Abstract
The success of any organism depends not only on niche adaptation but also the ability to survive environmental perturbation from homeostasis, a situation generically described as stress. Although species-specific mechanisms to combat “stress” have been described, the production of heat shock proteins (HSPs), such as HSP70, is universally described across all taxa. Members of the HSP70 gene family comprising the constitutive (HSC70) and inducible (HSP70) members, plus GRP78 (glucose-regulated protein, 78 kDa), a related HSP70 family member, were cloned using degenerate polymerase chain reaction (PCR) from two evolutionary divergent Antarctic marine molluscs (Laternula elliptica and Nacella concinna), a bivalve and a gastropod, respectively. The expression of the HSP70 family members was surveyed via quantitative PCR after an acute 2-h heat shock experiment. Both species demonstrated significant up-regulation of HSP70 gene expression in response to increased temperatures. However, the temperature level at which these responses were induced varied with the species (+6–8°C for L. elliptica and +8–10°C for N. concinna) compared to their natural environmental temperature). L. elliptica also showed tissue-specific expression of the genes under study. Previous work on Antarctic fish has shown that they lack the classical heat shock response, with the inducible form of HSP70 being permanently expressed with an expression not further induced under higher temperature regimes. This study shows that this is not the case for other Antarctic animals, with the two molluscs showing an inducible heat shock response, at a level probably set during their temperate evolutionary past.
Keywords: Antarctic, Heat shock protein, Stress, Temperature, Climate change
Introduction
All organisms are adapted to life within a constrained environmental envelope, with consequential specialisations in ecology, physiology and biochemistry. Associated with these adaptations is a species-specific capacity to cope with environmental change. If an organism is taken outside of its “normal” environmental envelope by, for example, changing temperature, salinity or oxygen availability, the organism becomes vulnerable, a situation generically described as stress. In turn, this environmental challenge triggers a biochemical response, the aim of which is to counteract or mitigate any potential cell damage caused by the environmental insult and to enhance survival.
At the molecular level, the production of stress proteins (reviewed in Gross 2004), such as the heat shock proteins (HSPs), is regarded as a classical response. These are a family of highly conserved proteins that act as chaperones to stabilise and refold denatured proteins, preventing the formation of cytotoxic aggregates (Parsell and Lindquist 1993; Hartl 1996; Fink 1999). Numerous families of heat shock proteins have been identified, the naming of which is related to their weight in kilodaltons. The most studied of these family members are the 70-kD heat shock proteins (HSP70s), comprising constitutive (HSC70, heat shock cognate 70), stress-inducible (HSP70s, heat shock protein 70) and glucose-regulated forms (GRP78, glucose-regulated protein, 78 kD). Whilst their action has been described in response to a wide variety of stresses, the classical activation of the inducible HSP70 genes is in response to elevated environmental temperatures and is tightly controlled by the heat shock transcription factor (HSF1; reviewed in Morimoto 1998).
The classic heat shock response involving a strong up-regulation of HSP70 production has been demonstrated in all organisms examined to date with the exception of Hydra oligactis (Bosch et al. 1988), an Antarctic ciliate Euplotes focardii (La Terza et al. 2001, 2004) and several species of Antarctic notothenioid fish (Place and Hofmann 2005). However, the fish case is complex. Three distantly related Antarctic species, Trematomus bernacchii, Pagothenia borchgrevinki and Lycodichthys dearborni permanently express the inducible form of HSP70 (Place and Hofmann 2005), but the Nototheniidae lack the ability to further up regulate this gene in response to elevated environmental temperatures (Place et al. 2004; Buckley et al. 2004). Given these data, the question arises as to whether the permanent expression of HSP70 and lack of a heat shock response in Antarctic Notothenioids is family specific and/or a consequence of adaptation to highly stable, cold Antarctic seawater temperatures and could therefore be a general phenomenon extending to other non-piscine Antarctic marine organisms.
Antarctic invertebrates are, in general, as stenothermal and, in some cases, more stenothermal than the endemic fish species (Somero and DeVries 1967; Peck and Conway 2000) with many species having survivable temperature envelopes between 5°C and 12°C above the minimum sea temperature of −1.86°C (Peck 2002). Even within this temperature range, animals start to lose critical biological functions such as swimming (scallops), righting responses (limpets) and reburying (clams; Peck et al. 2004), all of which are lost with temperature elevations of only 1–2°C above current summer maximum seawater temperatures (0–1.8°C). Many Antarctic invertebrates, including the groups mentioned above, die at temperatures below +10°C (Peck 1989; Peck et al. 2002; Pörtner et al. 2006). Shallow seawater temperatures along the west Antarctic Peninsula have risen in excess of 1°C over the last 50 years (Meredith and King 2005), whilst the IPCC (2001) Third Assessment Climate Model predicts a further 2°C increase in global seawater temperatures over the next 100 years, albeit with large regional variations and confidence intervals. In light of current and predicted seawater temperature increases, Antarctic stenotherms are, therefore, at considerable future risk of seasonal exposure to ambient water temperatures that exceed those known to result in the loss of critical biological functions (Peck et al. 2004).
In this study, members of the HSP70 gene family comprising the constitutive (HSC70) and inducible (HSP70) members, plus GRP78 (glucose-regulated protein, 78 kDa), a related HSP70 family member, were cloned using degenerate polymerase chain reaction (PCR) from two evolutionary divergent Antarctic marine molluscs [Laternula elliptica (a bivalve) and Nacella concinna (a gastropod)]. L. elliptica is a sediment burrowing mollusc, whereas N. concinna is a common inter-tidal species and can be found anywhere from mid-tide level (Walker 1972) to depths greater than 110-m depth (Powell 1951, 1973). The N. concinna used in this study were collected by divers at 8- to 10-m depths. Given these collection distributions, both species experience yearly water temperatures restricted between −1.86°C and +1°C. The expression of the HSP70 family members was assessed using quantitative PCR (Q-PCR) after an acute 2-h heat shock experiment. The data are discussed within the context of adaptation to life in an extreme and changing environment.
Methods
Animal sampling and experimental work
All animals used in experimental work were collected at Rothera Research Station, Adelaide Island, Antarctic Peninsula (67°34′07″ S, 68°07′30″ W), by scuba divers during the austral summer at depths of 10–15 m (L. elliptica) and 6 m (N. concinna). Seasonal water temperatures for this collection site are provided in Fig. 1. Laternula elliptica (bivalve) and Nacella concinna (gastropod) were collected and immediately returned to the laboratory where they were maintained in a through-flow aquarium under a simulated natural light/dark cycle. Predicted sunrise and sunset times (Poltips 3, Proudman Oceanographic Laboratory) were used in conjunction with a mechanical timer to control the lighting regime. During the time the animals were held in the aquarium, the water temperature was 0.75°C ± 0.0°C. All experimental work was carried out in January 2004. L. elliptica and N. concinna were not fed when held in the aquarium, but the former were observed filtering seawater in the tanks and would have obtained some food via this route, whereas the latter were observed grazing biofilms on the aquarium tanks. Animals were maintained for 5–7 days in the aquarium before the experimental work.
Fig. 1.
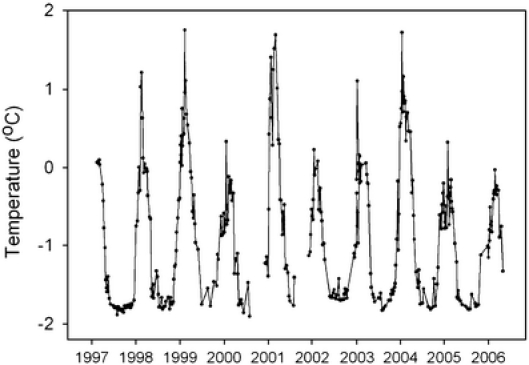
Ten-year time course series of annual seawater temperature fluctuations around Rothera Research Station at 15 M (scuba operation) depth. Range of temperatures over this whole period varied from a minimum of −1.8°C to a maximum of +1.7°C. Data provided by Professor Andrew Clarke from the RaTS (Rothera Time course Series) Long-Term Monitoring Programme
The two species were exposed to a thermal shock by immediate transfer to seawater maintained at a range of temperatures [1.33°C ± 0.07°C (control), 4.8°C ± 0.06°C, 6.0°C ± 0.03°C, 8.12°C ± 0.05°C, 10.16°C ± 0.07°C, 14.9°C ± 0.0°C and 20.09°C ± 0.04°C (N. concinna only)] for 2 h. Groups of five animals of each species were transferred to the experimental tanks. After the 2-h thermal shock, the animals were weighed (±0.1 g), the shell length was measured (±0.1 mm), the animals were killed and the tissues were collected and placed either in RNALater (Ambion) or snap frozen in liquid nitrogen for subsequent analysis. Tissue samples were collected from the foot of N. concinna, and gill, foot muscle, digestive gland, gonad, mantle and siphon from L. elliptica.
Sample analysis
RNA extraction and isolation of HSP genes Total RNA was extracted from tissues using TRI reagent (Sigma) according to the manufacturer’s instructions. One microgram of total RNA was DNAse treated using 0.4U DNase I (Ambion) in 10 mM DTT/100 mM MgCl2 buffer and reverse transcribed using a first-strand synthesis kit (Promega). Degenerate primers for HSP70 were designed from a protein alignment of HSP70 genes from a variety of species (H. sapiens to molluscs). The primers (HSP70F, ATCATCGCYAACGACCAGGGMRAC; HSP70R, GTTGTTGAAGTARGCDGGSACBGT) amplified a 500-bp fragment encompassing amino acids 30–125 (motifs used for primers: IIANDQGD and TVPAYFNN). PCR cycling conditions varied according to the organism: L. elliptica, 95°C, 5 min; 35 cycles of 95°C, 20 s; 55°C, 20 s; and 72°C for 40 s with a final elongation step of 72°C for 5 min. The same basic programme was kept for N. concinna but with the annealing temperature reduced to 45°C and the number of cycles increased to 40. Products were subcloned into p-GEMT-easy (Promega), transformed into Escherichia coli strain XL-2 Blue MRF’ (Stratagene) and a minimum of 48 clones sequenced from each organism. Sequence data was assembled using the phred, Phrap and consed packages (Ewing et al. 1998; Gordon et al. 1998). Consensus sequences were database searched using WU-blast2 (WU-blastx; Altschul et al. 1997) against Uniprot (Boeckmann et al. 2003; Wu et al. 2006) to assign their HSP identity. The nucleotide sequences were aligned using Clustal W (Thompson et al. 1994) and specific primers designed to each different member of the HSP family for each organism, all with an annealing temperature of 60°C. Amplified fragment sizes varied between 119 and 142 bp. The specificity of each of the primers was checked by amplification and sequencing of the products. All HSP sequence fragments have been submitted to the European Molecular Biology Laboratory database with the accession numbers AM293594–AM293601 inclusive.
Isolation of β actin genes For comparative analysis to be made between the different HSP genes, a housekeeping sequence β actin was isolated from both organisms. Degenerate primers were designed from a ClustalW alignment of a number of β actin genes from Takifugu (Venkatesh et al. 1996), two Asteroidea and one Orthogastropoda (accession numbers P53484, p12716, p123717 and P17304, respectively; SeaactinF, ACCGACTACYTSAKKAAGATCCT; SeaactinR, GAVGCVAGGATGGAGCCRCC). The PCR conditions for L. elliptica were as follows: 95°C, 5 min; 35 cycles of 95°C, 20 s; 60°C, 20 s; and 72°C for 40 s with a final elongation step of 72°C for 5 min. The same basic programme used for N. concinna but with a lower annealing temperature of 45°C, and the number of cycles increased to 40. PCR products were sequenced, assembled and checked as described above for the HSP genes. In this instance, if multiple β actin fragments were amplified from the same organism, primers were designed to regions of identity between the different family members. Primers were designed to anneal at 60°C. Expression levels of β actin between different tissues and different treatment states were checked to ensure constant expression and reproducibility. Sequences of all primers are listed in Table 1.
Table 1.
HSP and actin gene primer sets for L. elliptica and N. concinna
| Organism | Primer set | Gene | Primer sequence | RSq | PCR efficiency (%) | |
|---|---|---|---|---|---|---|
| Nacella concinna | 1F2 and 1Rev2 | HSP70A | Nco1F2 | ATTCGATGACGAGACGGTTCA | 0.968 | 134.90 |
| Nco1Rev2 | AACGTCTTCAATTCGCTTTTGTA | |||||
| 3F and 3Rev | HSP70B | Nco3F | AGTTCACCGACGACACAGTAC | 0.945 | 103.70 | |
| Nco3Rev | TATTTTAGTCTCTGATTTGTACTC | |||||
| 7F and 7Rev2 | GRP78 | Nco7F | CTTGGGATGATAAATCTGTCCA | 0.996 | 86.40 | |
| Nco7Rev2 | CTTTGTCAGAACCTTGTACATTA | |||||
| 9F and 9Rev | HSC70 | Nco9F | AATTTGACGATGGACACGTTCAA | 0.988 | 87.00 | |
| Nco9Rev | GGTCTTTTGTTCACCCTTGTAG | |||||
| ActinF and ActinR4 | Actin | NcoActinF | GAGAAATCGTCCGAGACATCAA | 0.983 | 95.00 | |
| NcoActinRev4 | CAGCAGATTCCATACCCAAGAA | |||||
| Laternula elliptica | 2F and 2Rev | HSP70A | Lel2F | CTGTCTTGAGCGATGGTGGC | 0.998 | 116.80 |
| Lel2Rev | TTTGTTACGGTCTTTCCTAAGTA | |||||
| 3F3 and 3Rev | HSC70 | Lel3F3 | CAATGACAACACTCGCCCCA | 0.996 | 84.30 | |
| Lel3Rev | TGTTGACAGTCTTTCCGAGGTA | |||||
| 4F and 4Rev | HSP70B | Lel4F | AAGCTTGTCAACCACGGCGG | 0.975 | 107.20 | |
| Lel4Rev | CCTTGACCCTTTGGCCAAGG | |||||
| 5F and 5Rev | GRP78 | Lel5F | GGTCAAGAACAAGAACAACAAAC | 1.000 | 94.90 | |
| Lel5Rev | TGACGATTTTCTCTCCCAGGAA | |||||
| Actin F and ActinRev | Actin | LelActinF | CGACGGTCAGGTCATCACCA | 0.999 | 95.90 | |
| LelActinRev | GACAGGACAGTGTTGGCGTA | |||||
RSq and PCR efficiency values are included, as calculated using the Stratagene MxPro–MX3000P v. 3.00 Build 311 Schema 74 software.
Quantitative PCR HSP and actin sequences were amplified from each organism under each treatment condition using specific primers, Brilliant SYBR® Green QPCR Master Mix (Stratagene) and an MX3000P (Stratagene). PCR conditions were as follows: 95°C, 10 min; 40 cycles of 95°C, 30 s; 60°C, 1 min; and 72°C for 1 min with a final dissociation curve step as per manufacturer’s recommendations. The plate setup for each Q-PCR experiment consisted of five control individuals and five experimental (treated) individuals; both sets were amplified with a specific HSP primer pair and an actin control primer set. All amplifications were reproduced in triplicate. Each primer set was checked to ensure that no primer dimers were produced during the course of the amplification reaction. RSq values and PCR efficiencies were checked over a fourfold 10× dilution series and the values calculated using the MxPro–MX3000P v 3.00 Build 311 Schema 74 software (Table 1). Primers producing low RSq values were discarded and new primers designed. Amplifications were analysed using the MxPro–MX3000P v 3.00 Build 311 Schema 74 software and Ct (dR) values exported into Excel. Relative expression ratios of the HSP genes compared to the actin housekeeping genes between the control and the treated samples were derived using the Relative Expression Software Tool (REST; http://www.gene-quantification.info/; Pfaffl 2001; Pfaffl et al. 2002). This is an excel macro that incorporates both a mathematical model to calculate relative expression ratios on the basis of the PCR efficiency and crossing point derivation of the investigated samples and a two-sided pairwise fixed reallocation randomisation test. This test makes no assumptions about distribution (such as normality of distribution) and assumes that treatments were randomly allocated. The randomisation test repeatedly and randomly reallocates the observed values to the two groups and notes the apparent effect (expression ratio). The proportion of these effects, which are as great as that actually observed in the experiment, provides the p value of the test. Two thousand randomisations were used in the test (Pfaffl 2001; Pfaffl et al. 2002). These results were then followed by further statistical analysis (Minitab v 14) using a two-way analysis of variance (ANOVA) to test for the significance of an effect of either temperature or tissue.
Results
Four members of the HSP70 gene family were isolated from both L. elliptica and N. concinna. These were defined according to their sequence similarity scores after searching the sequence databases using WU-blastx. In each animal, these comprised two members of the inducible form (HSP70, designated HSP70A and HSP70B), one heat shock cognate HSC70 gene and one for glucose-regulated protein, 78kDa (GRP78; Table 2). Meaningful phylogenetic comparisons were not possible, as there are only two other sequences for mollusc GRP78 and HSC70 genes in the public databases.
Table 2.
Designation of HSP gene family member status based on BLAST match results from database sequence similarity searches
| Organism | Primer set | Gene designation | Closest database match | Score | Percent identity | Probability |
|---|---|---|---|---|---|---|
| Nacella concinna | 1F2 and 1Rev2 | HSP70A | P08106: Gallus gallus (chicken) | 448 | 81 | 1.3e−40 |
| 3F and 3Rev | HSP70B | Q86QM8: Locusta migratoria (Migratory locust) | 557 | 81 | 3.6e−52 | |
| 7F and 7Rev2 | GRP78 | Q75W49: Crassostrea gigas (Pacific oyster) | 565 | 81 | 5.2e−53 | |
| 9F and 9Rev | HSC70 | Q9XZJ2: Crassostrea gigas (Pacific oyster) | 559 | 81 | 2.2e−52 | |
| Laternula elliptica | 2F and 2Rev | HSP70A | Q2MJK5: Haliotis discus hannai (Abalone) | 534 | 85 | 9.9e−50 |
| 3F3 and 3Rev | HSC70 | Q76N60: Paralichthys olivaceus (Japanese flounder) | 555 | 96 | 5.9e−52 | |
| 4F and 4Rev | HSP70B | Q86MC3: Balanus amphitite (barnacle) | 471 | 81 | 4.7e−43 | |
| 5F and 5Rev | GRP78 | Q75W49: Crassostrea gigas (Pacific oyster) | 451 | 76 | 9.1e−41 |
Nacella concinna Initially to obtain a general idea of the relative expression levels of each of the HSP genes in N. concinna, the genes were assayed using standard PCR and gel electrophoresis in a set of control animals (Fig. 2). Both inducible forms of the HSP70 genes were present but at a relatively low level. In contrast, GRP78 was more strongly expressed by approximately twofold, but the constitutive HSC70 gene was very strongly expressed at a level similar to that of the β actin gene. Neither HSC70 nor GRP78 showed any significant change in gene expression levels assessed using Q-PCR after exposure to 10°C, 15°C or 20°C. Although both HSP70 genes showed no significant change in level at 10°C, there was massive up-regulation of both genes at 15°C and 20°C. HSP70A was up regulated by almost 2,000-fold at both temperatures, whereas HSP70B was up regulated by approximately 350-fold at 15°C, which increased to almost 750-fold at 20°C (Fig. 3). A two-way ANOVA on the combined dataset showed both a significant difference between genes (F3,6 = 8.10, p = 0.016) and an effect of temperature (F2,6 = 5.38, p = 0.046). Partitioning the dataset (into HSP70 A and B; GRP78 and HSC70) and reanalysing shows that, with the inducible HSP70s, there is no effect of gene (F1,2 = 0.81, p = 0.462), but there is an effect of temperature (F2,2 = 29.97, p = 0.032), as evidenced by the massive up regulation of these genes; whereas with GRP78 and HSC70, there is no effect of either gene (F1,2 = 0.12, p = 0.76) or temperature (F2,2 = 0.70, p = 0.590).
Fig. 2.
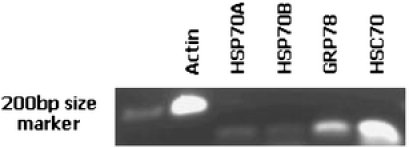
Control expression levels of Nacella concinna actin and HSP70 genes performed using PCR on 1 µg of total RNA/complementary DNA (cDNA)
Fig. 3.
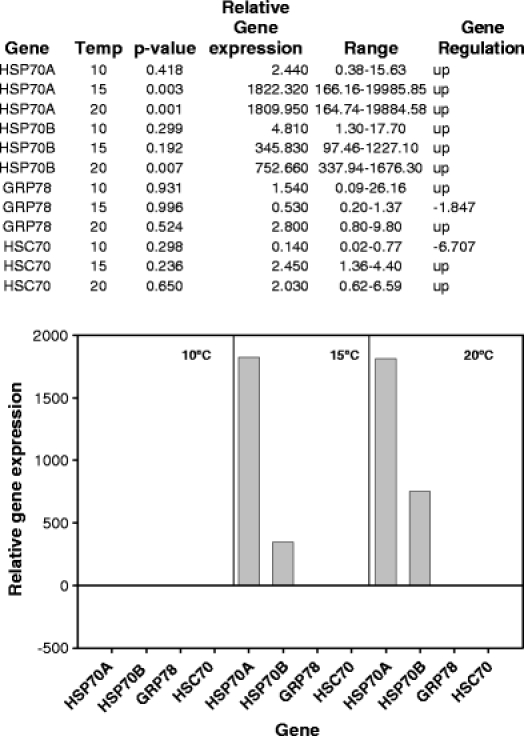
Q-PCR results for Nacella concinna over three different temperature heat shocks. Relative expression ratios of the HSP genes from the control compared to the experimental animals are shown both in table format and graphically
Laternula elliptica This mollusc was originally assayed for HSP expression across a range of five tissues (gill, digestive gland, mantle, foot and siphon) in control animals. Gonad tissue was not included in the heat shock evaluation because heat is one method of inducing spawning in a number of marine organisms, and as such, this would influence gene expression in this tissue. In L. elliptica, both inducible forms of the HSP70 gene were permanently expressed, with HSP70A expressed more strongly and uniformly than HSP70B. HSP70B showed tissue-specific expression levels with highest expression in mantle, followed by gut and very low levels in the foot, gill and siphon. GRP78 was fairly uniformly expressed at approximately two- to threefold higher levels than the HSP70A gene (Fig. 4). The gene designated as HSC70 showed a very low level of expression, which did not change appreciably after the heat shock treatment. Therefore, the data for this gene, whilst surveyed across all temperature ranges, and tissues have not been included in this analysis.
Fig. 4.
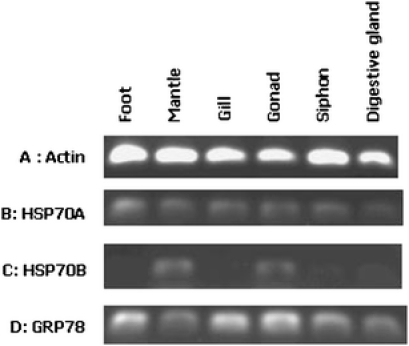
Control expression levels of Laternula elliptica actin and HSP70 genes performed using PCR on 1 µg of total RNA/cDNA. A actin, B HSP70A, C HSP70B, D GRP78
Samples were sequentially analysed starting from the highest temperature of 15°C and then surveyed at lower temperatures if the genes showed up-regulation. Hence, some tissues were checked at the lower temperatures of 6°C and 4°C.
All three genes (HSP70A, HSP70B and GRP78) under study appeared to show increased expression levels in response to temperature. The relative expression levels of HSP70A showed a significant response to temperature (F2,8 = 25.30, p = <0.001) and an effect of tissue specificity at the 10% level (F4,8 = 2.91, p = 0.093). This gene showed up-regulation from 10°C. The maximum increase in relative expression of this gene was approximately 12× (Fig. 5). HSP70B exhibited higher maximal expression levels, up to 86× (Fig. 6). There was relatively uniform expression in all tissues at both 15°C and 10°C as evidenced by the ANOVA results (F4,4 = 0.30, p = 0.862), with a significant effect of temperature (F1,4 = 23.97, p = 0.008). The survey of tissues at 8°C showed significant relative expression in both foot and gill (according to the individual p values). Foot muscle was further surveyed at lower temperatures and showed no appreciable up-regulation (Fig. 6). When the whole 8°C dataset is combined with that from 10°C and 15°C and subjected to a two-way ANOVA, the seemingly tissue-specific nature of the response at this lower temperature masks the effects of both tissue and temperature (F4,8 = 0.81, p = 0.550; F2,8 = 2.34, p = 0.158), respectively. Therefore, it is only possible to say that HSP70B is statistically significantly up regulated at 10°C. In the case of GRP78, there appeared to be differences in tissue-specific expression levels and a potentially higher uniform induction threshold of 15°C (Fig. 7). A two-way ANOVA test on the 8–15°C dataset produced no significant result for either tissue or temperature (F4,8 = 0.68, p = 0.626; F2,8 = 2.28, p = 0.165), respectively. Individual p values indicate that significant up-regulation of expression occurs in gill, mantle and siphon (p values of 0.48, 0.002 and 0.008, respectively) at 15°C; however, a two-way ANOVA test on the restricted 10–15°C dataset do not show an overall effect of either tissue or temperature (F4,4 = 1.69, p = 0.312; F1,4 = 3.15, p = 0.150). Foot tissue showed the highest expression at 8°C and so was surveyed at lower temperatures, but no up-regulation was indicated (Fig. 7).
Fig. 5.
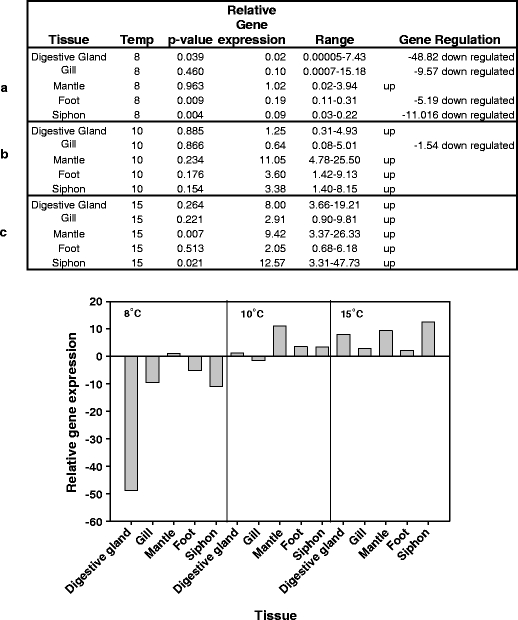
Q-PCR results for the Laternula elliptica HSP70A gene at 8°C (a), 10°C (b) and 15°C (c) in both tabular and graphical format
Fig. 6.
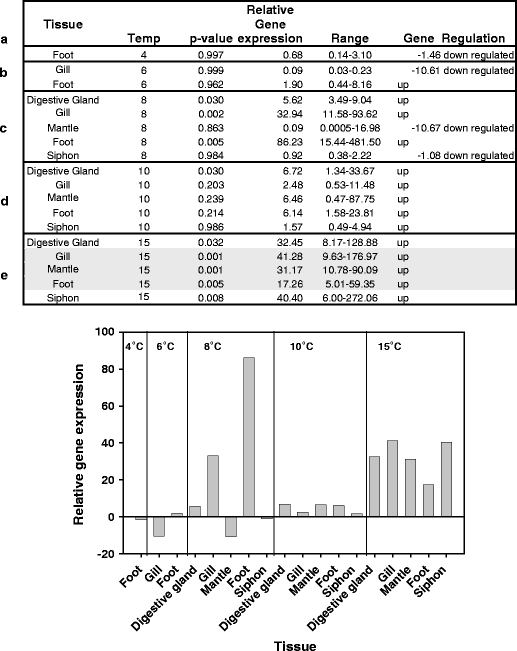
Q-PCR results for the Laternula elliptica HSP70B gene at 4°C (a), 6°C (b), 8°C (c), 10°C (d) and 15°C (e) in both tabular and graphical format. Significant individual p values at 15°C are shaded
Fig. 7.
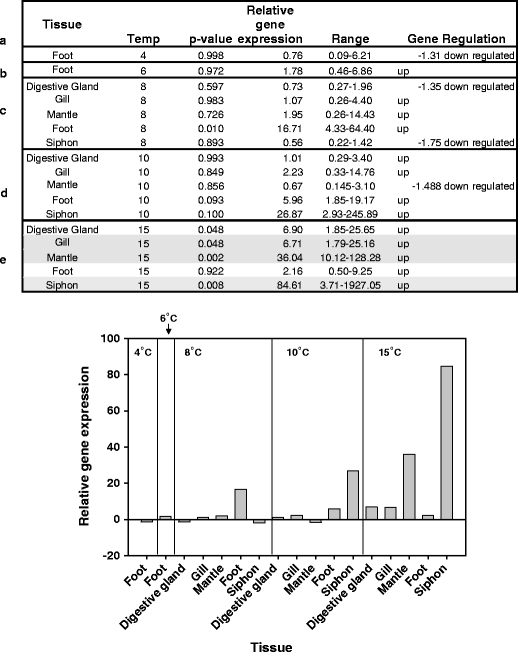
Q-PCR results for the Laternula elliptica GRP78 gene at 4°C (a), 6°C (b), 8°C (c), 10°C (d) and 15°C (e) in both tabular and graphical format. Significant individual p values at 15°C are shaded
Discussion
Both Antarctic marine molluscs in this study possess a quantifiable heat shock response, which varies in magnitude between the two species (maximum of 2,000-fold in N. concinna but only 40-fold in L. elliptica) and also in induction temperature. N. concinna exhibits a massive heat shock response at 15°C, whereas the up-regulation threshold for L. elliptica is statistically significant from 10°C depending on the gene.
Four genes, HSP70A, HSP70B, HSC70 and GRP78, were cloned from each of the two animals. The two inducible forms of HSP70 are very similar: The two N. concinna genes are 78.2% identical, whereas the two L. elliptica genes are 70.2% identical both at the DNA level. The most parsimonious explanation for which is propagation via gene duplication, either a gene-specific or a whole-genome event. Each of the HSP70 genes identified in each organism exhibits different control levels of gene expression compared to its paralogue in the same organism (in particular, HSP70B in L. elliptica is the subject of tissue-specific expression; for further discussion, see below). This is in line with the retention and evolution of duplicate genes via sub-functionalisation (Force et al. 1999), and maintenance of duplicates in this instance may reflect different tissue requirements to protect against low temperature or ice-nuclei insult.
GRP78 is located in the endoplasmic reticulum and is a classic marker of the unfolded protein response with an HSP-like chaperon function (Sommer and Jarosch 2002), which in mammals, is not classically activated in response to temperature (Hendershot et al. 1994). However, evidence from both the platyfish and Japanese oyster indicate that, in lower vertebrates/invertebrates, it is up regulated in response to temperature (Yamashita et al. 2004; Yokoyama et al. 2006). Our experiments substantiate these findings.
In the experiments with N. concinna, only foot muscle was surveyed. L. elliptica is much larger, and several different tissues could be dissected and examined. The magnitude of response varied between tissues, with some tissues showing a statistically significant difference in expression levels of some genes compared to others (cf. gill, mantle and siphon with GRP78). Certainly, in the control animals, HSP70A showed fairly uniform expression levels across tissues, whereas HSP70B showed some indication of tissue specificity, with high expression in the mantle carried through to the 15°C experiments. HSP70B shows statistically significant individual results for gill, mantle and foot (and siphon at the 10% level) at 15°C, whereas GRP78 shows significant up-regulation in gill, mantle and siphon at 15°C. This is perhaps not surprising, as the foot, in particular, is the crucial organ for mobility in this animal, and gills present a large surface area in contact with the surrounding environment and are critical for respiration. The siphon is the only part of the animal, which is in direct contact with flowing seawater when buried and would be the first contact for increased water temperatures. The results for mantle are interesting, as this organ is largely responsible for secretion of the shell; however, it does enclose the critical respiratory chamber containing the gills and is also continuous with the siphon, and so maybe it too acts as a first response to increased seawater temperatures.
When examining the HSP response in these animals using Q-PCR, care has to be taken with regard to the change in relative gene expression as an absolute figure rather than looking at the data overall and extracting general trends. This is because repeat sampling of animals before and after treatment was not possible. As a result, this meant that analysis of five “paired” results was, in fact, analysis of 10 different individuals; hence, the Q-PCR results were highly variable with large confidence intervals. Individual variation in gene expression is clearly high and may have been exacerbated by different sized animals being used in each set of experiments (for example, in the 10°C N. concinna cohort, weight varied from 1.3 to 7.1 g, and in the 10°C L. elliptica cohort, weight ranged from 23.7 to 75.8 g). Previous work examining physiological responses to increased water temperatures showed a correlation of response magnitude with size (Peck and Bailey in review; Peck et al. in review), and therefore, by inference, size may also affect gene expression levels or at least rate of change. This is clearly a factor that requires further investigation for future work but is impossible to eliminate completely, as it is not always possible to choose similar sized animals for the experiments when evaluating animals from extreme environments.
The two invertebrates studied demonstrated that they are capable of a heat shock response. This is in contrast to Antarctic Notothenioids, which show permanent expression of the inducible forms of the HSP70 genes and no heat shock response (Hofmann et al. 2000, Place et al. 2004; Place and Hofmann 2005). The two mollusc species in this study also show some permanent expression of the inducible forms of the HSP70 genes, albeit at a relatively low level. They also show permanent expression of the HSC70 genes; N. concinna, in particular, shows relatively high control levels of HSC70 similar to that of the actin gene. As the N. concinna foot was used for these experiments, actin would be expected to be one of the major genes expressed. The fact that the HSC70 gene is expressed at a similar level to actin suggests by inference that it is one of the most highly expressed genes in this animal. GRP78 is also constitutively expressed at high levels in this animal. As both HSC70 and GRP78 are expressed at such high-level constitutively, this may be the reason why they are not up regulated further in N. concinna in response to increased seawater temperatures, particularly when compared to the situation in L. elliptica where relative control levels of GRP78 are lower and HSC70 is poorly expressed.
Taking the data overall, the permanent expression of the inducible HSP70 genes, species-specific high expression of HSC70 (N. concinna) and permanent expression of GRP78 (N. concinna and L. elliptica) indicates that, as for Antarctic fish, chaperone proteins form an essential part of the adaptation of biochemical machinery of these Antarctic animals to low but stable temperatures. High constitutive levels of HSP gene family member expression may be a compensatory mechanism for coping with elevated protein damage at low temperatures analogous to the permanent expression of HSP70 in the Antarctic notothenioids. There is some evidence that protein degradation rates and protein carbonyl concentrations (as a measure of protein oxidation) appear comparatively higher in invertebrates at polar water temperatures than in species living at warmer temperatures (Fraser et al. 2002; Philipp et al. 2005). Other studies have also shown elevated levels of ubiquitin-conjugated proteins in polar fishes, a likely indication of increased levels of denatured proteins at polar water temperatures (Place et al. 2004). Taken together, this evidence suggests that transcribing, translating and folding proteins at polar water temperatures is problematic. Indeed, cold denaturation of proteins has previously been documented (Privalov 1990) and exposure of endotherm cells and ectotherms to cold shock can induce HSP70 expression (Ali et al. 2003; Laios et al. 1997).
Whilst both species show constitutive expression of HSP gene family members and up-regulation of HSP70 genes, there is a clear difference in the response between N. concinna and L. elliptica. This may be phylogenetically constrained but impossible to determine given the limited species sampling. Certainly, the level may be pre-determined according to the lifestyle of the organism. L. elliptica is a sediment-burrowing mollusc that experiences a seasonal sea temperature range of between −1.86°C to +1.86°C at Rothera Point (Peck et al. 2006; Fig. 1). This species has also been shown to be one of the most stenothermal Antarctic marine species studied to date with an effective temperature tolerance between −2°C to +2.5°C, an upper lethal temperature of +7.5°C and a critical temperature window of 5–7°C, above which anaerobiosis starts (Peck et al. 2002, 2004; S. Morley, personal communication). In contrast, N. concinna is a common inter-tidal species and can be found anywhere from mid-tide level (Walker 1972) to depths greater than 110-m depth (Powell 1951, 1973). The N. concinna used in this study were collected by divers at 8- to 10-m depths, where again, the yearly water temperature range only varies between −1.86°C and +1°C. Although data collected during the austral summer at South Cove, Rothera Point, on inter-tidal limpets demonstrated that, on a sunny day, the foot temperature of intertidal N. concinna exceeded 6.3°C, whereas the maximum shell temperatures reached 7.2°C (C. Waller, personal communication). According to the current study, these foot temperatures are still considerably lower than that required to induce HSP up-regulation (15°C). When examining the threshold for HSP induction in temperate marine organisms, a value of +8°C to +10°C over habitat temperature is common (Tomanek and Somero 1999; Buckley et al. 2001) and, therefore, the threshold at which HSPs are induced in these two molluscs is similar to the temperature rise required for the normal induction temperature for temperate species (Hofmann 2005). There are no accurate fossil records for N. concinna in the Antarctic (A. Crame, personal communication), but fossils of L. elliptica have been described from the late Pliocene (5 Ma; Soot-Ryen 1952; Jonkers 1999) Hence, these founder animals may have an HSP regulation that evolved in a warmer and more variable climate.
Given that the HSP response in these molluscs is induced at typical temperate habitat levels, the question remains as to why the level at which the thermostat has been set has not been reduced in line with the very narrow temperature range that these animals currently experience in their natural environment. Ultimately, there is an energetic cost associated with the production of heat shock proteins, and overproduction may be cytotoxic (Feder and Hofmann 1999; Sorensen et al. 2003). As both N. concinna and L. elliptica constitutively express members of the HSP70 family (HSC70 and GRP78: N. concinna and GRP78 L. elliptica) and are permanently investing heavily in their production, they may not have the spare energetic capacity for significant up-regulation without significant deleterious effects on other cellular processes.
A second explanation may be that the constitutive requirement for the HSP family to mitigate problems of protein conformity at low temperature decrease with small to moderate elevations in temperature, such as those below 10°C. This could either reduce the expression levels somewhat of the relevant genes or balance any increase in protein unfolding with rising temperature. Eventually, the insult from the temperature shock would overwhelm the balance, and a marked increase in HSP gene expression would result similar to that observed. We currently do not have data to differentiate the competing hypotheses.
In summary, these results show that, in contrast to Antarctic Notothenioids, two Antarctic molluscs exhibit the classical biochemical-based heat shock response to elevated environmental temperatures. The level and magnitude of the response varies with the species. The level at which this response is activated, under experimental conditions, indicates that the control of this function is probably a relic from temperate ancestors and, given the data presented here, probably would not be activated under increased seawater temperatures associated with global warming predictions. Therefore, genetic factors other than HSP production might be expected to play a more important role in the adaptation of these animals to life in higher temperatures should they survive. A more comprehensive investigation into the complex transcriptional changes that take place in these animals associated with increased environmental temperatures is currently underway in our laboratory.
Acknowledgement
This paper was produced within the BAS Q3 Latest and BAS Q4 Bioreach/Bioflame core programmes. Thanks to all members of the Rothera Dive Team for providing samples and to Pete Rothery for statistics advice. Overall diving support was provided by the NERC National Facility for Scientific Diving at Oban.
References
- Ali KS, Dorgai L, Abraham M, Hermesz E (2003) Tissue- and stressor-specific differential expression of two hsc70 genes in carp. Biochem Biophys Res Commun 307:503–509 [DOI] [PubMed]
- Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 [DOI] [PMC free article] [PubMed]
- Boeckmann B, Bairoch A, Apweiler R et al (2003) The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res 31:365–370 [DOI] [PMC free article] [PubMed]
- Bosch TCG, Krylow SM, Bode HR, Steele RE (1988) Thermotolerance and synthesis of heat-shock proteins—these responses are present in hydra-attenuata but absent in hydra-oligactis. Proc Natl Acad Sci U S A 85:7927–7931 [DOI] [PMC free article] [PubMed]
- Buckley BA, Owen M-E, Hofmann GE (2001) Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol 204:3571–3579 [DOI] [PubMed]
- Buckley BA, Place SP, Hofmann GE (2004) Regulation of heat shock genes in isolated hepatocytes from an Antarctic fish, Trematomus bernacchii. J Fish Biol 207:3649–3656 [DOI] [PubMed]
- Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185 [DOI] [PubMed]
- Feder ME, Hofmann GE (1999) Heat shock proteins, molecular chaperones and their stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282 [DOI] [PubMed]
- Fink AL (1999) Chaperone-mediated protein folding. Physiol Rev 79:425–449 [DOI] [PubMed]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J (1999) Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151:1531–1545 [DOI] [PMC free article] [PubMed]
- Fraser KPP, Clarke A, Peck LS (2002) Feast and famine in Antarctica: seasonal physiology in the limpet Nacella concinna. Mar Ecol Prog Ser 242:169–177 [DOI]
- Gordon D, Abajian C, Green P (1998) Consed: a graphical tool for sequence finishing. Genome Res 8:195–202 [DOI] [PubMed]
- Gross M (2004) Emergency services: a bird’s eye perspective on the many different functions of stress proteins. Current Protein and Peptide Science 5:213–223 [DOI] [PubMed]
- Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–580 [DOI] [PubMed]
- Hendershot LM, Valentine VA, Lee AS, Morris SW, Shapiro DN (1994) Localization of the gene encoding human bip/grp78, the endoplasmic-reticulum cognate of the hsp70 family, to chromosome-9q34. Genomics 20:281–284 [DOI] [PubMed]
- Hofmann GE (2005) Patterns of gene expression in ectothermic marine organisms on small to large-scale biogeographical patterns. Intergr Comp Biol 45:247–255 [DOI] [PubMed]
- Hofmann GE, Buckley BA, Airaksinen S, Keen JE, Somero GN (2000) Heat-shock protein expression is absent in the Antarctic fish Trematomus bernacchii family Nototheniidae. J Exp Biol 203:2331–2339 [DOI] [PubMed]
- IPCC (2001) Climate change: synthesis report. In: Watson RT, (eds) IPCC Third assessment Report. Cambridge University Press, Cambridge, UK
- Jonkers HA (1999) Aligned growth positions in Pliocene Laternula elliptica (King & Broderip) (Bivalvia: Anomalodesmata: Laternulidae). Antarct Sci 11:463–464 [DOI]
- Laios E, Rebeyka IM, Prody CA (1997) Characterization of cold-induced heat shock protein expression in neonatal rat cardiomyocytes. Mol Cell Biochem 173:153–159 [DOI] [PubMed]
- LaTerza AL, Miceli C, Luporini P (2001) Divergence between two Antarctic species of the ciliate Euplotes, E. focardii and E. nobilii, in the expression of heat-shock protein 70 genes. Mol Ecol 10:1061–1067 [DOI] [PubMed]
- LaTerza AL, Miceli C, Luporini P (2004) The gene for the heat-shock protein 70 of Euplotes focardii, an Antarctic psychrophilic ciliate. Antarct Sci 16:23–28 [DOI]
- Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Lett 32:L19604–L19609 [DOI]
- Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796 [DOI] [PubMed]
- Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance—degradation and reactivation of damaged proteins. Ann Rev Genet 27:437–496 [DOI] [PubMed]
- Peck LS (1989) Temperature and basal metabolism in two Antarctic marine herbivores. J Exp Mar Biol Ecol 127:1–12 [DOI]
- Peck LS (2002) Ecophysiology of Antarctic marine ectotherms: limits to life. Polar Biol 25:31–40 [DOI]
- Peck LS, Conway LZ (2000) The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalve molluscs. In: Harper E, Crame AJ (eds) Evolutionary biology of the bivalvia. vol. 177. Geological Society of London Special Publication, London, pp 441–450
- Peck LS, Convey P, Barnes DKA (2006) Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol Rev 81:75–109 [DOI] [PubMed]
- Peck LS, Pörtner HO, Hardewig I (2002) Metabolic demand, oxygen supply, and critical temperatures in the Antarctic bivalve Laternula elliptica. Physiol Biochem Zool 75:123–133 [DOI] [PubMed]
- Peck LS, Webb KE, Bailey DM (2004) Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct Ecol 18:625–630 [DOI]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007 [DOI] [PMC free article] [PubMed]
- Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:1–10 [DOI] [PMC free article] [PubMed]
- Philipp E, Brey T, Pörtner HO, Abele D (2005) Chronological and physiological ageing in a polar and a temperate mud clam. Mech Age Dev 126:598–609 [DOI] [PubMed]
- Place SP, Hofmann GE (2005) Constitutive expression of a stress-inducible heat shock protein gene, hsp70, in a phylogenetically distant Antarctic fish. Polar Biol 28:261–267 [DOI]
- Place SP, Zippay ML, Hofmann GE (2004) Constitutive roles for inducible genes: evidence for the alteration in expression of the inducible hsp70 gene in Antarctic notothenioid fishes. Am J Physiol Regul Integr Comp Physiol 287:R429–R436 [DOI] [PubMed]
- Pörtner H-O, Peck LS, Hirse T (2006) Hypoxia alleviates thermal stress in the Antarctic bivalve, Laternula elliptica: evidence for oxygen limited thermal tolerance. Polar Biol 29:688–693 [DOI]
- Powell AWB (1951) Antarctic and subanctarctic Mollusca: Pelecypoda and Gastropoda. Discov Rep 26:49–196
- Powell AWB (1973) The patellid limpets of the world (Patellidae). Indo-Pac Mollusca 3:75–206
- Privalov PL (1990) Cold denaturation of proteins. Crit Rev Biochem Mol Biol 25:281–305 [DOI] [PubMed]
- Somero GN, DeVries AL (1967) Temperature tolerance of some Antarctic fishes. Science 156:257–258 [DOI] [PubMed]
- Sommer T, Jarosch E (2002) BiP binding keeps ATF6 at bay. Dev Cell 3:1–2 [DOI] [PubMed]
- Soot-Ryen T (1952) Laternula elliptica (King & Broderip 1831) from the Pecten-conglomerate, Cockburn Island. Arkiv för Zoologi 4:163–164
- Sorensen JG, Kristensen TN, Loeschcke V (2003) The evolutionary and ecological role of heat shock proteins. Ecol Lett 6:1025–1037 [DOI]
- Thompson JD, Higgins DG, Gibson TJ (1994) Clustal-W—improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680 [DOI] [PMC free article] [PubMed]
- Tomanek L, Somero GN (1999) Evolutionary and acclimation induced variation in the heat-shock responses of congeneric marine snails (Genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol 202:2925–2936 [DOI] [PubMed]
- Venkatesh B, Tay BH, Elgar G, Brenner S (1996) Isolation, characterization and evolution of nine pufferfish (Fugu rubripes) actin genes. J Mol Biol 259:655–665 [DOI] [PubMed]
- Walker AJM (1972) Introduction to the Antarctic limpet Patinigera polaris (Hombron and Jacquinot) at Signy Island, South Orkney Islands. Br Antarct Surv Bull 28:49–69
- Wu CH, Apweiler R, Bairoch A et al (2006) The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic Acids Res 34:D187–D191 [DOI] [PMC free article] [PubMed]
- Yamashita M, Hirayoshi K, Nagata K (2004) Characterisation of multiple members of the HSP70 family in platyfish culture cells: molecular evolution of stress protein HSP70 in vertebrates. Gene 336:207–218 [DOI] [PubMed]
- Yokoyama Y, Hashimoto H, Kubota S, Kuriyama A, Ogura Y, Mizuta S, Yoshinaka R, Toyohara H (2006) cDNA cloning of Japanese oyster stress protein homologous to the mammalian 78-kDa glucose regulated protein and its induction by heatshock. Fish Sci 72:402–409 [DOI]


