Abstract
Larvae of the brine shrimp Artemia franciscana serve as important feed in fish and shellfish larviculture; however, they are subject to bacterial diseases that devastate entire populations and consequently hinder their use in aquaculture. Exposure to abiotic stress was shown previously to shield Artemia larvae against infection by pathogenic Vibrio, with the results suggesting a mechanistic role for heat shock protein 70. In the current report, combined hypothermic/hyperthermic shock followed by recovery at ambient temperature induced Hsp70 synthesis in Artemia larvae. Thermotolerance was also increased as was protection against infection by Vibrio campbellii, the latter indicated by reduced mortality and lower bacterial load in challenge tests. Resistance to Vibrio improved in the face of declining body mass as demonstrated by measurement of ash-free dry weight. Hypothermic stress only and acute osmotic insult did not promote Hsp70 expression and thermotolerance in Artemia larvae nor was resistance to Vibrio challenge augmented. The data support a causal link between Hsp70 accumulation induced by abiotic stress and enhanced resistance to infection by V. campbellii, perhaps via stimulation of the Artemia immune system. This possibility is now under investigation, and the work may reveal fundamental properties of crustacean immunity. Additionally, the findings are important in aquaculture where development of procedures to prevent bacterial infection of feed stock such as Artemia larvae is a priority.
Keywords: Temperature stress, Osmotic stress, Artemia franciscana, Hsp70, Cross-protection
Introduction
Artemia are predominantly found in extreme habitats where few animals exist (Van Stappen 2002), and the ability to tolerate environmental perturbations makes this aquatic crustacean an interesting model organism for stress response studies (Clegg and Trotman 2002). For example, numerous studies address the effects of temperature and salinity, important physical factors in the life of this organism, on the survival of Artemia cysts, larvae, and adults (Miller and McLennan 1988a, b; Liang and MacRae 1999; Frankenberg et al 2000; Browne and Wanigasekera 2000; Clegg et al 2000a, b). Moreover, in these studies, including the pioneering work by Miller and McLennan (1988a, b) on p68 and p89, apparent equivalents to Hsp70 and Hsp90, respectively, several Artemia Hsps were identified. This work is important fundamentally because it is related to stress physiology, and Artemia also have an essential role in aquaculture as a component of live diets, particularly in fish and shellfish larviculture (Sorgeloos et al 1986).
Stress is a state where organismal homeostasis is either threatened or interrupted by intrinsic and/or extrinsic stimuli or stressors (Chrousos and Gold 1992; Mercier et al 2006). Aquatic organisms are regularly exposed to severe environmental and pathophysiological stresses (Song et al 2006) that induce a cascade of molecular and physiological responses (Livingstone 1985). Animals survive adverse conditions in several ways, and one well-characterized mechanism is by the induction of stress proteins, also termed heat shock proteins (Hsps). These proteins are synthesized constitutively in cells, and they are induced after exposure to stress including heat, cold, oxygen deprivation, desiccation, infection, and disease (Lindquist and Craig 1988; Steinert and Pickwell 1993; Parsell and Lindquist 1994; Jolly and Morimoto 1999; Sørensen and Loeschcke 2001). Besides cell maintenance during stress, Hsps assist in proper folding of proteins, prevention of protein aggregation, and transport of proteins across membranes (Lindquist 1992). Moreover, Hsps play essential roles in immune reactions of animals against infection and disease (Robert 2003; Pockley 2003) and cross-tolerance to environmental perturbations (DuBeau et al 1998; Todgham et al 2005).
Cross-tolerance or cross-protection is a mechanism whereby a primary stress transiently increases the resistance to other insults of the same or a different nature (Volker et al 1992), thus, allowing cells or animals to survive subsequent, more severe, stress (Jean et al 2004). In line with these observations, induced Hsp synthesis confers secondary hyperthermic stress tolerance on Artemia (Clegg et al 2000a; Frankenberg et al 2000). In other work, a non-lethal heat shock promoted Hsp70 expression and cross-protected Artemia larvae against Vibrio challenge (Sung et al 2007). The present study extends this focus, featuring the differential expression of Hsp70 in Artemia larvae upon exposure to abiotic stressors. Additionally, the effects of these stresses on weight loss, induced thermotolerance, and immune response as revealed by survival after Vibrio challenge were determined, revealing a potential relationship between Hsp70 and enhanced immunity in Artemia larvae.
Materials and methods
Maintenance of bacteria
Vibrio campbellii (LMG 21363) isolates stored in 40% glycerol at −80°C were grown at 28°C on marine agar. Marine broth 2216 (Difco Laboratories, Detroit, MI) was then inoculated with single colonies and incubated overnight with constant shaking at 28°C to stationary phase. Bacterial cells were harvested under aseptic conditions by centrifugation at 2200×g for 15min before suspension in seawater which had been filtered and autoclaved as used throughout the study. Culture densities were determined spectrophotometrically at 550nm, and bacterial numbers were calculated according to the McFarland standard (BioMerieux, Marcy L’Etoile, France) where an optical density of 1.0 corresponds to 1.2 × 109 cells/ml.
Preparation of axenic Artemia larvae
Axenic Artemia larvae were obtained essentially as described (Marques et al 2004a). One gram of high-hatching A. franciscana cysts from the Great Salt Lake, Utah, USA (EG® Type, INVE Aquaculture, Belgium) was hydrated in 90ml of tap water for 1h with vigorous aeration and then transferred to a laminar flow hood for decapsulation. Fifty milliliters of cold sodium hypochlorite containing 15% (w/v) active chlorine and 3.3ml of 32% (w/v) sodium hydroxide were added to the hydrated cysts, followed after 150s by 70ml of autoclaved sodium thiosulfate pentahydrate at 10mg/l. Decapsulated cysts were washed several times with seawater and collected over 50-μm sterile sieves. A few milligrams of cysts were transferred to separate sterile 50-ml Falcon tubes containing 30ml of seawater and capped. The tubes were incubated at 28°C for 18–24h with rotation at four cycles per min and constantly exposed to incandescent light at ±41μEm−2. Subsequently, hatched larvae at stage 2 of development, when the mouth has opened and bacterial ingestion can occur, were harvested within the next 4–6h.
Thermal and osmotic stress of Artemia larvae
Axenically hatched larvae acclimated at 28°C were transferred to seawater at 4°C in sterile 500-ml bottles and held for 1h at 4°C with aeration before incubation at 28°C for 6h, a treatment termed CS1. Larvae incubated at 4°C were also exposed to a non-lethal heat shock of 37°C (Δt = 5°C min−1) for 30min, followed by recovery for 6h at 28°C, a procedure termed CS2. Air used during culturing was passed through a 0.22-μm filter.
Axenically hatched Artemia larvae, held normally at a salinity of 30g/l, were exposed for 30min to osmotic stress including a hypotonic shock at 4g/l and hypertonic shocks at 50, 100, and 150g/l. For osmotic stress under axenic conditions, larvae acclimated at 30g/l were collected on cloth filters and transferred to 500-ml aerated bottles containing 50ml of salt solutions at 4, 50, 100, and 150g/l. Salt solutions consisted of Instant Ocean® synthetic salt, Aquarium Systems Inc., France sterilized by autoclaving and diluted with autoclaved distilled water. After stress, larvae were acclimated to normal salinity for 6h either by adding salt to the hypotonic solution or by diluting the hypertonic solutions with distilled water. Larvae were then harvested and used for protein characterization, ash-free dry weight measurements, induced thermotolerance experiments, and V. campbellii challenge tests.
Protein extraction, SDS polyacrylamide gel electrophoresis and immunoprobing of Western blots
Protein extraction was performed essentially as described previously (Clegg et al 2000a; Sung et al 2007). Artemia larvae were collected on 50-μm sieves and rinsed with ice-cold distilled water. Two hundred milligrams per milliliter (wet weight) of tissue was homogenized in cold buffer K (150mM sorbitol, 70mM potassium gluconate, 5mM MgCl2, 5mM NaH2PO4, 40mM HEPES, pH7.4) and supplemented with protease inhibitor cocktail (Catalogue # P8340, Sigma-Aldrich, Inc. USA) as recommended by the manufacturer. Aliquots of homogenate were combined with equal volumes of sodium dodecyl sulfate (SDS) sample buffer, vortexed, heated at 95°C for 5min (Laemmli 1970), cooled, and centrifuged at 2,200×g for 1min.
Ten microliters samples representing 1.0mg (wet weight) of animals were applied to each lane of a 10% SDS polyacrylamide gel (Frankenberg et al 2000; Clegg et al 2000a) before electrophoresis. Two gels were run simultaneously, and one was stained with Coomassie Biosafe (BioRad Laboratories, USA). Proteins in the second gel were transferred to a polyvinylidene fluoride transfer membrane (BioRad Immun-Blot™ PVDF, USA) for antibody probing. Membranes were incubated in blocking buffer [50ml of phosphate-buffered saline containing 0.2% (v/v) Tween-20 and 5% (w/v) bovine serum albumin] for 60min at 25°C. Mouse monoclonal anti-Hsp70 antibody, clone 3A3 (Affinity BioReagents Inc., Golden, CO), which recognizes both constitutive and inducible Hsp70 (Sung et al. 2007), was used as primary antibody at the recommended dilution of 1:5,000. Donkey anti-mouse IgG coupled with horseradish peroxidase conjugate (Affinity BioReagents Inc.) was employed as secondary antibody at the recommended dilution of 1:2,500. Detection was performed with 0.7mM diaminobenzidine tetrahydrochloride dihydrate as substrate in association with 0.01% (v/v) H2O2 in 0.1M Tris–HCl (pH7.6).
Determination of larval ash-free dry weight
To determine ash-free dry weight, Artemia larvae were collected over a 50-μm cloth filter and washed several times with autoclaved distilled water. One hundred larvae from each treatment were placed in triplicate in separate porcelain cups cleaned previously with formic acids, dried, and weighed. The samples were heated 4h at 103°C for dry weight measurement, then combusted at 600°C for 6h to determine ash content. The ash-free dry weight was calculated as the difference between the total dry weight and the ash weight.
Determination of induced thermotolerance in Artemia larvae
After recovery from an initial stress, 50 larvae were transferred in triplicate into separate Falcon tubes containing 30ml of seawater, exposed to heat shock at 42°C for 30min (Clegg et al 2000a), and then transferred to 28°C. Induced thermotolerance was determined 12h after heating by counting actively swimming larvae. The experiments were repeated once.
V. campbellii challenge tests with Artemia larvae
For bacterial challenge, 50 larvae were incubated at 28°C in each of six sterile Falcon tubes containing 30ml of seawater. Three tubes received bacteria and three did not, the latter used for assessing bacterial contamination by incubating 100μl of culture medium on marine agar 2216 (Marques et al 2004a) for 5days at 28°C. If contamination was detected, the experimental results were discarded. Challenge tests were performed as described (Marques et al 2006a), except V. campbellii (LMG 21363), a relatively virulent pathogen for gnotobiotically grown Artemia larvae (Marques et al 2006a, b, c; Defoirdt et al 2006) was added to 1 × 107 cells/ml. Survival was determined 24 and 36h after challenge by collecting actively swimming larvae and fixing in Lugol’s solution before counting. Survival percentage was calculated as Nt × 100/No where Nt and No are final and initial numbers of larvae, respectively.
Colonization of Artemia larvae by V. campbellii
Artemia larvae subjected to treatments CS1 and CS2 were harvested 8h after Vibrio challenge by sieving on sterile 150-μm pore size nylon filters and then rinsed twice with 10ml of autoclaved nine-salts solution (NSS; 17.6g/l NaCl, 1.47g/l Na2SO4, 0.08g/l NaHCO3, 0.25g/l KCl, 0.04g/l KBr, 1.87g/l MgCl2, 0.41g/l CaCl2, 0.008g/l SrCl2, and 0.008g/l H3BO3). Ten larvae, transferred to sterile plastic bags containing 10ml of NSS, were homogenized with a stomacher blender (400SN, Seward Medical, UK) for 10min. The homogenates were transferred to Falcon tubes, tenfold serial dilutions were prepared, and samples were plated on marine agar with a Spiral-plater (Spiral Systems Inc., USA) before incubation at 28°C for 24h and colony counting. The experiments were conducted in duplicate with each test performed in triplicate.
Statistical analyses
Values for larval survival (%) were ArcSin to satisfy normality and homocedasticity requirements whenever necessary. For V. campbellii load, the CFU values were log-transformed. Significant differences in larval survival and V. campbellii colonization were determined by performing one-way analysis of variance followed by Tukey test at a significance level of 0.05. All statistical analyses were performed with software SPSS® version 11.5 for Windows®.
Results
Synthesis of Hsp70 in stressed Artemia larvae
Coomassie staining of SDS polyacrylamide gels clearly demonstrated increased amounts of a 70-kDa polypeptide in CS2 samples (Fig. 1a). Immunoprobing of Western blots with a monoclonal antibody to Hsp70 revealed a single co-migrating polypeptide of approximately 70kDa, which, as shown in stained gels, increased only in CS2 samples (Fig. 1b). In contrast, CS1 treatment yielded a minor reduction in the antibody-reactive 70-kDa protein, and the protein was indifferent to osmotic stress. The same results were obtained in two independent experiments for which Fig. 1 is a representative example.
Fig. 1.

a SDS polyacrylamide gel electrophoresis of protein samples from stressed Artemia larvae. Ten microliters samples containing equivalent amounts of protein were loaded in each lane. b Immunodetection of Hsp70 on western blots; CTR, non-stressed larvae; CS1, cold shock at 4°C for 1 h followed by recovery at 28°C for 6 h; CS2, cold shock at 4°C followed by heat shock at 37°C for 30 min and recovery at 28°C for 6 h; 4 g/l, hypotonically stressed larvae exposed to a 4 g/l salt solution for 30 min followed by recovery for 6 h; 50 g/l, 100 g/l and 150 g/l, hypertonically stressed larvae exposed to 50, 100 and 150 g/l salt solution for 30 min followed by recovery for 6 h; M, protein standards in kDa. Box, 70-kDa protein
Larval ash-free dry weight is stress-dependent
Animals osmotically stressed at 100 and 150g/l and those from the CS2 treatment had lower ash-free dry weights than non-stressed larvae (Fig. 2). In contrast, no significant differences were detected in ash-free dry weight of larvae subjected to CS1 treatments and osmotic stresses at 4 and 50g/l (P > 0.05).
Fig. 2.

Ash-free dry weight of stressed Artemia larvae. One hundred larvae were heated 4 h at 103°C for dry weight measurement, then combusted at 600°C for 6 h to determine ash content. The ash-free dry weight was calculated as the difference between the total dry weight and the ash weight. Refer to Fig. 1 for explanation of sample designations. Asterisk indicates a significant difference (p < 0.05) between the stress treatment and control
Induced thermotolerance in larvae occurred only in response to CS2 treatment
Thermotolerance in Artemia larvae exposed to the CS2 treatment was enhanced, whereas larvae subjected to CS1 treatment and osmotic stress were less resistant to temperature increase (Fig. 3).
Fig. 3.
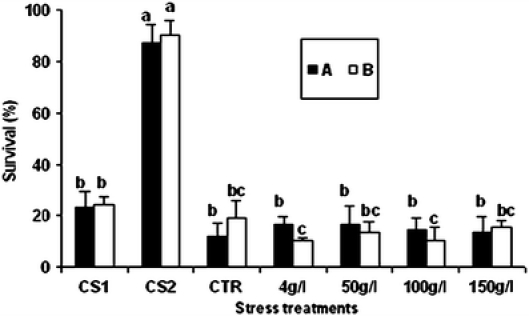
Thermotolerance induction in Artemia larvae. Non-stressed (CTR) and stressed Artemia larvae were exposed to heat shock at 42°C for 30 min. Induced thermal tolerance was based on larval survival after 12 h of heat shock. Experiments were repeated once with replicates indicated by a and b. Refer to Fig. 1 for explanation of sample designations. Values showing the same superscript letter for each experiment are not significantly different (p > 0.05)
Enhanced survival of CS2 stressed larvae in V. campbellii challenge tests
In replicate experiments, approximately 80% of non-stressed and unchallenged larvae survived incubation at 28°C (Fig. 4), whereas about 40% of non-stressed larvae challenged with V. campbellii were viable. CS2-treated larvae exhibited substantially higher survival as compared to non-stressed challenged controls, while a small but significantly reduced survival (p < 0.05) occurred for larvae experiencing CS1 treatment. Larvae osmotically stressed at 4, 100, and 150g/l salinity had significantly lower survival (p < 0.05) than non-stressed animals upon exposure to V. campbellii, and for the latter, viability was very low (Fig. 5). Approximately 90% and 72% of the unchallenged larvae were alive at 24 and 36h, respectively, in these experiments (not shown).
Fig. 4.

Survival of Artemia larvae in V. campbellii challenge tests. After CS1 and CS2 treatments, larvae were exposed to a V. campbellii challenge of 1 × 107 cells/ml. Live larvae were counted 24 h and 36 h after challenge, and each experiment was repeated once, with replicates labeled a and b. NC Non-stressed and unchallenged larvae, CTR non-stressed larvae with V. campbellii challenge, CS1 and CS2, refer to Fig. 1 for explanation. Values in matching time columns with the same superscript letter are not significantly different (p > 0.05)
Fig. 5.

Survival of osmotically stressed Artemia larvae after challenge tests with V. campbellii. Live larvae were counted 24 h and 36 h after challenge with 1 × 107V. campbellii per milliliter. Each experiment was repeated once and replicates are labeled a and b. Refer to Fig. 1 for explanation of sample designations. Values in the same column for each experiment with matching superscript letters are not significantly different (p > 0.05)
Reduced V. campbellii load in CS2-stressed larvae
Approximately 8.7 × 103V. campbellii accumulated per non-stressed larva (Fig. 6), with higher numbers generally present after CS1 treatment, although differences were not significant. In contrast, reductions of approximately 49% and 61% occurred in the number of V. campbellii per larva after CS2 treatments (p < 0.05).
Fig. 6.

Vibrio colonization of Artemia larvae. Larvae from CS1 and CS2 treatments were exposed to 1 × 107V. campbellii per milliliter for 8 h before collection of 10 animals and counting of bacteria. The experiment was repeated once with replicates labeled Exp 1 and Exp 2. CTR, non-stressed larvae with V. campbellii challenge; CS1 and CS2; refer to Fig. 1 for explanation of sample designations. Asterisk indicates significant difference (p < 0.05) between stress treatment and control
Discussion
Exposing gnotobiotic Artemia larvae to 4°C for 1h followed by an abrupt non-lethal heat shock at 37°C for 30min and a 6h recovery is shown in this report to induce Hsp70 production. Conversely, a small reduction in the amount of Hsp70 was observed in larvae cooled from 28 to 4°C and then warmed to 28°C. Stress often stimulates Hsp expression in aquatic organisms with Hsp70 induced in hypoxic Nile tilapia Oreochromis niloticus (L.) juveniles (Delaney and Klesius 2004), Indian major carp, Cirrhinus mrigala (Ham.; Das et al 2005), and the tiger prawn, P. monodon (de la Vega et al 2006). A temperature increase from 21 to 37°C for 30min followed by a 24-h recovery strongly induced Hsp70 in Artemia adults (Clegg et al 2000a), and a non-lethal heat shock from 28 to 37°C for 30min with a 6-h recovery triggered Hsp70 production in gnotobiotic-grown larvae (Sung et al 2007). In contrast to the situation with thermal perturbation, neither hypotonic nor hypertonic osmotic stress for 30min induced Hsp70 production in Artemia larvae. Likewise, the absence of Hsp70 induction was observed in hypo-osmotic stressed P. monodon obtained by switching from 35 to 10g/l for 8h (de la Vega et al 2006). Although Artemia normally adapt readily to changing salt concentrations (Browne and Wanigasekera 2000), larvae exposed to salinities of 100 and 150g/l for 30min experienced significant weight loss. This may have resulted from reduced energy reserves as a consequence of increased metabolic activity required to cope with the imposed stress, a result seen in rats (Harris et al 1998).
Stress may compromise organismal immune response and increase vulnerability to infection. As an example, temperature stress increases the susceptibility of sea bass, Dicentrarchus labrax, to nodavirus (Varsamos et al 2006). Additionally, thermal stress reduces resistance of the tiger shrimp P. monodon to P. damselae subsp. damselae (Wang and Chen 2006b), and the White shrimp L. vannamei is more prone to V. alginolyticus infection after heating (Cheng et al 2005). Transfer of P. monodon from 25 to 5ppt and from 15 to 35ppt salinity reduced immune capability and decreased resistance against P. damselae subsp. damselae infection (Wang and Chen 2006a). H2O2 accelerates the mortality of Tenacibaculum maritimum-infected turbot (Avendaño-Herrera et al 2006), while metal stress influences disease transmission and susceptibility of aquaculture species (Liao et al 2006). Furthermore, hypoxic-stressed Penaeus styliriostris are more sensitive to infection by V. alginolyticus (Le Moullac et al 1998). Collectively, these studies indicate that stress suppresses immunity, leading to increased vulnerability to infections and greater mortalities. In agreement with these data, gnotobiotic Artemia larvae experiencing CS1 treatment and exposure to high salt were less resistant to V. campbellii than were non-stressed animals.
Although many stresses reduce immunity, a non-lethal heat shock may cross-protect against further insult, a phenomenon usually correlated with Hsp production and defense against subsequent environmental disturbance. Thermal shock of salmon guards against a subsequent severe osmotic challenge, perhaps due to Hsp70 induction (DuBeau et al 1998), and the induced expression of Hsps in most fishes by high temperature is correlated with increased resistance to a second heat stress (Basu et al 2002). A sub-lethal heat shock promotes Hsp70 accumulation in adult Artemia and shields against exposure to lethal heat shock (Frankenberg et al 2000). In this study, stressed gnotobiotic Artemia larvae undergoing CS2 treatment produced Hsp70, indicating that this protein is directly involved in conferring enhanced heat tolerance. Furthermore, combined hypothermic and hyperthermic stress up-regulates Hsp70, and this is associated with cross-protection against V. campbellii. Unexpectedly, these animals exhibited a significantly lower ash-free dry weight, although a twofold increase in larval survival was recorded after V. campbellii challenge. Additionally, stressed larvae failing to accumulate Hsp70 lacked protection against V. campbellii, agreeing with earlier results indicating a role for Hsp70 in cross-protection of Artemia larvae (Sung et al 2007).
On an applied note, it is common practice in commercial aquaculture to store Artemia larvae in the cold, thus, reducing the number of daily harvests, preventing larvae from molting, prolonging storage, and preserving biomass, all with economic advantage (Merchie 1996). The transfer of cold-stored larvae to fish tanks often involves sudden temperature increases because 28°C is an optimal rearing condition for most warm water aquaculture species. Our results suggest that such larvae have increased sensitivity to opportunistic pathogens like Vibrio species.
How Hsp70 and other Hsps protect against pathogenic V. campbellii is unclear, but extracellular Hsps are known to regulate the innate immune response (Pockley 2003; Chen et al 1999). For instance, the heat-induced synthesis of small heat shock proteins and Hsp90 triggers C. elegans immunity to pathogenic Pseudomonas aeruginosa (Singh and Aballay 2006a). The mechanism may involve heat shock transcription factor-1 and the associated DAF2/DAF-16 pathway which regulates aging and immunity in nematodes (Singh and Aballay 2006b). Furthermore, extracellular Hsp72 robustly promotes inflammatory cytokine production (Johnson and Fleshner 2006) and may stimulate production of inducible nitric oxide synthase (Panjwani et al 2002; Campisi and Fleshner 2003), tumor necrosis factor α , interleukin-1β and IL-6 (Asea et al 2000; Campisi and Fleshner 2003), all known to modulate infection. Substantial evidence indicates that Toll-like receptors 2 and/or 4, which act as cell surface receptors for extracellular Hsp72, transduce inflammatory signals to innate immune cells such as macrophages, dendritic cells, and neutrophils (Visintin et al 2001; Vabulas et al 2002; Asea et al 2002; Ménoret 2004). Findings presented in this report support the emerging idea that Hsps activate innate immune responses in Artemia and other invertebrates, thereby protecting against pathogens such as V. campbellii. These observations are of fundamental importance in understanding invertebrate immune function, and they have significant potential for application in aquaculture.
Acknowledgments
This work was supported by Universiti Malaysia Terengganu (UMT; formerly known as University College of Science and Technology Malaysia, KUSTEM) through a doctoral grant to YYS and research funding supported by the Belgian Foundation for Scientific Research (FWO) through the project “Nutritional and immunostimulatory characteristics of isogenic yeast mutants in Artemia” (1.5.125.04).
References
- Avendaño-Herrera R, Magariños B, Irgang R, Toranzo AE (2006) Use of hydrogen peroxide against the fish pathogen Tenacibaculum maritimum and its effect on infected turbot (Scophthalmus maximus). Aquaculture 257:104–110 [DOI]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442 [DOI] [PubMed]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034 [DOI] [PubMed]
- Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, Schulte PM, Iwama GK (2002) Heat shock protein genes and their functional significance in fish. Gene 295:173–183 [DOI] [PubMed]
- Browne RA, Wanigasekera G (2000) Combined effects of salinity and temperature on survival and reproduction of five species of Artemia. J Exp Mar Biol Ecol 244:29–44 [DOI]
- Campisi J, Fleshner M (2003) The role of extracellular Hsp72 in acute stress-induced potentiation of innate immunity in physically active rats. J Appl Physiol 94:43–52 [DOI] [PubMed]
- Clegg JS, Jackson SA, Hoa NV, Sorgeloos P (2000a) Thermal resistance, developmental rate and heat shock proteins in Artemia franciscana, from San Francisco Bay and Southern Vietnam. J Exp Mar Biol Ecol 252:85–96 [DOI] [PubMed]
- Clegg JS, Jackson SA, Popov VI (2000b) Long-term anoxia in encysted embryos of the crustacean, Artemia franciscana: viability, ultrastructure and stress proteins. Cell Tissue Res 301:433–446 [DOI] [PubMed]
- Clegg JS, Trotman CNA (2002) Physiological and biochemical aspects of Artemia ecology. In: Abatzopoulos ThJ, Beardmore JA, Clegg JS, Sorgeloos P (eds) Artemia: basic and applied biology. Kluwer, Dordrecht, pp 129–170
- Chen W, Syldath U, Bellmann K, Burkart V, Kolb W (1999) Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol 162:3212–3219 [PubMed]
- Cheng W, Wang LU, Chen JC (2005) Effect of water temperature on the immune response of white shrimp Litopenaeus vamnnamei to Vibrio alginolyticus. Aquaculture 250:592–601 [DOI]
- Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioural homeostasis. J Am Med Assoc 267:1244–1252 [DOI] [PubMed]
- Das P, Gupta A, Manna SK (2005) Heat shock protein 70 expression in different tissues of Cirrhinus mrigala (Ham.) following heat stress. Aquaculture Res 36:525–529 [DOI]
- Defoirdt T, Halet D, Sorgeloos P, Bossier P, Verstraete W (2006) Short-chain fatty acids protect gnotobiotic Artemia franciscana from pathogenic Vibrio campbellii. Aquaculture 261:804–808 [DOI]
- Delaney MA, Klesius PH (2004) Hypoxic conditions induce Hsp70 production in blood, brain and head kidney of juvenile Nile tilapia Oreochromis niloticus (L.). Aquaculture 236:633–644 [DOI]
- de la Vega E, Hall MR, Degnan BM, Wilson KJ (2006) Short-term hyperthermic treatment of Penaeus monodon increases expression of heat shock protein 70 (HSP70) and reduces replication of gill associated virus (GAV). Aquaculture 253:82–90 [DOI]
- DuBeau SF, Pan F, Tremblay GC, Bradley TM (1998) Thermal shock of salmon in vivo induces the heat shock protein hsp 70 and confers protection against osmotic shock. Aquaculture 168:311–323 [DOI]
- Frankenberg MM, Jackson SA, Clegg JS (2000) The heat shock response of adult Artemia franciscana. J Thermal Biol 25:481–490 [DOI] [PubMed]
- Harris RBS, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH (1998) Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol Regul Integr Comp Physiol 275:1928–1938 [DOI] [PubMed]
- Jean S, De Jong L, Moreau X (2004) Chaetognaths: a useful model for studying heat shock proteins. Effect of wound healing. J Exp Mar Biol Ecol 312:319–332 [DOI]
- Johnson JD, Fleshner M (2006) Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 79:425–434 [DOI] [PubMed]
- Jolly C, Morimoto RI (1999) Stress and the cell nucleus: dynamics of gene expression and structural reorganization. Gene 7:261–270 [PMC free article] [PubMed]
- Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed]
- Le Moullac G, Soyez C, Saulnier D, Ansquer D, Avarre JC, Levy P (1998) Effect of hypoxic stress on the immune response and the resistance to vibriosis of the shrimp Penaeus stylirostris. Fish Shellfish Immunol 8:621–629 [DOI]
- Liang P, MacRae TH (1999) The synthesis of a small heat shock/a-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol 207:445–456 [DOI] [PubMed]
- Liao CM, Chang CF, Yeh CH, Chen SC, Chiang KC, Chio CP, Chou BYH, Jou LJ, Lien GW, Lin CM, Shen HH, Wu GD (2006) Metal stresses affect the population dynamics of disease transmission in aquaculture species. Aquaculture 257:321–332 [DOI]
- Lindquist S (1992) Heat-shock proteins and stress tolerance in microorganisms. Curr Opin Genet Dev 2:748–755 [DOI] [PubMed]
- Lindquist S, Craig EA (1988) The heat-shock proteins. Ann Rev Gen 22:631–677 [DOI] [PubMed]
- Livingstone DR (1985) Biochemical measurements. In: Bayne BL (ed) The effects of stress and pollution on marine animals. Praeger, NY, pp 81–132
- Marques A, Dhont J, Sorgeloos P, Bossier P (2004a) Evaluation of different yeast cell wall mutants and microalgae strains as feed for gnotobiotically grown brine shrimp Artemia franciscana. J Exp Mar Biol Ecol 321:115–136 [DOI]
- Marques A, Dhont J, Sorgeloos P, Bossier P (2006a) Immunostimulatory nature of β-glucans and baker’s yeast in the challenge test of Artemia. Fish Shellfish Immunol 20:682–692 [DOI] [PubMed]
- Marques A, Thanh TH, Sorgeloos P, Bossier P (2006b) Use of microalgae and bacteria to enhance protection of gnotobiotic Artemia against different pathogens. Aquaculture 258:116–126 [DOI]
- Marques A, Thanh TH, Verstraete W, Dhont J, Sorgeloos P, Bossier P (2006c) Use of selected bacteria and yeast to protect gnotobiotic Artemia against different pathogens. J Exp Mar Biol Ecol 334:20–30 [DOI]
- Ménoret A (2004) Purification of recombinant and endogenous HSP70s. Methods 32:7–12 [DOI] [PubMed]
- Merchie G (1996) Use of nauplii and meta-nauplii. In: Lavens P, Sorgeloos P (eds) Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper. No. 361, Rome, pp 137–163
- Mercier L, Palacios E, Campa-Córdova AI, Tovar-Ramírez D, Hernández-Herrera R, Racotta IS (2006) Metabolic and immune responses in Pacific whiteleg shrimp Litopenaeus vannamei exposed to a repeated handling stress. Aquaculture 258:633–640 [DOI]
- Miller D, McLennan AG (1988a) The heat shock response of the cryptobiotic brine shrimp Artemia—I. Thermotolerance. J Thermal Biol 13:119–123 [DOI]
- Miller D, McLennan AG (1988b) The heat shock response of the cryptobiotic brine shrimp Artemia—II. Heat shock proteins. J Thermal Biol 13:125–134 [DOI]
- Panjwani NN, Popova L, Srivastava PK (2002) Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol 168:2997–3003 [DOI] [PubMed]
- Parsell DA, Lindquist S (1994) Heat shock proteins and stress tolerance. In: Morimoto RI, Tissieres A, Georgopoulos C (eds.) The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, NY, pp 457–494
- Pockley AG (2003) Heat shock proteins as regulators of the immune response. Lancet 362:469–476 [DOI] [PubMed]
- Robert J (2003) Evolution of heat shock protein and immunity. Dev Comp Immunol 27:449–464 [DOI] [PubMed]
- Singh V, Aballay A (2006a) Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc Natl Acad Sci USA 103:13092–13097 [DOI] [PMC free article] [PubMed]
- Singh V, Aballay A (2006b) Heat-shock and gene activation of HSF-1 enhance immunity to bacteria. Cell Cycle 21:2443–2446 [DOI] [PubMed]
- Song LS, Wu LT, Ni DJ, Chang YQ, Xu W, Xing KZ (2006) The cDNA cloning and mRNA expression of heat shock protein 70 gene in the haemocytes of bay scallop (Argopecten irradians, Lamarck 1819) responding to bacteria challenge and naphthalin stress. Fish Shellfish Immunol 21:335–345 [DOI] [PubMed]
- Sorgeloos P, Lavens P, Léger P, Tackaert W, Versichele D (1986) Manual for the culture and use of brine shrimp Artemia in aquaculture. Artemia Reference Center Faculty of Agriculture State University of Ghent, Ghent, Belgium
- Sørensen JG, Loeschcke V (2001) Larval crowding in Drosophila melanogaster induces hsp70 expression, and leads to increased adult longevity and adult thermal stress resistance. J Insect Physiol 47:1301–1307 [DOI] [PubMed]
- Steinert SA, Pickwell GV (1993) Induction of hsp70 proteins in mussels by ingestion of tributyltin. Responses of marine organisms to pollutants. Part 2. Environ Res 35:89–93 [DOI]
- Sung YY, Van Damme EJM, Sorgeloos P, Bossier P (2007) Non-lethal heat shock protects gnotobiotic Artemia franciscana larvae against virulent Vibrios. Fish Shellfish Immunol 22:318–326 [DOI] [PubMed]
- Todgham AE, Schulte PM, Iwama GK (2005) Cross-tolerance in the tidepool sculpin: the role of heat shock proteins. Physiol Biochem Zool 78:133–144 [DOI] [PubMed]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H (2002) HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277:15107–15112 [DOI] [PubMed]
- Van Stappen G (2002) Zoogeography. In: Abatzopoulos ThJ, Beardmore JA, Clegg JS, Sorgeloos P (eds) Artemia: basic and applied biology. Kluwer, Dordrecht, pp 171–224
- Varsamos S, Flik G, Pepin JF, Wendelaar BSE, Breuil G (2006) Husbandry stress during early life stages affects the stress response and health status of juvenile sea bass, Dicentrarchus labrax. Fish Shellfish Immunol 20:83–96 [DOI] [PubMed]
- Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM (2001) Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol 166:249–255 [DOI] [PubMed]
- Volker U, Mach R, Schmid R, Hecker M (1992) Stress proteins and cross-protection by heat shock and salt stress in Bacillus subtilis. J Gen Microbiol 138:2125–2135 [DOI] [PubMed]
- Wang FI, Chen JC (2006a) Effect of salinity on the immune response of tiger shrimp Penaeus monodon and its susceptibility to Photobacterium damselae subsp. damselae. Fish Shellfish Immunol 20:671–681 [DOI] [PubMed]
- Wang FI, Chen JC (2006b) The immune response of tiger shrimp Penaeus monodon and its susceptibility to Photobacterium damselae subsp. damselae under temperature stress. Aquaculture 258:34–41 [DOI] [PubMed]


