Abstract
Objective
Alpha hemoglobin-stabilizing protein (AHSP) inhibits the production of reactive oxygen species in various cells, including erythrocytes. Reduced AHSP can mean reduced protection from stressors. Our objective was to investigate whether AHSP is involved in the response to stress in pregnancy.
Study design
Placentas were collected from normal term pregnancies (n = 10) and pregnancies complicated by HELLP (n = 10), intrauterine growth restriction (IUGR; n = 10) or fetal death (IUFD; n = 6). AHSP messenger RNA (mRNA) and protein were determined using real time quantitative polymerase chain reaction (PCR) and Western blot, respectively. All statistical analyses were performed by using the GraphPad Prism Software. Differences were considered significant at p < 0.05.
Results
Placental AHSP mRNA level in HELLP (4.16E10−4 ± 1.77) and IUFD (4.19E10−4 ± 3.37) were significantly decreased compared with controls (28.47E10−4 ± 14.86; p < 0.01), whereas levels in the IUGR group (7.55E10−4 ± 6.4) showed a trend toward being lower but the difference did not reach statistical significance. Western blot analysis results indicate a no significant increase of ASHP protein in the HELLP syndrome group and a significant decrease in the IUFD group compared with controls. There was no significant difference between the IUGR and control groups.
Conclusion
ASHP mRNA expression in the placenta is decreased in complicated pregnancies, and it may be involved in the pathogenic mechanisms leading to the adverse pregnancy outcome.
Keywords: HELLP syndrome, ASHP, Intra uterine growth restriction, Intra uterine fetal death, Placenta
Introduction
Alpha-hemoglobin-stabilizing protein (AHSP), formerly named erythroid differentiation-related factor (EDRF; Miele et al 2001), is a specific molecular chaperone that binds to the α-chains of hemoglobin and prevents α-globin precipitation in erythroid cell line (Kihm et al 2002; Gell et al 2002). Studies have shown that if ASHP is absent, the alpha-globin becomes unstable and generates reactive oxygen species (ROS) that damage it and other cellular constituents (Betten et al 2004). ASHP seems to inhibit ROS production from α-globin by reducing its inherent ability to participate in redox reactions (Dos Santos and Costa 2005). AHSP is part of the heat-shock protein family, proteins that are produced in response to stressors, such as heat, oxidizing conditions, and toxins. Such adaptive responses may take weeks before being manifested (Ferns et al 2006; Wang et al 2005; Papp et al 2003). Reduced AHSP can mean reduced protection from stressors.
ASHP knockout mice exhibit reticulocytosis, abnormal erythrocyte morphology, and increased ROS that lead to subsequent cellular oxidative damage (Kaul et al 2004).
Oxidative stress occurring in the placenta is believed to play an important role in the development of hemolysis, elevated liver enzyme, and low platelet (HELLP) syndrome (Herrmann et al 2004); intrauterine growth restriction (IUGR; Myatt 2006), and intrauterine fetal death (IUFD) (Gareskog et al 2006). Oxidative stress in the placenta leads to thrombosis, inflammation, and endothelial cell dysfunction. Together, these factors result in the untoward pregnancy outcomes (Rehberg et al 2006).
A relative deficiency of AHSP in the placenta or in the erythrocytes within the placenta can result in oxidative stress de novo or magnify the effect of uteroplacental insufficiency. Our aim in this study was to determine AHSP messenger RNA (mRNA) and protein expression in the placenta from pregnancies complicated by HELLP syndrome, IUGR, and IUFD.
Materials and methods
Tissue collection and population
Placentas were collected immediately after caesarean section from 10 normal term pregnancies, 10 pregnancies complicated by HELLP syndrome, 10 with IUGR, and after delivery, from 6 pregnancies with IUFD.
All specimens were snap frozen in liquid nitrogen and stored at −80°C until use.
To eliminate or reduce the impact of fetal death on placental analysis, we recruited only the patients with a demise-delivery interval within 12 h.
The study was approved by the Institutional Review Board and informed consent was obtained in all patients.
HELLP syndrome was defined by the presence of all three of the following criteria: hemolysis (diagnosed by peripheral blood smear and a serum lactate dehydrogenase level ≥600 U/l), elevated liver enzymes (serum aspartate aminotransferase concentration ≥70 U/l), and low platelet count (<100,000 cells/μl; Rodgers et al 1988).
Gestational age was determined according to the best obstetric criteria based on last menstrual period or ultrasonography at <20 weeks’ gestation. Intrauterine growth restriction was defined by a fetal abdominal circumference less than the fifth percentile for the gestational age based on standard growth curves (al-Eissa et al 1995). In the IUFD group, patients with underlying maternal diseases or other obstetrical complications were excluded (Table 1).
Table 1.
Demographic characteristics
| Control, n = 10 | HELLP, n = 10 | IUGR, n = 10 | IUFD, n = 6 | |
|---|---|---|---|---|
| Maternal age (years) | 29.1 ± 3.01 | 29.4 ± 2.63 | 28.7 ± 1.2 | 30.7 ± 2.0 |
| Parity | 1.29 ± 0.65 | 1.25 ± 0.46 | 1.4 ± 0.9 | 1.5 ± 0.6 |
| Gestational age at delivery (weeks) | 37.1 ± 0.8 | 28 ± 3.9* | 30.3 ± 1.2* | 27.3 ± 3.2* |
| Systolic blood pressure (mmHg) | 118.25 ± 4.3 | 165.74 ± 23.2* | 116.0 ± 3.2 | 115.14 ± 3.0 |
| Diastolic blood pressure (mmHg) | 68. 70 ± 2.1 | 107.15 ± 9.10* | 70 ± 0.9 | 78.0 ± 2.4 |
| Birth weight (g) | 3231.7 ± 436.1 | 1052.8 ± 847.1* | 650.4 ± 350.2* | 730.7 ± 350.2* |
| Placental weight (g) | 658.43 ± 97.81 | 513.25 ± 111.19* | 354.4 ± 65.2* | 300.4 ± 95.2* |
*p < 0.001 versus control
RNA extraction
A piece of the frozen tissue (20–40 mg) was homogenized in lysis buffer, and the total RNA was extracted with a RNA isolation kit (Promega, Madison, WI, USA). RNA samples were tested by ultraviolet absorption at 260 nm to determine RNA concentration. The quality and concentration of the RNA samples were further confirmed by electrophoresis on denaturated 1% agarose gels. Two micrograms of RNA were reverse transcribed in a total volume of 25 μl for 60 min at 37°C with M-MLV reverse transcriptase (Promega) using random nonamers to obtain complementary DNA (cDNA).
Real-time quantitative polymerase chain reaction
cDNA was used for real-time quantitative polymerase chain reaction (PCR). To avoid false-positive results attributable to the amplification of contaminating genomic DNA in the cDNA preparation, the primers were selected to flank an intron, and PCR efficiencies were tested and found to be close to 1.
The following primers were used:
5′-CCAACCGCGAGAAGATGAC-3′(forward) and 5′-GAGGCGTACAGGGATAGCACA-3′ (reverse) for β-actin, and 5′-TGTCACCTGCTGCCTGTAAT-3′(forward) and 5′-AAGGAGTTCAGCGTTCTGCT-3′ (reverse) for AHSP.
The genes were run in duplicate using SYBR Green chemistry. All samples were tested in triplicate using β-actin as the reference gene for data normalization to correct for variations in RNA quality and quantity. A serial dilution of the standard plasmid (harboring AHSP and b-actin cDNAs, respectively) was included in each run to obtain an estimate of absolute gene expression levels (under the assumption that reverse transcription efficiency is 100%). Direct detection of PCR products was monitored by measuring the fluorescence produced by SYBR Green I dye binding to double-stranded DNA after every cycle.
The copy number of AHSP mRNA was then determined in each sample and divided by the amounts of β-actin mRNA to give copy number per unit β-actin mRNA.
Western blotting
To confirm the results, we made a Western blot. Tissue extracts were prepared with lysis buffer (phosphate-buffered saline containing 1% Nonidet P40, 0.1% sodium dodecyl sulfate, 1 mM phenylmethylsulfonylfluoride, and 2 μg/ml aprotinin). Samples containing 45 μg proteins were subjected to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. As a positive control, cell extracts from Escherichia coli BL21-expressing recombinant AHSP were also loaded in all Western blot. After regular blocking and washing, the membranes were incubated with rabbit polyclonal antibody (1:3,000 dilution) against human AHSP (Dr. Mitchell Weiss, Children’s Hospital of Philadelphia, Philadelphia, PA, USA) for 1 h followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG (Pierce, Rockford, IL, USA; 1:2,000 dilution) for 1 h. AHSP protein was visualized using an enhanced chemiluminescent substrate (SuperSignal West Femto Maximum Sensitivity Substrate, Pierce).
The intensities of AHSP bands were evaluated from pictures obtained using Quantity One 1-D analysis software.
Statistical analysis
All values were expressed as mean ± standard deviation of the mean (SD). Results of Western blot are expressed as mean and SD in arbitrary units of densitometry. Results in each of the adverse outcomes group were compared to the control group using the Student’s t test. All statistical analyses were performed by using the GraphPad Prism software. Differences were considered significant at p < 0.05.
Results
Real-time PCR assay for AHSP gene expression
Placental AHSP mRNA level in HELLP (4.16E10−4 ± 1.77) and IUFD (4.19E10−4 ± 3.37) were significantly decreased compared with the controls (28.47E10−4 ± 14.86; p < 0.01), whereas levels in the IUGR group (7.55E10−4 ± 6.4) showed a trend toward being lower but the difference did not reach statistical significance (Fig. 1).
Fig. 1.
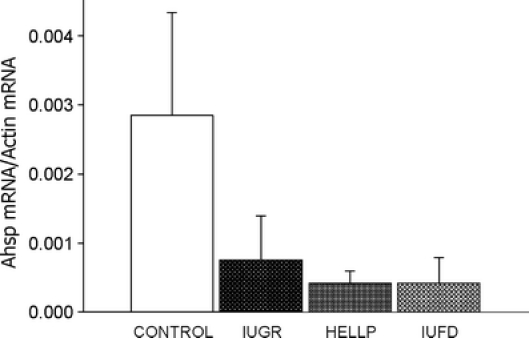
Real-time quantitative PCR. AHSP mRNA levels in the placenta of patients with normal or pathological pregnancies. Results are expressed as mean ± SD
Western blot assay for AHSP protein production
A representative Western blot is shown in Fig. 2. As it is illustrated in Fig. 3, we found no significant increase of ASHP protein in the HELLP syndrome group (654.80 ± 49.30) and a significant decrease in the IUFD group (332.30 ± 26.24) compared with the controls (576.10 ± 30.09), whereas there was no significant difference between this latter and the IUGR group (568.10 ± 48.76).
Fig. 2.
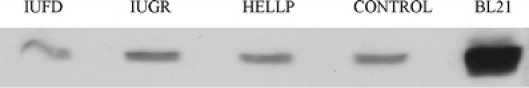
Representative Western blot for AHSP. Protein lysate from placentas are shown on the first four lanes. The last lane shows the protein lysate from BL21 after induction of AHSP expression (positive control). The control lane shows the protein lysate that was present in all Western blots and used for normalization of expression
Fig. 3.
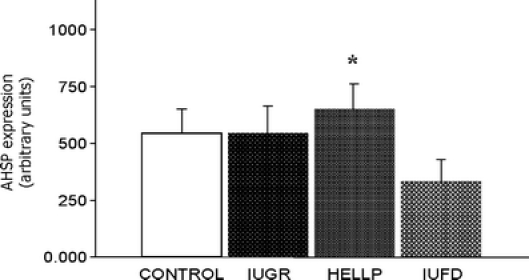
Western blot data analysis. Expression of AHSP protein in placentas of patients with normal or pathological pregnancies. Results are expressed as mean ± SD. *p < 0.05
In all the groups for both ASHP mRNA and protein, no significant differences were found in the correlation analysis between AHSP protein and other variables including placental weight and gestational age at delivery.
Discussion
Several studies have shown a role for AHSP in hematological diseases, such as beta thalassemia. Mice lacking AHSP have abnormal red-cell production and lifespan, caused by a relatively small excess of unchaperoned globing chains. Moreover, thalassemia intermedia in mice are exacerbated by the concomitant loss of AHSP (protein or gene; Kong et al 2003).
Our results support the idea that a pro-oxidant state occurs in pregnancies complicated by HELLP syndrome (Chen et al 1994; Wisdom et al 1991). There is evidence of increased lipid peroxidation in preeclampsia, both in the placenta (Walsh and Wang 1993) and in the circulation (Uotila et al 1993; Garzetti et al 1993). Consistent with our findings, there is an association between abnormal function of red cells and preeclampsia (Cunningham et al 1985). Spickett et al. suggested that oxidative stress in preeclampsia leads to erythrocyte damage, which subsequently results in the hemolysis observed in HELLP syndrome (Spickett et al 1998).
Our findings raise the possibility that placental ASHP plays a role in adverse pregnancy outcomes.
The underexpression of mRNA in the IUFD group translated into lower protein in the IUFD group but not in the HELLP group. The exact meaning of the finding is that protein levels of ASHP did not decrease, as their mRNA expression in HELLP placenta is uncertain, and it is not clear from these data alone whether this discrepancy may be caused by post-transcriptional regulation, stabilization of the protein AHSP, and/or alterations in its turnover rate. Therefore, we can hypothesize that, despite the same transcriptional activity of AHSP mRNA in extreme degrees of placental function, also due to high oxidative stress rate, the protein could have a rapid degradation. This condition is more likely to happen in IUFD than in HELLP placentas, in which the mechanisms of response to oxidative stress could still be relatively efficacious.
Although the regulatory network that underlies the temporal control of transcript expression and gene function remains to be determined, the identification of hypoxic trophoblast transcripts is likely to shed light on the mechanisms underlying trophoblast response to villous hypoxia.
As within the HELLP group no significant differences were found in the correlation analysis between both AHSP mRHA and protein and placental weight or gestational age at delivery, we may presume that the results are not related to differences in gestational age.
With regards to the IUFD group, we can presume that our findings are not secondary to alterations secondary to fetal death because placentas were collected after a very short death-to-delivery/collection interval (less than 12 h).
This study, like others, relies on findings in human placentas obtained in the third trimester, and therefore, we cannot speculate about the oxidative stress of trophoblast tissue in early gestation. The causation hypothesis would, therefore, need to be investigated in animal models that reproduce the human condition.
Acknowledgment
We are indebted to Dr. Mitchell Weiss (Children’s Hospital of Philadelphia) for providing AHSP antibody. This work was supported by the Italian Ministry for University and Scientific Research through PRIN 2004-068971-007.
Footnotes
This paper was presented as a poster at the 27th Annual Meeting of the Society for Maternal Fetal Medicine; San Francisco, CA, USA, February 5–10, 2007.
Monica Emanuelli and Davide Sartini contributed equally to this paper.
References
- al-Eissa YA, Ba’Aqeel HS, Haque KN, AboBakr AM, al-Kharfy TM, Khashoggi TY, al-Husain MA (1995) Determinants of term intrauterine growth retardation: the Saudi experience. Am J Perinatol 12:278–281 [DOI] [PubMed]
- Betten A, Dahlgren C, Mellqvist UH, Hermodsson S, Hellstrand K (2004) Oxygen radical-induced natural killer cell dysfunction: role of myeloperoxidase and regulation by serotonin. J Leukoc Biol 75:1111–1115 [DOI] [PubMed]
- Chen G, Wilson R, Cumming G, Walker JJ, Smith WE, McKillop JH (1994) Intracellular and extracellular antioxidant buffering levels in erythrocytes from pregnancy-induced hypertension. J Hum Hypertens 8:37–42 [PubMed]
- Cunningham FG, Lowe T, Guss S, Mason R (1985) Erythrocyte morphology in women with severe preeclampsia and eclampsia. Preliminary observations with scanning electron microscopy. Am J Obstet Gynecol 153:358–363 [DOI] [PubMed]
- Dos Santos CO, Costa FF (2005) ASHP and beta-thalassemia: a possible genetic modifier. Hematology 10:157–161 [DOI] [PubMed]
- Ferns G, Shams S, Shafi S (2006) Heat shock protein 27: its potential role in vascular disease. Int J Exp Pathol 87:253–274 [DOI] [PMC free article] [PubMed]
- Gareskog M, Eriksson UJ, Wentzel P (2006) Combined supplementation of folic acid and vitamin E diminishes diabetes-induced embryotoxicity in rats. Birth Defects Res A Clin Mol Teratol 76:483–490 [DOI] [PubMed]
- Garzetti GG, Tranquilli AL, Cugini AM, Mazzanti L, Cester N, Romanini C (1993) Altered lipid composition, increased lipid peroxidation, and altered fluidity of the membrane as evidence of platelet damage in preeclampsia. Obstet Gynecol 81:337–340 [PubMed]
- Gell D, Kong Y, Eaton SA, Weiss MJ, Mackay JP (2002) Biophysical characterization of the alpha-globulin binding protein ASPH. J Biol Chem 277:40602–40609 [DOI] [PubMed]
- Herrmann W, Hubner U, Koch I, Obeid R, Retzke U, Geisel J (2004) Alteration of homocysteine catabolism in pre-eclampsia, HELLP syndrome and placental insufficiency. Clin Chem Lab Med 42:1109–1111 [DOI] [PubMed]
- Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME (2004) Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest 114:1136–1145 [DOI] [PMC free article] [PubMed]
- Kihm AJ, Kong W, Russel JE et al (2002) An abundant erythroid protein that stabilizes free alpha-hemoglobin. Nature 417:758–763 [DOI] [PubMed]
- Kong Y, Katein AM, Louden CS, Weiss MJ (2003) Loss of alpha hemoglobin stabilizing protein exacerbates thalassemia phenotypes in mice. Blood 102:46a [DOI] [PMC free article] [PubMed]
- Miele G, Manson J, Clinton M (2001) A novel erythroid-specific marker of transmissible spongiform encephalopathies. Nat Med 7:361–364 [DOI] [PubMed]
- Myatt L (2006) Placental adaptive responses and fetal programming. J Physiol 572:25–30 [DOI] [PMC free article] [PubMed]
- Papp E, Nardai G, Soti C, Csermely P (2003) Molecular chaperones, stress proteins and redox homeostasis. Biofactors 17:249–257 [DOI] [PubMed]
- Rehberg JF, Briery CM, Hudson WT, Bofill JA, Martin JN (2006) Thrombotic thrombocytopenic purpura masquerading as hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome in late pregnancy. Obstet Gynecol 108:817–820 [DOI] [PubMed]
- Rodgers GM, Taylor RN, Roberts GM (1988) Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells. Am J Obstet Gynecol 159:908–914 [DOI] [PubMed]
- Spickett CM, Reglinski J, Smith WE, Wilson R, Walker JJ, McKillop J (1998) Erythrocyte glutathione balance and membrane stability during preeclampsia. Free Radic Biol Med 24:1049–1055 [DOI] [PubMed]
- Uotila JT, Tuimala RJ, Aarnio TM, Pyykkö KA, Ahotupa MO (1993) Findings on lipid peroxidation and antioxidant function in hypertensive complications of pregnancy. Br J Obstet Gynaecol 100:270–276 [DOI] [PubMed]
- Walsh SW, Wang Y (1993) Deficient glutathione peroxidase activity in preeclampsia is associated with increased placental production of thromboxane and lipid peroxides. Am J Obstet Gynecol 169:1456–1461 [DOI] [PubMed]
- Wang G, Guo X, Floros J (2005) Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. Am J Physiol Lung Cell Mol Physiol 289:497–508 [DOI] [PubMed]
- Wisdom SJ, Wilson SR, McKillop JH, Walker JJ (1991) Antioxidant systems in normal pregnancy and pregnancy-induced hypertension. Am J Obstet Gynecol 165:1701–1704 [DOI] [PubMed]


