Abstract
Non-invasive gamete and embryo assessment is considered an important focus in assisted reproductive technologies (ART). Currently, the selection of embryos for transfer is based on morphological indices. Though successful, the field of ART would benefit from a non-invasive quantitative method of viability determination. Omics technologies, including transcriptomics, proteomics and metabolomics, have already begun providing evidence that viable gametes and embryos possess unique molecular profiles with potential biomarkers that can be utilized for developmental and/or viability selection. Unlike the human genome that is relatively fixed and steady throughout the human body, the human proteome, estimated at over a million proteins, is more complex, diverse and dynamic. It is the proteins themselves that contribute to the physiological homeostasis in any cell or tissue. Of particular interest in ART is the secretome, those proteins that are produced within the embryo and secreted into the surrounding environment. Defining the human embryonic secretome has the potential to expand our knowledge of embryonic cellular processes, including the complex dialogue between the developing embryo and its maternal environment, and may also assist in identifying those embryos with the highest implantation potential. Advances in proteomic technologies have allowed the non-invasive profiling of the human embryonic secretome with ongoing research focused on correlation with outcome. From a clinical perspective, embryo selection based on morphological assessment and non-invasive analysis of the human embryonic secretome may improve IVF success and lead to routine single embryo transfers.
Keywords: proteomics, embryo, secretome, non-invasive assessment
Introduction
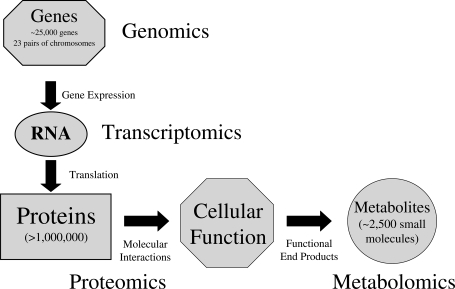
Over the last decade, there has been a strong focus on the molecular characterization of various biological systems and disease states. The human genome consists of ∼25 000 genes with the human transcriptome providing researchers with valuable gene expression data. However, it is the human proteome defined as the PROTEin complement to the human genOME that actually dictates cellular function and ultimately determines phenotype (Fig. 1). The proteome itself is dynamic, constantly changing through its own interactions, impacted by both internal and external stimuli. Advances in proteomic technologies have begun to shed light on the diversity and complexity of the human proteome, estimated at over 1 million proteins. A variety of biological samples are under investigation, with an emphasis towards identifying proteins involved in specific disease states, to develop new diagnostic and prognostic assays (Dominguez et al., 2007). Of particular interest to investigators is the secretome, those proteins that are produced by cells and secreted at any given time or under certain physiological conditions (Hathout, 2007). Several types of biological fluids, including serum and conditioned medium, have been analyzed for secreted proteins that may define a particular disease state or progression (Hu et al., 2006; Kulasingam and Diamandis, 2007). A number of putative cancer biomarkers have been discovered from analysis of serum proteomes from a variety of cancer patients. Although very promising, some of the candidate biomarkers are relatively abundant, shared among different human cancers and overexpressed in other human diseases such as autoimmune diseases (Hu et al., 2006). Future validation of candidate cancer biomarkers on large independent patient cohorts is critical. With the development of new sensitive proteomic technologies, investigators are concentrating on discovering low abundant serum proteins that are more sensitive and cancer specific. In this review, we discuss the role of proteomics in examining the mammalian embryonic secretome. Defining and characterizing the mammalian embryonic secretome will expand our knowledge of early embryogenesis, advance our understanding of the embryonic role during implantation and potentially contribute to the development of non-invasive viability assessment.
Figure 1.
A schematic diagram outlining the complexity of cellular function including associated omics technologies; genomics, transcriptomics, proteomics and metabolomics that contribute to the study and understanding of biological systems.
Embryo assessment
The selection of embryos for transfer is currently based on detailed morphological criteria (Ebner et al., 2003). Though relatively successful, the field of assisted reproductive technologies (ART) would benefit from more quantitative methods of viability determination to improve IVF success and reduce the incidence of multiple pregnancies (Sakkas and Gardner, 2005). Over the last decade, a decrease in the number of embryos for transfer has correlated with a significant reduction in higher order pregnancies (Stern et al., 2007). However, twin gestations continue to reflect a high percentage of IVF pregnancies with high health risks to both the mother and fetuses, including preterm delivery, low birthweight, perinatal mortality and other pregnancy-related complications (Pinborg, 2005). Hence, the promise of improving IVF pregnancy rates with more quantitative methods of embryo selection has the ability to optimize successful single embryo transfer.
Proteomics and the mammalian embryo
Proteins translated from RNA transcripts are directly responsible for cellular function as illustrated in Fig. 1. Gene expression studies of these RNA transcripts, though valuable, have been shown to often not predict protein abundance or function. For example, low levels of correlation were observed in a study where paired comparisons of changes in mRNA and protein expression levels were investigated in embryonic stem cells (Williamson et al., 2008). Furthermore, while examining the relationship between proteins and mRNA abundance in yeast, mRNA transcript analyses were found to be insufficient in predicting protein expression (Gygi et al., 1999). Several mechanisms, including targeted mRNA degradation and protein degradation, can be utilized by the cell during regulation of transcription/translation resulting in a lack of association between gene expression, RNA transcripts and protein expression. Consequently, in order to understand cellular function and comprehend biological processes and/or disease states, an in depth investigation of the mammalian proteome is vital.
Despite new advances in proteomic technologies, knowledge of the proteome of the mammalian preimplantation embryo remains limited. The combined effect of limited template, low protein expression and the lack of sensitivity of proteomics platforms have contributed as the main hurdles. In spite of these obstacles, there have been several excellent publications, a selection of these are described in Table I. Earlier proteomic studies utilized two-dimensional (2D) gel electrophoresis in combination with computerized analysis of gel images to construct and analyze protein databases of the preimplantation mouse embryo (Latham et al., 1992; Shi et al., 1994). Western blotting has also been used to identify the expression of known proteins (Navarrete Santos et al., 2004), or to detect post-translational modifications, like phosphorylation, in relation to embryo development (Wang et al., 2005). More recently, advances in proteomic technologies, with the development of mass spectrometry (MS), have made it possible to identify groups of proteins within limited amounts of complex biological fluids and tissues (Gutstein et al., 2008; Jansen et al., 2008).
Table I.
Selected publications highlighting the use of proteomics in embryo assessment/development
| Model | Characterization | Key findings | Reference |
|---|---|---|---|
| Mouse | 2D gel electrophoresis | Constructed a 2D gel protein database for the mouse embryo. Characterized changes in protein synthesis patterns during normal preimplantation development from fertilization to blastocyst stage | Latham et al. (1992) |
| Mouse | 2D gel electrophoresis | Constructed protein databases for compacted 8-cell and blastocyst stage mouse embryos. Documented the overall quantitative changes in protein levels | Shi et al. (1994) |
| Rabbit | Western blot | Insulin-responsive glucose transporter protein isoforms (GLUT4 and GLUT8) and the insulin receptor protein were expressed in rabbit blastocysts | Navarrete Santos et al. (2004) |
| Mouse | Western blot | An increase in phosphorylation of SAPK/JNK and p38MAPK negatively correlate with mouse embryo development | Wang et al. (2005) |
| Porcine | MALDI-TOF MS western blot | Major vault protein (MVP) accumulated in embryos that failed to develop normally in vitro | Sutovsky et al. (2005) |
| Mouse | SELDI-TOF MS | Embryos generated under 5% (low) oxygen more closely resembled in vivo-derived mouse embryos. High (20%) oxygen conditions were associated with down-regulation of 10 proteins/biomarkers | Katz-Jaffe et al. (2005) |
| Human | SELDI-TOF MS | Different protein profiles were identified between early and expanded blastocysts, and between developing blastocysts and degenerating embryos | Katz-Jaffe et al. (2006a) |
SAPK/JNK, stress-activated protein kinase/Jun kinase; p38 MAPK, 38 mitogen-activated protein kinase; MALDI-TOF MS, matrix-assisted laser desorption ionization–time-of-flight mass spectrometry; SELDI-TOF MS, surface-enhanced laser desorption/ionization–time of flight mass spectrometry.
MS: a powerful tool in global proteomics
MS has rapidly become an important technology in proteomic profiling. Searching for consistent and significant alteration in protein expression profiling between specific groups of samples have revealed important features of a disease state or physiological process. MS involves an ion source for production of a charged species in the gas phase, and an analyzer, which can separate ions by their mass-to-charge (m/z) ratio. Several commonly used ionization methods include electrospray ionization and surface-enhanced laser desoprtion/ionization (SELDI), which are coupled to time-of-flight (TOF), ion trap or quadrupole analyzers. SELDI-TOF MS, with specific capture affinity protein chips, has allowed fast, cost-effective, high-throughput analysis of small sample volumes (microliter range) and enables sensitivity to be in the picomole to femtomole range. Bound proteins are laser activated thereby liberating gaseous ions by desorption/ionization. The TOF tube is under a vacuum which causes smaller ions to travel faster towards the detector thereby allowing a separation of these ions according to m/z ratio represented as a protein profile (Seibert et al., 2004). The technology has been applied to a variety of biological tissues and fluids with specific focus on oncoproteomics including the early detection, metastases ability and therapeutic outcome of an assortment of different cancers (Cho, 2007). Identification of proteins following MS profiling is typically performed by protease digestion generating distinct cleavage products identifiable by tandem MS analysis and protein database searching (Liebler, 2002).
MS technology has begun to be utilized as a proteomic platform in the study of the developing preimplantation embryo (Table I). The major vault protein (MVP) was identified using matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) MS and western blotting as a protein expressed in porcine preimplantaion embryos. MVP was shown to accumulate in poor-quality porcine embryos that failed to develop normally in vitro (Sutovsky et al., 2005). In addition, SELDI-TOF MS has recently been used to generate protein profiles at all stages of mammalian embryonic development (Katz-Jaffe et al., 2005, 2006a). Differences in protein profiles were observed between early and expanded blastocysts as well as between developing blastocysts and degenerate embryos. Preliminary database searching identified some of the protein candidates to be directly linked to embryogenesis (Katz-Jaffe et al., 2006a).
The dynamic and sensitive nature of the proteome to variables during sample collection, storage, handling and processing needs to be considered and a consistent protocol needs to be followed for reproducible proteomic data (Hu et al., 2005). Data prejudice caused by MS calibration and instrument drift overtime and across labs can also introduce artifacts. This can be controlled for by running samples in replicates, routinely performing internal and external calibrations and including suitable control samples with every run.
The embryonic secretome
It is proposed that viable embryos possess a unique proteome with potentially some of these proteins secreted into the surrounding culture medium contributing to the secretome. Consequently, a non-invasive proteomic analysis of the secretome of mammalian embryos throughout preimplantation development may assist in revealing secreted factors that reflect developmental competence and viability.
One of the earliest studies of the human embryonic secretome revealed the release of 1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine (paf), a soluble factor that is produced and secreted by mammalian preimplantation embryos. paf operates in an autocrine manner as a survival factor during embryonic development. It also creates a range of alterations to maternal physiology including platelet activation (O'Neill, 2005). Leptin, a small pleiotrophic peptide, has also been observed in the conditioned medium from blastocysts in a human in vitro model studying the interactions between the embryo and endometrial epithelial cells (Gonzalez et al., 2000). It was shown that competent human blastocysts secreted higher leptin concentrations into the surrounding medium than arrested embryos. It has been hypothesized that the leptin secreted by the blastocyst initiates a ligand receptor-mediated effect with leptin receptors in the maternal endometrium establishing a molecular dialogue during the window of implantation (Cervero et al., 2005).
Acrogranin, a protein that regulates epithelial cell growth, has been shown to be secreted into the surrounding media by preimplantation mouse embryos (Díaz-Cueto et al., 2000). Upon addition of acrogranin to culture medium, blastocyst formation was promoted. Acrogranin is believed to act directly on the trophectoderm cells in an autocrine manner. Furthermore, this study revealed that addition of anti-acrogranin antibodies to the surrounding media delayed the onset of blastocyst formation. It has been suggested that acrogranin is expressed by the uterus and influences embryo development similar to that of exogenous added acrogranin that promotes blastocyst formation in culture (Díaz-Cueto et al., 2000).
More recently, Sakkas et al. (2003) characterized a soluble molecule secreted by human blastocysts that modulates regulation of HOXA10 expression in an epithelial endometrial cell line. This form of reciprocal embryo-endometrial interaction could transform the local uterine environment impacting both embryo development and the implantation process. In addition, the detection of soluble human leukocyte antigen G (sHLA-G) in spent IVF medium of Day 3 embryos has indicated higher pregnancy rates from sHLA-G-positive embryos (Noci et al., 2005; Sher et al., 2005). These results however have not been absolute with pregnancies established from sHLA-G-negative embryos. Additionally, technical differences have been recorded including the inability to measure sHLA-G production in some supernatants (Sargent et al., 2007). Overall, each of these individual secretome studies has focused on only the single variable. Considering the multifactorial nature of mammalian embryonic development, it would be reasonable to assume that more than one single factor would be required to predict developmental competence and/or implantation potential.
MS and the embryonic secretome
Using SELDI-TOF MS, distinctive secretome signatures were observed at each 24 h embryonic developmental stage from the time of fertilization to the blastocyst stage. These secretome signatures could uniquely identify the embryonic stages of development, independent of morphology. Some proteins were observed across several embryonic stages, whereas others were more stage-specific (Katz-Jaffe et al., 2006b). Interestingly, species comparison between the mouse and human embryonic secretome revealed limited diversity; similar to the protein paf which has been observed in the secretome of all mammalian species studied to date (O'Neill, 2005).
The transition from maternal inherited transcripts and proteins to the activation of the embryonic genome and the expression of key embryonic proteins must occur for continued embryonic development (Telford et al., 1990). In particular, unique proteins were observed in the human embryonic secretome after the activation of the embryonic genome (Katz-Jaffe et al., 2006b). Thus, embryos with a correctly activated embryonic genome, and hence, a fully functional embryonic proteome and secretome may have a higher potential of developmental competence. Correlation of secretome signatures with ongoing blastocyst development revealed an 8.5 kDa protein. The limited expression of this 8.5 kDa protein from the secretome of degenerating embryos, in conjunction with its significantly high expression from the secretome of developing blastocysts, potentially indicates an association between this protein and developmental competence (P < 0.05). Tandem MS and database peptide sequence identification indicated that the best candidate for this 8.5 kDa protein was ubiquitin (Katz-Jaffe et al., 2006b). Ubiquitin is a component of the ubiquitin-dependent proteosome system, targeting proteins for degradation. The system is involved in a number of physiological processes including proliferation and apoptosis. Secreted ubiquitin has been shown to be up-regulated in body fluids in certain disease states and this accumulation provides evidence for an increased protein turnover (Sandoval et al., 2006; Delbosc et al., 2008). Ubiquitin has also been implicated to play a crucial role during mammalian implantation by controlling the activities and turnover of key signaling molecules (Wang et al., 2004). Ongoing research is focused on identification of the remaining secreted proteins as well as correlation with both developmental competence and implantation.
Another aspect of ART that could benefit from non-invasive analysis is preimplantation genetic screening (PGS). PGS involves the biopsy of polar bodies or embryonic cells and has become a routine clinical procedure in many IVF clinics screening for specific chromosomal abnormalities in human embryos. Biopsy procedures are invasive to the growing embryo and could potentially compromise further development. An initial investigation into whether embryos with chromosomal abnormalities could be distinguished from euploid embryos by their respective secretome identified hatching blastocysts euploid for 10 chromosomes to exhibit notably different secretome profiles to hatching blastocysts identified as aneuploid for 10 chromosomes. In addition, degenerating aneuploid embryos exhibited a significantly different secretome profile to hatching aneuploid blastocysts (Katz-Jaffe et al., 2006c).
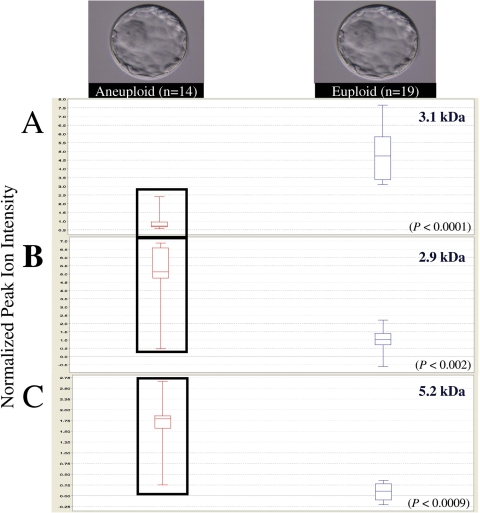
With further advances in genomics technologies allowing for comprehensive chromosomal analysis of all 23 pairs of chromosomes on a single cell (Fragouli et al., 2008), an integrated ‘omics’ approach has become realistic, discriminating secretome signatures between individual euploid and aneuploid blastocysts. Microdrops of spent IVF culture medium from individual blastocysts of transferable quality were processed (n = 33) and analyzed by SELDI-TOF MS determining a blastocyst secretome fingerprint. Each individual blastocyst was then subjected to comparative genomic hybridization for comprehensive chromosomal analysis of all 23 pairs of chromosomes; with n = 19 identified as euploid and n = 14 as aneuploid (Fragouli et al., 2008). Of the 14 aneuploid blastocysts, 9 had a single chromosomal aneuploidy and 5 were chaotic with more than two chromosomes involved. Secretome fingerprints from individual blastocysts identified protein signatures that allowed discrimination between euploid and aneuploid chromosomal constitutions (Katz-Jaffe et al., 2008). Following statistical analysis, a novel set of nine differentially expressed biomarkers was identified (P < 0.05). Each of the nine biomarkers was reproducible in all 33 secretome samples classifying a euploid secretome/blastocyst from an aneuploid secretome/blastocyst (Katz-Jaffe et al., 2008). Figure 2 displays box plots of three examples from this set, including a biomarker that displayed a decrease in expression in the secretome of aneuploid blastocysts (P < 0.05) (Fig. 2A), and two biomarkers that were shown to have increased expression in the secretome of aneuploid blastocysts (P < 0.05) (Fig. 2B and C). Ongoing research is focused on increasing the sample size to confirm reproducibility including a prospective analysis to validate the discrimination of euploid and aneuploid blastocysts. The ability to non-invasively assay for the combination of developmental competence and chromosomal constitution could represent a powerful viability selection tool in ART.
Figure 2.
Examples of biomarkers that were differentially expressed in the secretome signatures of euploid blastocysts (n = 19) compared with the secretome signature of aneuploid blastocysts (n = 14) (P < 0.05).
(A) Box plot revealing significant decrease in the expression of a 3.1 kDa biomarker in the secretome of aneuploid blastocysts compared with the secretome of euploid blastocysts (P < 0.0001) (B and C) Two box plots exhibiting significant increase in expression of 2.9 and 5.2 kDa biomarkers in the secretome of aneuploid blastocysts compared with the secretome of euploid blastocysts (P < 0.002 and P < 0.0009, respectively). The upper hinge of the box plot indicates the 75th percentile set, and the lower hinge the 25th percentile of the data. The internal line represents the median.
Protein microarray
In addition to MS technology, there are other proteomics platforms under investigation that show promise for defining the human embryonic secretome. In particular, protein microarrays have the added advantage of offering complimentary information to gene transcriptome studies as well as eliminating the need for follow-up protein identification. Recently, a study using protein microarrays compared pooled blastocyst conditioned media with control medium revealing the increased expression of the soluble tumor necrosis factor (TNF) receptor 1 and interleukin (IL)-10, and the decreased expression of macrophage-stimulating protein (MSP)-α, stem cell factor (SCF), chemokine (C-X-C motif) ligand 13 (CXCL13), TNF-related apoptosis inducing ligand receptor 3 (TRAILR3) and macrophage inflammatory protein (MIP)-1β (Dominguez et al., 2008). Further analysis investigating potential differences between pooled conditioned media from blastocysts that implanted versus pooled media that resulted in failed implantation revealed the presence of two significantly decreased proteins, CXCL13 and GM-CSF, in the pooled implanted blastocyst media. GM-CSF is a protein that has previously been identified in both human and mice blastocyst spent media culture to promote embryo development and implantation (Robertson, 2007). The decrease in GM-CSF protein expression, observed by Dominguez et al. (2008), may indicate an autocrine mechanism in supporting blastocyst development and potential implantation. None of the 120 proteins included in this protein microarray was observed to be significantly increased in the pooled conditioned media from implanted blastocysts (Dominguez et al., 2008). Larger sample volume requirements and other platform sensitivity limitations restricted the study to investigate pooled samples in contrast to individual media samples. Other platform concerns include the high cost of protein microarrays, the lower number of targets in comparison to MS or other technologies, and the dependence on the existence of antibodies and potential non-specific interactions (Spisak et al., 2007).
Metabolites in the human embryonic secretome
Metabolites, the functional end products of biological processes, are also under investigation in the secretome using spectroscopy-based methods coupled with targeted bioinformatics (Brison et al., 2007). These low molecular weight metabolites have been shown to radically change to reflect a particular metabolic and environmental state. Raman and Near Infrared Spectroscopy are two methods that have been used to detect specific oxidative stress biomarkers in spent culture medium with highlighted differences in algorithms generated for positive versus negative IVF outcomes. Viability indices generated from spent culture media analysis were observed to be higher for human embryos that went on to produce pregnancies and live births, compared with those that failed to implant (Seli et al., 2007). In addition, a blinded pilot study on retrospectively collected data, using the Raman spectroscopy platform, revealed an overall diagnostic accuracy for predicting embryonic reproductive potential, either delivery or a failed implantation, at 80.5% (Scott et al., 2008).
Proteomics and metabolomics are two complementary omics platforms in the investigation of the human embryonic secretome that show promise for the development of non-invasive methods for embryo selection in the field of ART. With the ability to assay both proteins and metabolites in spent culture media, generating complimentary but different molecular profiles, it is feasible to propose that embryo viability assessment may include a combination of both omics platforms.
Discussion
Developments in the field of proteomics, in particular improvements in MS instrument detection, have allowed the analysis of limited complex biological fluids, including spent culture media, and have facilitated fast, sensitive and high-throughput secretome analysis. Ongoing secretome research using a variety of proteomic platforms will continue to provide valuable data increasing our understanding of the biological processes involved in mammalian embryonic development (Table II). This knowledge may contribute to the evolution of non-invasive embryo viability assessment for use in ART. Alongside morphology, a non-invasive quantitative method for embryo selection may optimize successful single embryo transfers, reduce early pregnancy losses and increase live births. Defining the embryonic secretome will also provide researchers with insight into the unique sequences of events that are fundamental for successful implantation, including the most critical requirements of the blastocyst. Taking into account the complexity and diversity of the human embryo, it would seem reasonable to envisage a combined ‘omics’ contribution to the characterization of the human embryonic secretome.
Table II.
Selected publications highlighting the use of proteomics in defining the embryonic secretome.
| Model | Characterization | Key findings | Reference |
|---|---|---|---|
| Mouse | Western blot and immunoprecipitation | The protein acrogranin was secreted by embryos into the surrounding media. Addition of acrogranin to the culture media stimulated blastocyst formation. Addition of anti-acrogranin antibodies to culture media delayed blastocyst formation | Díaz-Cueto et al. (2000) |
| Human | ELISA | Competent blastocysts secreted higher levels of leptin than arrested embryos | Gonzalez et al. (2000) |
| Human | ELISA | Detection of soluble human leukocyte antigen G (sHLA-G) in spent IVF medium had a positive correlation to pregnancy outcome | Noci et al. (2005) and Sher et al. (2005) |
| Human/mouse | SELDI-TOF MS | Identified different secretome profiles for each stage of embryonic development. Ubiquitin, an 8.5 kDa protein, was up-regulated in the Day 5 secretome of developing blastocysts | Katz-Jaffe et al. (2006b) |
| Human | Protein microarray | Increased expression of soluble TNF receptor 1 and IL-10, and a decreased expression of MSP-α, SCF, CXCL13, TRAILR3 and MIP-1β in blastocyst culture media. Pooled implanted blastocyst media showed a decrease expression of CXCL13 and GM-CSF | Dominguez et al. (2008) |
ELISA, enzyme-linked immunosorbent assay; TNF, tumor necrosis factor.
Funding
Funding to pay the Open Access publication charges for this article was provided by BioSymposia Inc.
References
- Brison DR, Hollywood K, Arneson R, Goodacre R. Predicting human embryo viability: the road to non-invasive analysis of the secretome using metabolic footprinting. Reprod Biomed Online. 2007;15:296–302. doi: 10.1016/s1472-6483(10)60342-2. [DOI] [PubMed] [Google Scholar]
- Cervero A, Horcajadas JA, Dominguez F, Pellicer A, Simon C. Leptin system in embryo development and implantation: a protein in search of a function. Reprod Biomed Online. 2005;10:217–223. doi: 10.1016/s1472-6483(10)60943-1. [DOI] [PubMed] [Google Scholar]
- Cho WC. Contribution of oncoproteomics to cancer biomarker discovery. Mol Cancer. 2007;6:25. doi: 10.1186/1476-4598-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbosc S, Haloui M, Louedec L, Dupuis M, Cubizolles M, Podust VN, Fung ET, Michel J, Meilhac O. Proteomic analysis permits the identification of new biomarkers of arterial wall remodeling in hypertension. Mol Med. 2008;14:383–394. doi: 10.2119/2008-00030.Delbosc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Cueto L, Stein P, Jacobs A, Schultz RM, Gerton GL. Modulation of mouse preimplantation embryo development by acrogranin (epithelin/granulin precursor) Dev Biol. 2000;217:406–418. doi: 10.1006/dbio.1999.9564. [DOI] [PubMed] [Google Scholar]
- Dominguez DC, Lopes R, Torres ML. Proteomics: clinical applications. Clin Lab Sci. 2007;20:245–248. [PubMed] [Google Scholar]
- Dominguez F, Gadea B, Esteban FJ, Horcajadas JA, Pellicer A, Simon C. Comparative protein-profile analysis of implanted versus non-implanted human blastocysts. Hum Reprod. 2008;23:1993–2000. doi: 10.1093/humrep/den205. [DOI] [PubMed] [Google Scholar]
- Ebner T, Moser M, Sommergruber M, Tews G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review. Hum Reprod Update. 2003;9:251–262. doi: 10.1093/humupd/dmg021. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Lenzi M, Ross R, Katz-Jaffe MG, Schoolcraft WB, Wells D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum Reprod. 2008;23:2596–2608. doi: 10.1093/humrep/den287. [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Caballero-Campo P, Jasper M, Mercader A, Devoto L, Pellicer A, Simon C. Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. J Clin Endocrinol Metab. 2000;85:4883–4888. doi: 10.1210/jcem.85.12.7060. [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Morris JS, Annangudi SP, Sweedler JV. Microproteomics: analysis of protein diversity in small samples. Mass Spectrom Rev. 2008;27:316–330. doi: 10.1002/mas.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout Y. Approaches to the study of the cell secretome. Expert Rev Proteomics. 2007;4:239–248. doi: 10.1586/14789450.4.2.239. [DOI] [PubMed] [Google Scholar]
- Hu J, Coombes KR, Morris JS, Baggerly KA. The importance of experimental design in proteomic mass spectrometry experiments: some cautionary tales. Brief Funct Genomic Proteomic. 2005;3:322–331. doi: 10.1093/bfgp/3.4.322. [DOI] [PubMed] [Google Scholar]
- Hu S, Loo JA, Wong DT. Human body fluid proteome analysis. Proteomics. 2006;6:6326–6353. doi: 10.1002/pmic.200600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C, Hebeda KM, Linkels M, Grefte JM, Raemaekers JM, van Krieken JH, Groenen PJ. Protein profiling of B-cell lymphomas using tissue biopsies: A potential tool for small samples in pathology. Cell Oncol. 2008;30:27–38. doi: 10.1155/2008/898356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Jaffe MG, Linck DW, Schoolcraft WB, Gardner DK. A proteomic analysis of mammalian preimplantation embryonic development. Reproduction. 2005;130:899–905. doi: 10.1530/rep.1.00854. [DOI] [PubMed] [Google Scholar]
- Katz-Jaffe MG, Gardner DK, Schoolcraft WB. Proteomic analysis of individual human embryos to identify novel biomarkers of development and viability. Fertil Steril. 2006;a 85:101–107. doi: 10.1016/j.fertnstert.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Katz-Jaffe MG, Schoolcraft WB, Gardner DK. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil Steril. 2006;b 86:678–685. doi: 10.1016/j.fertnstert.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Katz-Jaffe MG, Stevens J, Kearns WG, Gardner DK, Schoolcraft WB. Relationship between embryonic secretome and chromosomal abnormalities in human IVF. Fertil Steril. 2006;c 86:S57. [Google Scholar]
- Katz-Jaffe MG, Fagouli E, Fillipovits J, Wells D, Schoolcraft WB. Relationship between the human blastocyst secretome and chromosomal constitution. Fertl Steril. 2008;90:S80. [Google Scholar]
- Kulasingam V, Diamandis EP. Proteomics analysis of conditioned media from three breast cell lines: a mine for biomarkers and therapeutic targets. Mol Cell Proteomics. 2007;6:1997–2011. doi: 10.1074/mcp.M600465-MCP200. [DOI] [PubMed] [Google Scholar]
- Latham KE, Garrels JI, Chang C, Solter D. Analysis of embryonic mouse development: construction of a high-resolution, two-dimensional gel protein database. Appl Theor Electrophor. 1992;2:163–170. [PubMed] [Google Scholar]
- Liebler DC. Introduction to Proteomics: Tools for the New Biology. Ottowa, New Jersey: Humana Press; 2002. [Google Scholar]
- Navarrete Santos A, Tonack S, Kirstein M, Kietz S, Fischer B. Two insulin-responsive glucose transporter isoforms and the insulin receptor are developmentally expressed in rabbit preimplantation embryos. Reproduction. 2004;128:503–516. doi: 10.1530/rep.1.00203. [DOI] [PubMed] [Google Scholar]
- Noci I, Fuzzi B, Rizzo R, Melchiorri L, Criscuoli L, Dabizzi S, Biagiotti R, Pellegrini S, Menicucci A, Baricordi OR. Embryonic soluble HLA-G as a marker of developmental potential in embryos. Hum Reprod. 2005;20:138–146. doi: 10.1093/humrep/deh572. [DOI] [PubMed] [Google Scholar]
- O'Neill C. The role of paf in embryo physiology. Hum Reprod Update. 2005;11:215–228. doi: 10.1093/humupd/dmi003. [DOI] [PubMed] [Google Scholar]
- Pinborg A. IVF/ICSI twin pregnancies: risks and prevention. Hum Reprod Update. 2005;11:575–593. doi: 10.1093/humupd/dmi027. [DOI] [PubMed] [Google Scholar]
- Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007;18:287–298. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Sakkas D, Gardner DK. Noninvasive methods to assess embryo quality. Curr Opin Obstet Gynecol. 2005;17:283–288. doi: 10.1097/01.gco.0000169106.69881.3e. [DOI] [PubMed] [Google Scholar]
- Sakkas D, Lu C, Zulfikaroglu E, Neuber E, Taylor HS. A soluble molecule secreted by human blastocysts modulates regulation of HOXA10 expression in an epithelial endometrial cell line. Fertil Steril. 2003;80:1169–1174. doi: 10.1016/s0015-0282(03)02163-0. [DOI] [PubMed] [Google Scholar]
- Sandoval JA, Hoelz DJ, Woodruff HA, Powell RL, Jay CL, Grosfeld JL, Hickey RJ, Malkas LH. Novel peptides secreted from human neuroblastoma: useful clinical tools? J Pediatr Surg. 2006;41:245–251. doi: 10.1016/j.jpedsurg.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Sargent I, Swales A, Ledee N, Kozma N, Tabiasco J, Le Bouteiller P. sHLA-G production by human IVF embryos: can it be measured reliably? J Reprod Immunol. 2007;75:128–132. doi: 10.1016/j.jri.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Scott R, Seli E, Miller K, Sakkas D, Scott K, Burns DH. Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study. Fertil Steril. 2008;90:77–83. doi: 10.1016/j.fertnstert.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Seibert V, Wiesner A, Buschmann T, Meuer J. Surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI TOF-MS) and ProteinChip technology in proteomics research. Pathol Res Pract. 2004;200:83–94. doi: 10.1016/j.prp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Seli E, Sakkas D, Scott R, Kwok SC, Rosendahl SM, Burns DH. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2007;88:1350–1357. doi: 10.1016/j.fertnstert.2007.07.1390. [DOI] [PubMed] [Google Scholar]
- Sher G, Keskintepe L, Fisch JD, Acacio BA, Ahlering P, Batzofin J, Ginsburg M. Soluble human leukocyte antigen G expression in phase I culture media at 46 hours after fertilization predicts pregnancy and implantation from day 3 embryo transfer. Fertil Steril. 2005;83:1410–1413. doi: 10.1016/j.fertnstert.2004.11.061. [DOI] [PubMed] [Google Scholar]
- Shi CZ, Collins HW, Garside WT, Buettger CW, Matschinsky FM, Heyner S. Protein databases for compacted eight-cell and blastocyst-stage mouse embryos. Mol Reprod Dev. 1994;37:34–47. doi: 10.1002/mrd.1080370106. [DOI] [PubMed] [Google Scholar]
- Spisak S, Tulassay Z, Molnar B, Guttman A. Protein microchips in biomedicine and biomarker discovery. Electrophoresis. 2007;28:4261–4273. doi: 10.1002/elps.200700539. [DOI] [PubMed] [Google Scholar]
- Stern JE, Cedars MI, Jain T, Klein NA, Beaird CM, Grainger DA, Gibbons WE. Assisted reproductive technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril. 2007;88:275–282. doi: 10.1016/j.fertnstert.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Manandhar G, Laurincik J, Letko J, Caamaño JN, Day BN, Lai L, Prather RS, Sharpe-Timms KL, Zimmer R, et al. Expression and proteasomal degradation of the major vault protein (MVP) in mammalian oocytes and zygotes. Reproduction. 2005;129:269–282. doi: 10.1530/rep.1.00291. [DOI] [PubMed] [Google Scholar]
- Telford NA, Watson AJ, Schultz GA. Transition from maternal to embryonic control in early mammalian development: a comparison of several species. Mol Reprod Dev. 1990;26:90–100. doi: 10.1002/mrd.1080260113. [DOI] [PubMed] [Google Scholar]
- Wang HM, Zhang X, Qian D, Lin HY, Li QL, Liu DL, Liu GY, Yu XD, Zhu C. Effect of ubiquitin-proteasome pathway on mouse blastocyst implantation and expression of matrix metalloproteinases-2 and -9. Biol Reprod. 2004;70:481–487. doi: 10.1095/biolreprod.103.021634. [DOI] [PubMed] [Google Scholar]
- Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;1:1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Williamson AJ, Smith DL, Blinco D, Unwin RD, Pearson S, Wilson C, Miller C, Lancashire L, Lacaud G, Kouskoff V, et al. Quantitative proteomics analysis demonstrates post-transcriptional regulation of embryonic stem cell differentiation to hematopoiesis. Mol Cell Proteomics. 2008;7:459–472. doi: 10.1074/mcp.M700370-MCP200. [DOI] [PubMed] [Google Scholar]