Abstract
Evidence that membrane-bound and extracellular heat shock proteins (HSPs) with molecular weights of 70 and 90 kDa are potent stimulators of the immune responses has accumulated over the last decade. In this review, we discuss the modulation of Hsp70 expression, a major stress-inducible member of the HSP70 family, in the cytoplasm and on the plasma membrane of tumor cells by clinically applied interventions such as radio- and chemotherapy.
Keywords: Hsp70, Membrane expression, Stress response modifiers, NK cell, Tumor
Cytosolic heat shock proteins (HSPs)
Heat shock proteins (HSPs), also termed stress proteins, are evolutionary highly conserved and ubiquitously distributed molecules that are present in nearly all cellular and subcellular compartments (cytosol, endoplasmatic reticulum, mitochondria, endosomal compartment, lysosome, nucleus, and membranes). Intracellularly, HSPs play pivotal roles in protein homeostasis and are intimately involved in the survival of prokaryotic and eukaryotic cells that have been subjected to various unfavorable insults. As molecular chaperones, these molecules support and promote the correct folding of nascent proteins and misfolded proteins, prevent undesirable protein aggregation, assist protein transport across membranes, and mediate antigen processing under physiological conditions in the cytosol (Hartl and Hayer-Hartl 2002). The synthesis of HSPs is highly up-regulated after thermal stress, whereas de novo protein synthesis is generally reduced. In addition to heat, a variety of non-physiological events, including nutritional deprivation, physical (i.e., UV light, gamma-irradiation), or chemical stressors (i.e., heavy metals, oxidative stress, cytostatic drugs, amino acid analogues, anti-inflammatory drugs, and alkyl-lysophospholipids) have been identified as potent inducers of a classical heat shock (stress) protein response. Elevated cytosolic HSP70 levels mediate protection of tumor cells from stress-induced lethal damage by interfering with apoptotic pathways (Wei et al. 1995; Jaattela et al. 1998) and, thus, may induce resistance to chemoradiotherapy. Therefore, high cytosolic levels of HSP70 are frequently associated with a negative clinical outcome and a higher frequency of metastasis in human cancer patients (Ciocca and Calderwood 2005). In contrast, membrane-bound and extracellular-located HSP70 is known to elicit cancer immunity and, thus, might be beneficial for the clinical outcome.
Membrane-bound and extracellular Hsp70 as mediators of an anti-tumor immunity
Although lacking a transmembrane domain, HSPs with molecular weights of 70 and 90 kDa have been found in the plasma membrane and in the extracellular milieu of cultured viable cells (Barreto et al. 2003). The initial identification of HSP70 family members in the plasma membrane was achieved using several methods including selective cell surface iodination (Multhoff et al. 1995) or by biotinylation followed by proteomic profiling of surface-bound proteins (Shin et al. 2003). Their presence was subsequently demonstrated by flow cytometry using a monoclonal antibody, which is highly specific for surface Hsp70 on viable tumor cells (cmHsp70.1, multimmune GmbH, Munich, Germany). Other commercially available Hsp70 antibodies recognize Hsp70 on the surface of pre-apoptotic cells interacting with phosphatidylserine (PS) on the outer membrane leaflet or when Hsp70 is bound from outside to receptors such as scavenger or C-type lectin receptors (Calderwood et al. 2007) or when it is associated with toll-like receptors 2 and 4 (TLR2/4; Triantafilou and Triantafilou 2004; Asea et al. 2000). This is because of the fact that their recognition epitopes differ from that of the antibody cmHsp70.1, recognizing an 8-mer amino acid sequence 453–461 NLLGRFEL, which is exposed to the extracellular milieu of viable tumor cells. A broad screening program of more than 1,000 different tumors and their corresponding normal tissues has revealed that about 50–70% of those tumors tested, including head and neck, colorectal, gastric, liver, pancreas, mammary, urogenital carcinomas, and leukemic blasts of hematological malignancies, are Hsp70 membrane-positive, as determined by Ab cmHsp70.1. In contrast, none of the corresponding normal tissues exhibited an Hsp70 membrane-positive phenotype (Gehrmann et al. 2003; Multhoff et al. 1995). Quantitative analysis revealed that about 15 to 20% of the total Hsp70 is present in tumor cell membranes (manuscript in preparation). It appears that the membrane expression of Hsp70, as determined by the antibody cmHsp70.1, is restricted to malignantly transformed cells. These data led us to the hypothesis that membrane-bound Hsp70 is highly tumor-specific and that it might, thus, provide a target structure for an appropriately targeted immunotherapeutic strategy.
A potential prognostic value for the presence of Hsp70 on the plasma membrane has also been identified. This was demonstrated using a xenograft SCID/beige mouse model (Gehrmann et al. 2003), in which human tumors were orthotopically implanted into immunodeficient mice. In this model, the surface density of Hsp70 expression on metastases was greater than that on primary tumors (Multhoff et al. 2000; Stangl et al. 2006). These findings might be explained by the fact that an Hsp70 membrane-positive phenotype facilitates metastatic dissemination, enables adherence to endothelial cells and organs, or that it might confer resistance to an unfavorable milieu that is present during the metastatic process. Supporting these results is the finding that tumor patients with Hsp70 membrane-positive squamous cell carcinomas of the lung and lower rectal carcinomas have a significantly decreased overall survival (Pfister et al. 2007).
It appears that extracellular HSPs with molecular weights of about 20 (Hsp27), 60 (cpn60; Quintana et al. 2004), 70 (Hsc70, Hsp70, Bip), 90 (gp96; Srivastava et al. 1998), 110 (Wang et al. 2006), and 170 kDa (grp170; Park et al. 2006) can affect the immune system. Small HSPs including human Hsp27 (Bandyopadhyay et al. 2007), as well as BiP (Panayi and Corrigal 2006), an ER-residing member of the HSP70 family, have been found to suppress immune functions of T cells and, thus, might provide an anti-inflammatory tool.
Even in the absence of immunogenic peptides recombinant Hsp70 protein, the major stress-inducible member, in combination with pro-inflammatory cytokines including IL-2 and IL-15, can stimulate NK cells and, thus, might lead to the induction of a protective anti-tumor immunity (Multhoff et al. 1997; Multhoff et al. 2001). The mechanism of tumor cell killing has been shown to be perforin-independent granzyme B-mediated apoptosis (Gross et al. 2003a). The expression of several activating NK cell receptors, including CD94/NKG2C, NKG2D, NKp30, NKp44, and NKp46 is concomitantly up-regulated by a treatment of NK cells with Hsp70 protein or a 14-mer peptide derived from the C terminus (aa 450–463; Gross et al. 2003a, b). Cell surface binding and antibody blocking studies have identified the Hsp70 interacting receptor as being the C-type lectin receptor CD94. Unlike NK cells, T cells and NK-like T (NKT) cells do not recognize Hsp70 membrane-positive tumor cells (Gross et al. 2003b). In line with these findings, the mouse homologue of the human TKD peptide, TRDNNLLGRFELSG, has been found to stimulate cytotoxic activity in mouse NK cells against tumors (Zhang and Huang 2007).
Peptide-free, full-length Hsp70 also activates the innate immune system via direct effects. Tumor-derived chaperone-rich cell lysates containing HSP70, HSP90, grp94, and calreticulin have been shown to mediate anti-cancer immunity, which requires the involvement of NK cells (Zeng et al. 2006). Binding of HSP70 to APCs such as monocytes/macrophages and dendritic cells (DCs) results in secretion of pro-inflammatory cytokines including IL-1ß, IL-6, and TNF-α (Asea et al. 2000) and, thus, stimulate the innate immune system non-specifically. Presently, scavenger receptors, C-type lectin receptors, and Toll-like receptors 2 and 4 (TLR2/4; Asea et al. 2002) are discussed as binding partners for HSPs (Calderwood et al. 2007; Triantafilou and Triantafilou 2004, Asea et al. 2000). Pramod Srivastava and colleagues have shown that HSP70- (Hsp70 and Hsc70) and Hsp90 (gp96)-chaperoned peptides derived from the cytosol of human tumors can activate a classical protective T cell-mediated immune response (Srivastava et al. 1998). The induction of such immune responses involves the uptake of HSP–peptide complexes by antigen-presenting cells (APCs) and the transfer of these peptides into the MHC presentation pathway for subsequent cross-presentation on MHC class I molecules to appropriately specific CD8+ T cell populations (Srivastava et al. 1998). A short overview about of the predominant location and the immunological effects of different members of the HSP70 family is shown in Table 1.
Table 1.
Immunological effects of members of the HSP70 superfamily in different subcellular compartments
| Location | Hsp70 | BiP | Grp170 |
|---|---|---|---|
| Cytosol | Protection | Not present | Not present |
| ER | Not present | Protection | Protection |
| Nucleus | Protection | Not present | Not present |
| Membrane | Pro-immune | Not present | Not present |
| Extracellular | Pro-immune | Anti-immune | Pro-immune |
Apart from affecting the effector side of the immune system, it is also possible to modify tumor cells so that they become better targets for the immune system. Transfection of mouse mastocytoma cells with membrane-bound Hsp70 results in tumor regression mediated by T and NK cells (Chen et al. 2002). Secretion of Hsp70 by genetically engineered mouse tumor cells results in tumor rejection through a mechanism that involves both T lymphocytes and NK cells (Massa et al. 2004; Wang et al. 2004). Grp170, the largest ER-residing Hsp70-related family member, has been shown to elicit innate as well as the adaptive immune responses in poorly immunogenic melanoma when it gets released (Wang et al. 2006).
Regarding these results, we have evaluated the capacity of various clinically applied methods such as radiation and anti-cancer reagents to induce a tumor-selective Hsp70 membrane expression (Table 2). According to their mode of action, anti-cancer drugs can be grouped as follows: (1) membrane-interactive substances, (2) drugs with anti-inflammatory capacity, (3) anti-neoplastic agents, and (4) agents that support redifferentiation (Table 3). We have demonstrated that all of the approaches tested exhibit specific features and have defined effects on Hsp70 expression in the cytoplasm or on the membrane (Gehrmann et al. 2004, 2005a, b; Multhoff et al. 1996).
Table 2.
Effects of clinically applied reagents on intracellular and membrane-bound Hsp70 levels and its immunological consequences
| Treatment | Intracellular | Membrane | Immunology |
|---|---|---|---|
| Radiation | Increase | Increase | Better NK target |
| Membrane drugs | No change | Increase | Better NK target |
| Anti-inflammatory | No change | Increase | Better NK target |
| Anti-neoplastic | Increase | Increase | Better NK target |
| Re-differentiation. | No change | Decrease | Worse NK target |
Table 3.
Reagents tested for their capacity to modulate the Hsp70 expression in tumor cells
| Component | Medicinal or trade name | Application | Mode of action | Reference |
|---|---|---|---|---|
| Membrane-interactive substances | ||||
| Alkyl-lysophospholipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3) | Edelfosine | Inhibitor of the PI3K pathway, inhibitor of PKC, inhibitor of PI PLC | Dimanche-Boitrel et al. (2005), Heczkova and Slotte (2006) | |
| Anti-inflammatory agents | ||||
| Acetyl-salicylic acid | Aspisol® | Analgesic, anti-phlogistic, anti-pyretic, inhibitor of platelet aggregation | COX-1/COX-2 inhibition | Ulrich et al. (2006) |
| Celecoxib | Celebrex™ | Osteoarthrosis, chronic polyarthritis | COX-2 inhibition | Kismet et al. (2004), Mardini and FitzGerald (2001) |
| Rofecoxib | Vioxx® | Osteoarthrosis, analgesic | COX-2 inhibition | Brune and Hinz (2004) |
| Pioglitazone | Actos™ | Type 2 diabetes | Insulin-sensitizer with anti-inflammatory capacity | Lee et al. (2006) |
| Anti-neoplastic agents | ||||
| Vincristine | Vincristin liquid, Lilly™ | Solid tumors, ALL, NHL | Tubulin binding, inhibiting mitotic spindle formation resulting in deadlock of mitosis in the metaphase | Gidding et al. (1999) |
| Paclitaxel | Taxol®-100 | Ovarian carcinoma, mammary carcinoma | Binding to microtubules, disturbing its dynamic reorganization in interphase and mitosis | Blagosklonny and Fojo (1999) |
| Carboplatin | Ribocarbo®-L | Epithelial ovary carcinoma, small cell bronchial carcinoma, head-and-neck tumors, urinary bladder carcinoma, tumors of the testis | Crosslinking of DNA single and double strands resulting in inhibiting the template function of the DNA | O’Dwyer et al. (2000) |
| Doxorubicin | Doxo-cell™ | Different haematological diseases and solid tumors | Intercalation into DNA, resulting in steric hindrance of DNA and RNA synthesis | Ciocca et al. (2003) |
| Fludarabine | Fludara® | B-CLL | Metabolite interferes with DNA synthesis by inhibiting ribonucleic reductase, DNA polymerase α/δ and ɛ, DNA primase, DNA ligase; reducing protein synthesis by partially inhibiting RNA polymerase II | Eissner et al. (2002), Parker et al. (2004) |
| Ifosfamide | IFEX® | ALL, different solid tumors | DNA alkylation, DNA strand cross-linking | Botzler et al. (1997) |
| Cytarabine | Ara-cell™ | ALL, NHL, AML | Metabolite ARA-CTP competitively inhibits DNA polymerase, inhibiting DNA synthesis by building-in in DNA | Grant (1998) |
| Redifferentiating agents | ||||
| Retinoids | ATRA: APL (AML-M3); 13-cis retinoic acid: leukoplakia, melanoma, ENT tumor, neuroblastoma, non-small cell bronchial carcinoma | Regulation of cell growth, apoptosis, homeostasis. promoting differentiation of tumor cells to a more benign cell type | Callari et al. (2003), Freemantle et al. (2003), Gendimenico and Mezick (1993) | |
Radiation
Radiation is frequently used in cancer therapy, either as a single regimen or in combination with chemotherapy. In elderly patients with non-small lung cell carcinomas and significantly reduced lung capacity, hypofractionated stereotactic radiotherapy has been shown to be safe, well tolerated, and to lead to a high local tumor control (Zimmermann et al. 2006). Despite these promising results, radiation-resistant tumor clones can limit the long-term therapeutic efficacy of this approach. It is, therefore, important to understand the molecular nature of radiation-induced effects that might be responsible for radiation resistance. High cytosolic levels of Hsp70 are known to confer resistance against apoptotic cell death induced by a variety of environmental stress stimuli (Jaattela 1999). In vitro, it has been shown that non-lethal doses of gamma irradiation increase the cytosolic Hsp70 levels in Chinese hamster ovary (CHO) cells (Sierra-Rivera et al. 1993). Also, UV light has the capacity to elevate cytosolic levels of Hsp70 in human fibroblasts (Matsumoto et al. 1995; Suzuki and Watanabe 1992). Photodynamic therapy has been found to increase the cell-surface expression and release of HSP70 and, thus, induces an inflammatory immune response that contributes positive to the clinical outcome (Korbelik et al. 2005).
Our group has demonstrated that, apart from the cytosol, a non-lethal dose of gamma irradiation (total of 1 × 10 Gy) profoundly enhances membrane-bound Hsp70 levels in human colon and pancreas carcinoma cells, which initially express low levels of Hsp70 on the cell membrane (Gehrmann et al. 2005a, b). A representative view of the plasma membrane expression of Hsp70 in untreated, and sublethally irradiated tumor cells is shown in Fig. 1. Functionally, these tumors appear to be better protected against a second stressful stimulus such as a second hit by gamma irradiation or by cytostatic drugs. Although these findings indicate that non-lethal gamma irradiation might confer resistance to standard therapy on certain tumor cell lines, the increased amounts of Hsp70 expressed on the plasma membrane also serve as a target recognition structure for the innate immune system. The amount of membrane-bound Hsp70 correlates with an increased kill mediated by NK cells. It, therefore, appears that membrane Hsp70 fulfills a dual role: on the one hand, as a protector against radiation/chemotherapy-induced apoptosis and, on the other hand, as a target structure for NK cell-mediated cytolysis. One might speculate that clinical investigations should, therefore, consider therapeutic protocols that combine standard radiochemotherapy with appropriately designed immunotherapies based on membrane-bound HSPs.
Fig. 1.
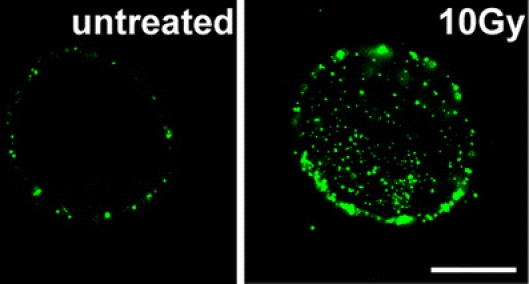
Immunohistochemical visualization of Hsp70 on the plasma membrane of CX+ tumor cells using Hsp70-specific antibody cmHsp70.1-FITC, either untreated or irradiated with the sublethal dose of 1 × 10 Gy, followed by a recovery period of 12 h
Membrane-interactive substances
The membrane-interactive substance alkyl-lysophospholipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3 or edelfosine) is used in the treatment of hematological diseases (Dimanche-Boitrel et al. 2005; Mollinedo et al. 2004; Heczkova and Slotte 2006). We tested its effects on the modulation of Hsp70 expression in the cytosol and on the cell surface of tumor cells (Botzler et al. 1999). We have shown that, in addition to its direct cytotoxic activity toward leukemic blasts, sublethal concentrations of this agent can increase membrane expression of Hsp70 on the myeloid leukemic cell line K562 (Botzler et al. 1999). However, this agent does not effect the cytosolic levels of Hsp70. These data led us to conclude that edelfosine and its derivatives primarily might act by changing the intracellular distribution of Hsp70 from the cytosol to the plasma membrane. In line with the effects induced by radiotherapy, an edelfosine-initiated increase in membrane-bound Hsp70 enhances the sensitivity of tumor cells to the cytotolytic response mediated by NK cells.
Anti-inflammatory drugs
In contrast to the supposed beneficial effects of acute inflammation, it has been suggested that chronic inflammation has a negative impact on the clinical outcome of cancer patients. The impact of non-steroidal anti-inflammatory drugs (Ulrich et al. 2006) in tumor therapy has, therefore, been tested, and beneficial effects of a long-term application of acetyl salicylic acid have been demonstrated for colorectal (Giovannucci et al. 1994; Thun et al. 1991), stomach (Farrow et al. 1998), esophagus (Corley et al. 2003), and breast (Johnson et al. 2002) cancer. Currently, two isoforms of cyclooxygenases (Brune and Hinz 2004) that play key roles in the conversion of arachidonic acid to prostaglandins have been characterized. The constitutive isoform COX-1 is known to interfere with housekeeping functions in the gastrointestinal tract, and the inducible isoform COX-2 (Mardini and FitzGerald 2001) has been reported to be overexpressed in different human diseases, including pancreatitis (Song et al. 2002), pancreatic carcinoma (Okami et al. 1999), colorectal cancer (Cao et al. 2000; Richter et al. 2001), and esophageal squamous cell carcinoma (Shamma et al. 2000).
It was, therefore, proposed that there is an association between COX-2 expression and tumorigenesis. Increasing doses of celecoxib, a COX-2 inhibitor, reduces breast cancer in rat models (Abou-Issa et al. 2001), and these beneficial effects have been shown to be caused by the anti-angiogenic efficacy of COX-2 inhibitors (Masferrer et al. 2000; Wang et al. 2002). COX-2 inhibitors also induce tumor cell apoptosis and inhibit cell proliferation (Patti et al. 2002; Yamazaki et al. 2002). We were, therefore, interested to test the effects of several non-steroidal anti-inflammatory drugs and pioglitazone (PIO; Lee et al. 2006), an insulin sensitizer with anti-inflammatory properties, on the expression of Hsp70 in human colon carcinoma cell lines, which exhibited differential Hsp70 membrane expression pattern (Multhoff et al. 1997). For these studies, colon cancer cells were treated with varying doses of the COX-1/COX-2 inhibitor aspisol (ASA), the COX-2 inhibitors celecoxib (CLX) and rofecoxib (RFX) and PIO. Non-lethal doses of these reagents resulted in an increase in membrane-bound Hsp70 in tumor cells with initially low expression levels. With respect to function, an anti-inflammatory drug-mediated increase in membrane-bound Hsp70 increased the sensitivity of tumor cells to NK cell-mediated lysis (Gehrmann et al. 2004). Antibody blocking studies demonstrated that the elevated density of Hsp70 on the plasma membrane of treated tumor cells was responsible for their increased sensitivity to the cytolytic effects of NK cells. The cytosolic levels of Hsp70 remained unaffected by sublethal doses of COX inhibitors.
Anti-neoplastic agents
Hsp70 that are released from tumor cells undergoing chemotherapy may mediate a danger signal to the host`s immune system (Calderwood et al. 2005; Ciocca et al. 2003). Cytostatic drugs are frequently used in the therapy of rapidly replicating tumor cells, and they act in two different ways. Either they directly interfere with DNA during the replication process or they interact with proteins or enzymes that are involved in the process of cell cycle, proliferation, and differentiation. We have investigated the effects of DNA-interfering drugs (including cytarabine, ifosfamide, carboplatin, doxorubicin, and fludarabine; Botzler et al. 1997; Eissner et al. 2002) and substances that interfere with the cell cytoskeleton (vincristine and paclitaxel; Gidding et al. 1999) on the fast-growing leukemic cell line K562. Treatment of K562 cells with non-lethal doses of each compound revealed that only drugs that interfere with cytoskeletal proteins such as tubulin and actin (vincristine, paclitaxel) are able to increase the amounts of cytosolic and membrane-bound Hsp70. In contrast, DNA-interfering reagents did not affect the Hsp70 levels (Gehrmann et al. 2002).
It is worth noting that, in contrast to the leukemic cell line, Hsp70 was only elevated in the cytosol but not on the plasma membrane of peripheral blood lymphocytes derived from healthy individuals (Botzler et al. 1999).
On the basis of these results, we speculate that the differences in the Hsp70 cell surface expression in tumor and normal cells might result from differences in the composition of the membrane lipids in the two cell types. Already in 1989, an interaction of Hsp70 with fatty acids of the membrane has been hypothesized by Hightower and Guidon (1989). The increase in cytosolic Hsp70 levels in both cell types might be caused by the fact that vincristine and paclitaxel are known to interact with tubulin filaments and, thus, to cause protein aggregates. As shown in Fig. 2, both reagents are indeed able to induce tubulin aggregates that act as inducers for the Hsp70 synthesis (Gehrmann et al. 2003).
Fig. 2.
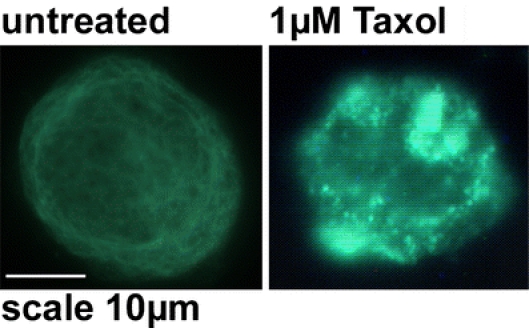
Immunohistochemical visualization of tubulin aggregates induced by Taxol (paclitaxel) in K562 tumor cells. Left graph Untreated K562 cells with a regular tubulin network; right graph K562 cells treated with Taxol (1 μM) with tubulin aggregates; scale bar, 10 μm
Redifferentiating agents
Another approach for the treatment of cancer is to induce redifferentiation in tumor cells. Retinoids are synthetic derivatives of vitamin A that have such a redifferentiation capacity (Freemantle et al. 2003; Gendimenico and Mezick 1993). Clinically, retinoids are used in the treatment of distinct hematological diseases such as acute promyelocytic leukemia (Park et al. 2003) and some solid tumors (Altucci and Gronemeyer 2001; Callari et al. 2003). Retinoids are also known to play a role in post-natal development, regulation of cell growth (Nagpal et al. 1996), apoptosis (Massaro and Massaro 2000), and homeostasis (Wan et al. 2000).
We were interested to determine whether there is any association between retinoid-induced loss in tumorigenicity and Hsp70 expression in the cytosol and on the plasma membrane of tumor cells. As membrane Hsp70 expression is associated with malignancy, we speculated that retinoids might reduce the amount of membrane-bound Hsp70. As with the other drugs, we analyzed the differentiating capacity of sublethal concentrations of 13-RA and ATRA on membrane Hsp70 levels and correlated them with the cytolytic capacity of NK cells. Furthermore, the retinoid-induced effects were compared to those of sodium butyrate, which also exhibits a redifferentiating capacity in colon carcinoma cell lines with a differential membrane Hsp70 expression pattern (Multhoff et al. 1997).
For these experiments, exponentially growing, adherent tumor cells were incubated for up to 6 weeks with non-lethal concentrations of 13-RA or ATRA at (Gehrmann et al. 2005a, b). Such treatment significantly reduced the number of Hsp70-positive tumor cells, and it appeared that retarded cell growth but not apoptotic cell death was responsible for the loss of Hsp70 membrane-positive tumor cells (Gehrmann et al. 2005a, b). Concomitant with the decreased cell growth and the loss of Hsp70 membrane positivity, the phenotype of the tumor cells changed from a three-dimensional growth kinetic to a monolayer (Gehrmann et al. 2005a, b). Cytosolic Hsp70 levels remained unaffected. Taken together, these data provide further evidence that the Hsp70 membrane positivity is associated with a malignant cell phenotype.
Induced overexpression of membrane Hsp70 as a therapeutical approach
In the cytosol, Hsp70 is essential for protein folding, unfolding, and transport, and it interferes with anti-apoptotic pathways (Jaattela 1999; Gabai et al. 1998). Therefore, a reduction of the cytosolic Hsp70 content by small interfering RNA might be beneficial for the therapy of cancer. However, most therapeutical interventions, including radiochemotherapy, cause an upregulation of Hsp70 in the cytosol, on the plasma membrane and also in the extracellular milieu (Todryk et al. 2003). Because of the dual role of Hsp70 inside and outside of tumor cells, a therapy-induced induction of extracellular Hsp70 can elicit an anti-cancer immune response. The tumor-specific membrane expression pattern and the ubiquitous distribution of Hsp70 in different tumor entities make membrane-bound Hsp70 an attractive target structure for immunotherapeutic approaches. As detailed earlier, extracellular Hsp70 binds to receptors such as TLR2 and TLR4 and non-specifically stimulates the innate immune system (Asea et al. 2002). In addition to TLRs, Hsp70 binding to APCs can be mediated via the lipopolysaccharide receptor CD14 (Asea et al. 2000), scavenger receptors (Vabulas et al. 2002; Calderwood et al. 2007), or CD40 on B cells (Becker et al. 2002), and C-type lectin receptors (Theriaults et al. 2006). A peptide-dependent binding of Hsp70 to the alpha-2 macroglobulin receptor CD91 has also been reported (Basu et al. 2001).
We have shown that Hsp70 in combination with proinflammatory cytokines can directly induce the proliferation and migration of NK cells and increase their cytolytic activity. Such stimulation also increases the cell surface density of CD56, CD94, and stimulatory NK cell receptors such as the NCRs and NKG2D (unpublished data). The C-type lectin receptor CD94 is essential for a quantitative binding of Hsp70 to NK cells and for the initiation of intracellular signaling pathways that result in the perforin-independent, granzyme B-mediated cytotoxicity toward membrane Hsp70-positive tumor cells (Gross et al. 2003b, c).
In addition to the full-length Hsp70 protein, an Hsp70-peptide termed TKD, isolated from the C terminus of Hsp70, which shows similar stimulatory activity on NK cells has been identified (Multhoff et al. 2001). A phase I clinical trial has demonstrated that ex vivo stimulation of patients’ NK cells with TKD is feasible, safe, well tolerated, and initiates Hsp70 reactivity in patient-derived NK cells (Krause et al. 2004). These encouraging data have initiated a broad screening program, the aim of which is to test a variety of different clinically applied reagents for their capacity to modulate membrane Hsp70 expression and influence the susceptibility to cytokine/TKD-activated NK cells. We are currently considering the mechanisms via which alterations in Hsp70 expression might be explained. These reagents might (1) increase transcription or influence associated processes, (2) increase translation or influence associated processes, (3) decrease degradation processes, (4) increase in the transport to the membrane or decrease release/retransport from the membrane, or (5) modify the membrane composition to facilitate the binding and integration of Hsp70.
With respect to the increase of membrane-bound Hsp70 levels, a large number of different stress stimuli including physical (UV light, gamma irradiation, photodynamic therapy), chemical (DeMaio 2000), geranylgeranylactone (Hirakawa et al. 1996), and cytokines including IFN-gamma (Asea et al. 2000) have been described. It is apparent that membrane-bound and extracellular HSPs have an immunological impact, and herein, we have described clinically applied reagents with the capacity to increase or decrease membrane Hsp70 expression and, thereby, influence their susceptibility to NK cell-mediated lysis (Fig. 3). These insights and findings might provide the basis for the development of clinical trials and therapeutic protocols that combine standard chemo/radiotherapy with immunological approaches.
Fig. 3.
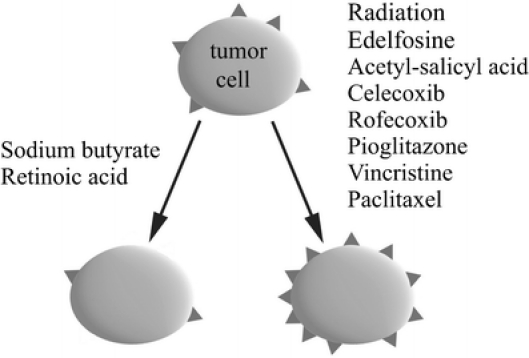
Clinically applied modifiers of the Hsp70 membrane expression on tumor cells. In vitro, the substances on the left side decrease the expression of Hsp70 on the membrane of tumor cells, symbolized as triangles, whereas those on the right side increase it. In all cases, optimization of the treatment is essential, e.g., concerning solvent, concentration, duration of treatment, recovery time. Subject to these conditions, treatment influences neither the viability nor other vital parameters of the tumor cells, including membrane markers
Acknowledgment
This work was supported in part by the EU-Transnet project MRTN-2004-512253, the DFG project MU1238 7/2, and the multimmune GmbH, Munich. We want to thank Prof. Graham Pockley (University of Sheffield, UK, presently Technical University Munich) for constructive suggestions and for proofreading of the manuscript.
Abbreviations
- 13-RA
13-cis-retinoic acid
- ALL
acute lymphoid leukemia
- AML
acute myeloid leukemia
- APC(s)
antigen presenting cell(s)
- APL
acute promyelocytic leukemia
- ARA-CTP
arabinosyl-cytosin-triphosphate
- ASA
acetyl-salicylic acid
- ATRA
all-trans-retinoic acid
- B-CLL
B cell chronic lymphoid leukemia
- CD
cluster of differentiation (nomenclature for surface proteins of human leucocytes)
- CLX
celecoxib
- COX
cyclooxygenase
- ER
endoplasmic reticulum
- Hsp
heat shock protein
- IL
interleukin
- NCR
natural cytotoxicity receptor
- NHL
non-Hodgkin lymphoma
- NK
natural killer cell
- NKG2D
C-type lectin-like immunoreceptor on NK cells
- NKT cells
natural killer-like T cells
- PI3K
phosphatidyl-inositol-3-phosphat kinase
- PIO
pioglitazone
- PIPLC
phosphatidyl-inositol-specific phospholipase C
- PKC
protein kinase C
- RFX
rofecoxib
- SBU
sodium butyrate
- TKD
14-mer Hsp70 peptide with the amino acid sequence T-K-D-N-N-L-L-G-R-F-E-L-S-G
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
Contributor Information
Jürgen Radons, Phone: +49-3834-865412, FAX: +49-3834-865402, Email: juergen.radons@uni-greifswald.de.
Gabriele Multhoff, Phone: +49-89-41404514, FAX: +49-89-41404299, Email: gabriele.multhoff@lrz.tu-muenchen.de.
References
- Abou-Issa HM, Alshafie GA, Seibert K, Koki AT, Masferrer JL, Harris RE (2001) Dose-response effects of the COX-2 inhibitor, celecoxib, on the chemoprevention of mammary carcinogenesis. Anticancer Res 21:3425–3432 [PubMed]
- Altucci L, Gronemeyer H (2001) Nuclear receptors in cell life and death. Trends Endocrinol Metab 12:460–468 [DOI] [PubMed]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442 [DOI] [PubMed]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034 [DOI] [PubMed]
- Bandyopadhyay G, Laudanski K, Li F, Lentz C, Bankey P, Miller-Graziano C (2007) Negative signalling contributes to T cell energy in trauma patients. Crit Car Med 13:794–801 [DOI] [PubMed]
- Barreto A, Gonzalez JM, Kabingu E, Asea A, Fiorentino S (2003) Stress-induced release of Hsc70 form human tumors. Cell Immunol 222:97–104 [DOI] [PubMed]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK (2001) CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14:303–313 [DOI] [PubMed]
- Becker T, Hartl FU, Wieland F (2002) CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol 158:1277–1285 [DOI] [PMC free article] [PubMed]
- Blagosklonny MV, Fojo T (1999) Molecular effects of paclitaxel: myths and reality (a critical review). Int J Cancer 83:151–156 [DOI] [PubMed]
- Botzler C, Kis K, Issels R, Multhoff G (1997) A comparison of the effects of ifosfamide vs. mafosfamide treatment on intracellular glutathione levels and immunological functions of immunocompetent lymphocyte subsets. Exp Hematol 25:338–344 [PubMed]
- Botzler C, Ellwart J, Gunther W, Eissner G, Multhoff G (1999) Synergistic effects of heat and ET-18-OCH3 on membrane expression of hsp70 and lysis of leukemic K562 cells. Exp Hematol 27:470–478 [DOI] [PubMed]
- Brune K, Hinz B (2004) Selective cyclooxygenase-2 inhibitors: similarities and differences. Scand J Rheumatol 33:1–6 [DOI] [PubMed]
- Calderwood SK, Theriault JR, Gong J (2005) Message in a bottle: role of the 70 kDa HSP in anti-tumor immunity. Eur J Cancer 35:2518–2527 [DOI] [PubMed]
- Calderwood SK, Mambula SS, Gray PJ, Theriault JR (2007) Extracellular HSP in cell signalling. FEBS Lett 581:3689–3694 [DOI] [PubMed]
- Callari D, Sinatra F, Paravizzini G et al (2003) All-trans retinoic acid sensitizes colon cancer cells to hyperthermia cytotoxic effects. Int J Oncol 23:181–188 [PubMed]
- Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM (2000) Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci U S A 97:11280–11285 [DOI] [PMC free article] [PubMed]
- Chen X, Tao Q, Yu H, Zhang L, Cao X (2002) Tumor cell membrane-bound Hsp70 elicits antitumor immunity. Immunol Lett 84:81–87 [DOI] [PubMed]
- Ciocca DR, Calderwood SK (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10:86–103 [DOI] [PMC free article] [PubMed]
- Ciocca DR, Rozados VR, Cuello Carrion FD, Gervasoni SI, Matar P, Scharovsky OG (2003) Hsp25 and Hsp70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones 8:26–36 [DOI] [PMC free article] [PubMed]
- Corley DA, Kerlikowske K, Verma R, Buffler P (2003) Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 124:47–56 [DOI] [PubMed]
- DeMaio A (2000) Heat shock proteins, oxygen radicals, and apoptosis: the conflict between protection and destruction. Crit Care Med 28:1679–1681 [DOI] [PubMed]
- Dimanche-Boitrel MT, Meurette O, Rebillard A, Lacour S (2005) Role of early plasma membrane events in chemotherapy-induced cell death. Drug Resist Updat 8:5–14 [DOI] [PubMed]
- Eissner G, Multhoff G, Gerbitz A, Kirchner S, Bauer S, Haffner S, Sondermann D, Andreesen R, Holler E (2002) Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide. Blood 100:334–340 [DOI] [PubMed]
- Farrow DC, Vaughan TL, Hansten PD et al (1998) Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarker Prev 7:97–102 [PubMed]
- Freemantle SJ, Spinella MJ, Dmitrovsky E (2003) Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene 22:7305–7315 [DOI] [PubMed]
- Gabai VL, Meriin AB, Yaglom JA, Volloch VZ, Sherman MY (1998) Role of Hsp70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett 438:1–4 [DOI] [PubMed]
- Gehrmann M, Pfister K, Hutzler P, Gastpar R, Margulis B, Multhoff G (2002) Effects of antineoplastic agents on cytoplasmic and membrane-bound heat shock protein 70 (Hsp70) levels. Biol Chem 383:1715–1725 [DOI] [PubMed]
- Gehrmann M, Schmetzer H, Eissner G, Haferlach T, Hiddemann W, Multhoff G (2003) Membrane-bound heat shock protein 70 (Hsp70) in acute myeloid leukemia: a tumor specific recognition structure for the cytolytic activity of autologous NK cells. Haematologica 88:474–476 [PubMed]
- Gehrmann M, Brunner M, Pfister K, Reichle A, Kremmer E, Multhoff G (2004) Differential up-regulation of cytosolic and membrane-bound heat shock protein 70 in tumor cells by anti-inflammatory drugs. Clin Cancer Res 10:3354–3364 [DOI] [PubMed]
- Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, Zilch T, Multhoff G (2005a) Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ 12:38–51 [DOI] [PubMed]
- Gehrmann M, Schonberger J, Zilch T, Rossbacher L, Thonigs G, Eilles C, Multhoff G (2005b) Retinoid- and sodium-butyrate-induced decrease in heat shock protein 70 membrane-positive tumor cells is associated with reduced sensitivity to natural killer cell lysis, growth delay, and altered growth morphology. Cell Stress Chaperones 10:136–146 [DOI] [PMC free article] [PubMed]
- Gendimenico GJ, Mezick JA (1993) Pharmacological effects of retinoids on skin cells. Skin Pharmacol 6(Suppl 1):24–34 [DOI] [PubMed]
- Gidding CE, Kellie SJ, Kamps WA, de Graaf SS (1999) Vincristine revisited. Crit Rev Oncol Hematol 29:267–287 [DOI] [PubMed]
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC (1994) Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med 121:241–246 [DOI] [PubMed]
- Grant S (1998) Ara-C: cellular and molecular pharmacology. Adv Cancer Res 72:197–233 [DOI] [PubMed]
- Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G (2003a) Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem 278:41173–41181 [DOI] [PubMed]
- Gross C, Schmidt-Wolf IG, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart LA, Multhoff G (2003b) Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones 8:348–360 [DOI] [PMC free article] [PubMed]
- Gross C, Hansch D, Gastpar R, Multhoff G (2003c) Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem 384:267–279 [DOI] [PubMed]
- Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858 [DOI] [PubMed]
- Heczkova B, Slotte JP (2006) Effect of anti-tumor ether lipids on ordered domains in model membranes. FEBS Lett 580:2471–2476 [DOI] [PubMed]
- Hightower L, Guidon PT (1989) Selective release from cultured mammalian cells of heat shock (stress) proteins that resemble glia-axxon transfer protein. J Cell Physiol 138:257–266 [DOI] [PubMed]
- Hirakawa T, Rokutan K, Nikawa T, Kishi K (1996) Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology 111:345–357 [DOI] [PubMed]
- Jaattela M (1999) Escaping cell death: survival proteins in cancer. Exp Cell Res 248:30–43 [DOI] [PubMed]
- Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M (1998) Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J 17:6124–6134 [DOI] [PMC free article] [PubMed]
- Johnson TW, Anderson KE, Lazovich D, Folsom AR (2002) Association of aspirin and nonsteroidal anti-inflammatory drug use with breast cancer. Cancer Epidemiol Biomark Prev 11:1586–1591 [PubMed]
- Kismet K, Akay MT, Abbasoglu O, Ercan A (2004) Celecoxib: a potent cyclooxygenase-2 inhibitor in cancer prevention. Cancer Detect Prev 28:127–142 [DOI] [PubMed]
- Korbelik M, Sun J, Cecic I (2005) Photodynamic therapy-induced cell surface expression and release of HSPs: relevance for tumor response. Cancer Res 65:1018–1026 [PubMed]
- Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, Pfister K, Multhoff G (2004) Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase I trial. Clin Cancer Res 10:3699–3707 [DOI] [PubMed]
- Lee CJ, Han JS, Seo CY, Park TH (2006) Pioglitazone, a synthetic ligand for PPARgamma, induces apoptosis in RB-deficient human colorectal cancer cells. Apoptosis 11:401–411 [DOI] [PubMed]
- Mardini IA, FitzGerald GA (2001) Selective inhibitors of cyclooxygenase-2: a growing class of anti-inflammatory drugs. Mol Interv 1:30–38 [DOI] [PubMed]
- Masferrer JL, Leahy KM, Koki AT (2000) Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 60:1306–1311 [PubMed]
- Massa C, Guiducci C, Arioli I, Parenza M, Colombo MP, Melani C (2004) Enhanced efficacy of tumro cell vaccines transfected with secretable hsp70. Cancer Res 64:15023–1508 [DOI] [PubMed]
- Massaro GD, Massaro D (2000) Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol 278:955–960 [DOI] [PubMed]
- Matsumoto H, Wang X, Ohnishi T (1995) Binding between wild-type p53 and hsp72 accumulated after UV and gamma-ray irradiation. Cancer Lett 92:127–133 [DOI] [PubMed]
- Mollinedo F, Gajate C, Martin-Santamaria S, Gago F (2004) ET-18-OCH3 (edelfosine): a selective antitumour lipid targeting apoptosis through intracellular activation of Fas/CD95 death receptor. Curr Med Chem 11:3163–3184 [DOI] [PubMed]
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD (1995) A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 61:272–279 [DOI] [PubMed]
- Multhoff G, Botzler C, Allenbacher A, Issels R (1996) Effects of ifosfamide on immunocompetent effector cells. Cancer Immunol Immunother 42:251–254 [DOI] [PMC free article] [PubMed]
- Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R (1997) Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol 158:4341–4350 [PubMed]
- Multhoff G, Pfister K, Botzler C, Jordan A, Scholz R, Schmetzer H, Burgstahler R, Hiddemann W (2000) Adoptive transfer of human natural killer cells in mice with severe combined immunodeficiency inhibits growth of Hsp70-expressing tumors. Int J Cancer 88:791–797 [DOI] [PubMed]
- Multhoff G, Pfister K, Gehrmann M, Hantsche lM, Gross C, Hafner M, Hiddemann W (2001) A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones 6:337–344 [DOI] [PMC free article] [PubMed]
- Nagpal S, Thacher SM, Patel S et al (1996) Negative regulation of two hyperproliferative keratinocyte differentiation markers by a retinoic acid receptor-specific retinoid: insight into the mechanism of retinoid action in psoriasis. Cell Growth Differ 7:1783–1791 [PubMed]
- O’Dwyer PJ, Stevenson JP, Johnson SW (2000) Clinical pharmacokinetics and administration of established platinum drugs. Drugs 59(Suppl 4):19–27 [DOI] [PubMed]
- Okami J, Yamamoto H, Fujiwara Y et al (1999) Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res 5:2018–2024 [PubMed]
- Panayi GS, Corrigal VM (2006) Bip regulates autoimmune inflammation and tissue damage. Autoimmun Rev 5:140–142 [DOI] [PubMed]
- Park DJ, Vuong PT, de Vos S, Douer D, Koeffler HP (2003) Comparative analysis of genes regulated by PML/RAR alpha and PLZF/RAR alpha in response to retinoic acid using oligonucleotide arrays. Blood 102:3727–3736 [DOI] [PubMed]
- Park JE, Facciponte J, Chen X, MacDonald I, Repasky EA, Manjili MH, Wang XY, Subjeck JR (2006) Chaperoning function of stress protein grp170, a member of the HSP70 superfamily, is responsible for its immuno-adjuvant activity. Cancer Res 66:1161–1168 [DOI] [PubMed]
- Parker WB, Secrist JA, Waud WR (2004) Purine nucleoside antimetabolites in development for the treatment of cancer. Curr Opin Investig Drugs 5:592–596 [PubMed]
- Patti R, Gumired K, Reddanna P, Sutton LN, Phillips PC, Reddy CD (2002) Overexpression of cyclooxygenase-2 (COX-2) in human primitive neuroectodermal tumors: effect of celecoxib and rofecoxib. Cancer Lett 180:13–21 [DOI] [PubMed]
- Pfister K, Radons J, Busch R, Tidball J, Pfeifer M, Freitag L, Feldman HJ, Milani V, Issels R, Multhoff G (2007) Patient survival by Hsp70 membrane-phenotype: association with different routes of metastasis. Cancer 110:926–935 [DOI] [PubMed]
- Quintana FJ, Carmi P, Mor F, Cohen IR (2004) Inhibition of adjuvant-induced arthritis by DNA vaccination with the 70 kDa or the 90 kDA HSP: immune cross-regulation with the 60 kDa HSP. Arthritis Rheum 50:3712–3720 [DOI] [PubMed]
- Richter M, Weiss M, Weinberger I, Furstenberger G, Marian B (2001) Growth inhibition and induction of apoptosis in colorectal tumor cells by cyclooxygenase inhibitors. Carcinogenesis 22:17–25 [DOI] [PubMed]
- Shamma A, Yamamoto H, Doki Y et al (2000) Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin Cancer Res 6:1229–1238 [PubMed]
- Shin BK, Wang H, Yim AM et al (2003) Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem 278:7607–7616 [DOI] [PubMed]
- Sierra-Rivera E, Voorhees GJ, Freeman ML (1993) Gamma irradiation increases hsp-70 in Chinese hamster ovary cells. Radiat Res 135:40–45 [DOI] [PubMed]
- Song AM, Bhagat L, Singh VP, Van Acker GG, Steer ML, Saluja AK (2002) Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 283:G1166–G1174 [DOI] [PubMed]
- Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL (1998) Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity 8:657–665 [DOI] [PubMed]
- Stangl S, Wortmann A, Guertler U, Multhoff G (2006) Control of metastasized pancreatic carcinomas in SCID/beige mice with human IL-2/TKD-activated NK cells. J Immunol 176:6270–6276 [DOI] [PubMed]
- Suzuki K, Watanabe M (1992) Augmented expression of HSP72 protein in normal human fibroblasts irradiated with ultraviolet light. Biochem Biophys Res Commun 186:1257–1264 [DOI] [PubMed]
- Theriaults JR, Adachi H, Calderwood SK (2006) Role of scavenger receptors in the binding and internalization of HSP70. J Immunol 177:8604–8611 [DOI] [PubMed]
- Thun MJ, Namboodiri MM, Heath CW Jr (1991) Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 325:1593–1596 [DOI] [PubMed]
- Todryk SM, Gough MJ, Pockley G (2003) Facets of Hsp70 show immunotherapeutic potentialImmunology. 110:1–9 [DOI] [PMC free article] [PubMed]
- Triantafilou M, Triantafilou K (2004) Hsp70 and Hsp90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans 32:636–639 [DOI] [PubMed]
- Ulrich CM, Bigler J, Potter JD (2006) Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 6:130–140 [DOI] [PubMed]
- Vabulas RM, Wagner H, Schild H (2002) Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol 270:169–184 [DOI] [PubMed]
- Wan YJ, Cai Y, Cowan C, Magee TR (2000) Fatty acyl-CoAs inhibit retinoic acid-induced apoptosis in Hep3B cells. Cancer Lett 154:19–27 [DOI] [PubMed]
- Wang Z, Fuentes CF, Shapshay SM (2002) Antiangiogenic and chemopreventive activities of celecoxib in oral carcinoma cell. Laryngoscope 112:839–843 [DOI] [PubMed]
- Wang M-H, Grossmann ME, Young CYF (2004) Forced expression of Hsp70increases the secretion of Hsp70 and provides protection against tumour growth. Brit J Cancer 90:926–931 [DOI] [PMC free article] [PubMed]
- Wang XY, Arnouk H, Chen X, Kazim L, Repasky EA, Subjeck JR (2006) Extracellular targeting of ER chaperone glucose-related protein 170 enhances tumor immunity to a poorly immunogenic melanoma. J Immunol 177:1543–1551 [DOI] [PubMed]
- Wei YQ, Zhao X, Kariya Y, Teshigawara K, Uchida A (1995) Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol Immunother 40:73–78 [DOI] [PMC free article] [PubMed]
- Yamazaki R, Kusunoki N, Matsuzaki T, Hashimoto S, Kawai S (2002) Selective cyclooxygenase-2 inhibitors show a differential ability to inhibit proliferation and induce apoptosis of colon adenocarcinoma cells. FEBS Lett 531:278–284 [DOI] [PubMed]
- Zhang H, Huang W (2007) A 14-mer peptide form Hsp70 protein is the critical epitope which enhances NK activity against tumor cells in vivo. Immunol Invest 36:233–246 [DOI] [PubMed]
- Zeng Y, Chen X, Larmonier N, Li G, Sepassi M, Marron M, Andraeansky S, Katsanis E (2006) NK cells play a key role in the antitumor immunity generated by chaperone-rich lysate vaccination. Int J Cancer 119:2624–2631 [DOI] [PubMed]
- Zimmermann FB, Geinitz H, Schill S, Thamm R, Nieder C, Schratzenstaller U, Molls M (2006) Stereotactic hypofractionated radiotherapy in stage I (T1–2 N0 M0) non-small-cell lung cancer (NSCLC). Acta Oncol 45:796–801 [DOI] [PubMed]


