult C57BL/6 mice was removed using a rosewax depilation procedure. Heat was administered to the area 13–14 days later using an IR laser, and cyclophosphamide was injected i.p. the following day (Fig. 2a). In another model, the lower backs of adult animals were shaved 5–7 days before intradermal (i.d.) injection of 17-(allylamino)-17-demethoxygeldanamycin (17AAG), a GA derivative with reduced toxicity (Fig. 2b).
Fig. 2.
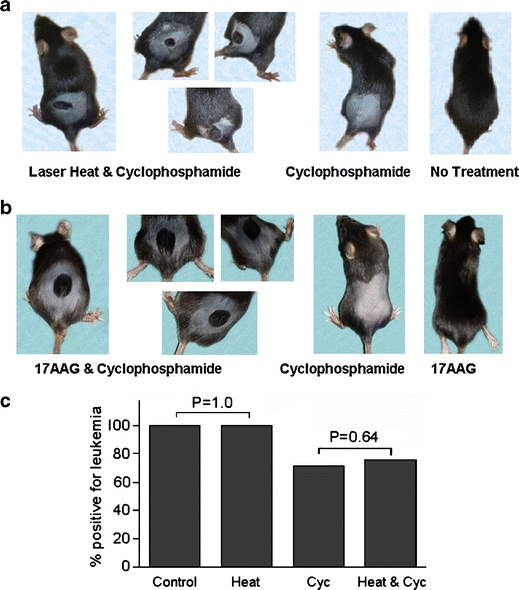
a and b Localized protection of hair on the lower back of adult mice from cyclophosphamide toxicity. Protective effects were induced in a by laser heat and in b by 17AAG. c Model cancer chemotherapy experiment using the young rat model. 17AAG 17-(allylamino)-17-demethoxygeldanamycin, Cyc cyclophosphamide (for experimental detail see the text)
Finally, we carried out model cancer chemotherapy experiments to demonstrate that induced alopecia could be prevented without sacrificing chemotherapy efficacy. Targeted delivery of heat to skin areas in which alopecia induction is to be inhibited will preclude the induction of a stress protein response in tumors not located in the heated areas. However, although the stress protein response is an intracellular protective response, the possibility needed to be considered that heat exposure of skin and embedded structures could induce signals that could affect chemotherapy of a tumor located elsewhere. To address this question, 8-day-old rats randomly assigned to 4 groups (n = 45, each) received an i.p. injection of MIA C51 rat chloroleukemic cells. One group was subjected to topical heat treatment (48°C/20 min) 6 h later and administered a single dose (35.5 μg/g) of cyclophosphamide 24 h after heat treatment. Control groups received either only a heat treatment or cyclophosphamide, or remained untreated. Animals were killed 30 days later, and the presence of leukemia was determined from an analysis of bone marrow aspirates (>30% nonerythroid blasts). Results revealed that localized heat treatment did not significantly reduce the antineoplastic effect of cyclophosphamide (Fig. 2c; see p values included in the graph). This finding was confirmed in a second, similarly powered experiment.
In summary, our data indicate that localized activation of a stress protein response is an effective new method for preventing chemotherapy-induced hair loss in animal models. As was hoped based on the known broad protective effects of an activated stress protein response, the method appears to afford protection against a diverse range of antineoplastic agents and combinations. Chemotherapy protocols utilized in the clinic not only differ in the drug or drug combination used but also in the number of drug doses administered per treatment cycle, the interval between doses and the duration of drug administration (i.e., injection or infusion). A preliminary experiment suggested that a one-time activation of a stress protein response protects the hair follicles of young rats from the toxic effects of a 5-day regimen of daily etoposide. If this result translates to humans, the present heat preconditioning method for preventing chemotherapy-induced alopecia should be compatible with or capable of adaptation to many of the chemotherapy protocols in clinical use.
Although heat preconditioning was utilized primarily to induce a protective response, several experiments were conducted in which a similar response was obtained following s.c. or i.d. administration of GA or 17AAG, respectively. Effective methods of liposomal delivery of compounds deep into the hair follicles were developed in recent years (Li and Hoffman 1995; Hoffman 2006; Jung et al. 2006). It appears, therefore, feasible to develop a preventative therapy that is based on the activation of a stress protein response in the scalp either by administration of an appropriate heat dose or by delivery of an effective dose of an inducer such as GA or 17AAG by means of a liposomal vehicle.
Acknowledgments
We thank M. Fenna, J. He, J. Xie, and S.L. Hsia for their help with certain experiments, and Walter Scott and David Barber for the critical review of the manuscript. This study was supported in part by the Rockefeller Brothers Fund (02-237), NCI grant R01 CA093489, and HSF Pharmaceuticals S.A.
References
- Ali A, Bharadwaj S, O’Carroll R, Ovsenek N (1998) HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol Cell Biol 18:4949–4960 [DOI] [PMC free article] [PubMed]
- Christodoulou C, Klouvas G, Efstathiou E, Zervakis D, Papazachariou E, Plyta M, Skarlos DV (2002) Effectiveness of the MSC cold cap system in the prevention of chemotherapy-induced alopecia. Oncology 62:97–102 [DOI] [PubMed]
- Dorr VJ (1998) A practitioner’s guide to cancer-related alopecia. Semin Oncol 25:562–570 [PubMed]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E (1999) HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J 13:2061–2070 [DOI] [PubMed]
- Gibbons NB, Watson RWG, Coffey RNT, Brady HP, Fitzpatrick JM (2000) Heat-shock proteins inhibit induction of prostate cancer cell apoptosis. Prostate 45:58–65 [DOI] [PubMed]
- Hesketh PJ, Batchelor D, Golant M, Lyman GH, Rhodes N, Yardley D (2004) Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support Care Cancer 12:543–549 [DOI] [PubMed]
- Hidalgo M, Rinaldi D, Medina G, Griffin T, Turner J, von Hoff DD (1999) A phase I trial of topical topitriol (calcitriol, 1,25-dihydroxyvitamin D-3) to prevent chemotherapy-induced alopecia. Anti-cancer Drugs 10:393–395 [DOI] [PubMed]
- Hoffman RM (2006) The hair follicle and its stem cells as drug delivery targets. Expert Opin Drug Deliv 34:37–443 [DOI] [PubMed]
- Huot J, Roy G, Lambert H, Chretien P, Landry J (1991) Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res 51:5245–5252 [PubMed]
- Hussein AM, Jimenez JJ, McCall CA, Yunis AA (1990) Protection from chemotherapy-induced alopecia in a rat model. Science 24:91564–1566 [DOI] [PubMed]
- Inoue Y, Sato Y, Nishimura M, Seguchi M, Zaitsu Y, Yamada K, Oka Y (1999) Heat-induced drug resistance is associated with increased expression of Bcl-2 in HL60. Anticancer Res 19:3989–3992 [PubMed]
- Ito H, Shimojo T, Fujisaki H et al (1999) Thermal preconditioning protects rat cardiac muscle cells from doxorubicin-induced apoptosis. Life Sci 64:755–761 [DOI] [PubMed]
- Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M (1998) Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J 17:6124–6134 [DOI] [PMC free article] [PubMed]
- Jimenez JJ, Yunis AA (1988) Treatment with monocyte-derived partially purified GM-CSF but not G-CSF aborts the development of transplanted chloroleukemia in rats. Blood 72:1077–1080 [PubMed]
- Jimenez JJ, Alvarez E, Bustamante CD, Yunis AA (1995) Pretreatment with 1,25(OH)2D3 protects from Cytoxan-induced alopecia without protecting the leukemic cells from Cytoxan. Am J Med Sci 310:43–47 [DOI] [PubMed]
- Jung S, Otberg N, Thiede G, Richter H, Sterry W, Panzner S, Lademann J (2006) Innovative liposomes as a transfollicular drug delivery system: penetration into porcine hair follicles. J Invest Dermatol 126:1728–1732 [DOI] [PubMed]
- Kampinga HH (1995) Hyperthermia, thermotolerance and topoisomerase II inhibitors. Br J Cancer 72:333–338 [DOI] [PMC free article] [PubMed]
- Katsimbri P, Bamias A, Pavlidis N (2000) Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer 36:766–771 [DOI] [PubMed]
- Kwak HJ, Jun CD, Pae HO et al (1998) The role of inducible 70-kDa heat shock protein in cell cycle control, differentiation, and apoptotic cell death of the human myeloid leukemic HL-60 cells. Cell Immunol 187:1–12 [DOI] [PubMed]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J (1993) Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem 268:3420–3429 [PubMed]
- Li L, Hoffman RM (1995) The feasibility of targeted selective gene therapy of the hair follicle. Nat Med 1:705–706 [DOI] [PubMed]
- Li GC, Werb Z (1982) Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A 79:3218–3122 [DOI] [PMC free article] [PubMed]
- Liu RY, Li X, Li L, Li GC (1992) Expression of human hsp70 in rat fibroblasts enhances cell survival and facilitates recovery from translational and transcriptional inhibition following heat shock. Cancer Res 52:3667–3673 [PubMed]
- Moloney WC, Dorr AO, Dowd G, Boschetti AE (1962) Myelogenous leukemia in the rat. Blood 19:45–59 [PubMed]
- Munstedt K, Manthey N, Sachsse S, Vahrson H (1997) Changes in self-concept and body image during alopecia induced cancer chemotherapy. Support Care Cancer 5:139–143 [DOI] [PubMed]
- Oesterreich S, Weng CN, Qiu M, Hilsenbeck SG, Osborne CK, Fuqua SA (1993) The small heat shock protein hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Res 53:4443–4448 [PubMed]
- Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496 [DOI] [PubMed]
- Paus R, Stenn KS, Link RE (1990) Telogen skin contains an inhibitor of hair growth. Br J Dermatol 122:777–784 [DOI] [PubMed]
- Paus R, Handjiski B, Eichmueller S, Czarnetzki BM (1994) Chemotherapy-induced alopecia in mice. Induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am J Pathol 144:719–734 [PMC free article] [PubMed]
- Pratt WB, Toft DO (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18:306–360 [DOI] [PubMed]
- Roigas J, Wallen ES, Loening SA, Moseley PL (1998) Effects of combined treatment of chemotherapeutics and hyperthermia on survival and the regulation of heat shock proteins in Dunning R3327 prostate carcinoma cells. Prostate 34:195–202 [DOI] [PubMed]
- Salminen WF, Voellmy R, Roberts SM (1996) Induction of hsp 70 in HepG2 cells in response to hepatotoxicants. Toxicol Appl Pharmacol 141:117–123 [PubMed]
- Samali A, Cotter TG (1996) Heat shock proteins increase resistance to apoptosis. Exp Cell Res 223:163–170 [DOI] [PubMed]
- Schilli MB, Paus R, Menrad A (1998) Reduction of intrafollicular apoptosis in chemotherapy-induced alopecia by topical calcitriol-analogs. J Invest Dermatol 111:598–604 [DOI] [PubMed]
- Vilaboa NE, Galan A, Troyano A, de Blas E, Aller P (2000) Regulation of multidrug resistance 1 (MDR1)/P-glycoprotein gene expression and activity by heat-shock transcription factor 1 (HSF1). J Biol Chem 275:24970–24976 [DOI] [PubMed]
- Wang J, Lu Z, Au JLS (2006) Protection against chemotherapy-induced alopecia. Pharm Res 23:2505–2514 [DOI] [PubMed]
- Whitesell L, Mimnaugh EG, de Costa B, Myers CE, Neckers LM (1994) Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A 91:8324–8328 [DOI] [PMC free article] [PubMed]
- Xia W, Vilaboa N, Martin JL, Mestril R, Guo Y, Voellmy R (1999) Modulation of tolerance by mutant heat shock transcription factors. Cell Stress Chaperones 4:8–18 [DOI] [PMC free article] [PubMed]
- Yunis AA, Arimura GK, Haines HG, Ratzan RJ, Gross MA (1975) Characteristics of rat carcinoma in culture. Cancer Res 35:337–345 [PubMed]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94:471–480 [DOI] [PubMed]


