Abstract
We have conducted nationwide surveys of primary central nervous system lymphoma (PCNSL) treated since 1985. In the present study, we newly collected data between 2000 and 2004 and investigated changes in clinical features and outcome over time. A total of 739 patients with histologically proven PCNSL undergoing radiotherapy were analyzed. Seventeen institutions were surveyed, and data on 131 patients were collected. These data were compared with updated data that were previously obtained for 466 patients treated during 1985–1994 and 142 patients treated during 1995–1999. Recent trends toward decrease in male/female ratio, increase in aged patients, and increase in patients with multiple lesions were seen. Regarding treatment, decrease in attempts at surgical tumor removal and increases in use of systemic chemotherapy and methotrexate (MTX)–containing regimens were observed. The median survival time was 18, 29, and 24 months for patients seen during 1985–1994, 1995–1999, and 2000–2004, respectively, and the respective 5-year survival rates were 15%, 30%, and 30%. In groups seen during 1995–1999 and during 2000–2004, patients who received systemic or MTX-containing chemotherapy had better prognosis than those who did not. Multivariate analysis of all patients seen during 1985–2004 suggested the usefulness of MTX-containing chemotherapy as well as the importance of age, lactate dehydrogenase level, and tumor multiplicity as prognostic factors. Thus, this study revealed several notable changes in clinical features of PCNSL patients. The prognosis improved during the last 10 years. Advantage of radiation plus chemotherapy, especially MTX-containing chemotherapy, over radiation alone was suggested.
Keywords: brain neoplasm, chemotherapy, lymphoma, primary CNS lymphoma, radiotherapy
Primary central nervous system lymphoma (PCNSL) is becoming one of the most important tumors in neuro-oncology. It was rare previously but has increased during the last two decades.1 With the increase in incidence, clinical features, diagnostic procedures, and physicians’ recognition and treatment policy for the disease seemed to have changed considerably. With widespread recognition of the disease and improvement of diagnostic modalities, the disease may be diagnosed more readily than before. Treatment policy appears to have also changed considerably with respect to the role of surgical resection and the use of chemotherapy and radiotherapy.2,3 Unfortunately, however, randomized studies on treatment have been scarce, and uncertainties still remain regarding the efficacy of chemotherapy, appropriate chemotherapy regimens, and appropriate use of radiation therapy.2–5 Many studies using high-dose methotrexate (MTX)–containing chemotherapy have reported favorable treatment outcome,6–17 whereas other studies have not necessarily supported the results.1,18–20 Also, high toxicity of an MTX-containing regimen has been reported.21
In view of the relative rarity but importance of the disease, we have conducted nationwide studies on it. The purposes of the studies were to analyze clinical features and treatment characteristics and evaluate patient outcomes. The first study was conducted for the patients seen between 1985 and 1994 by Hayabuchi et al.22 The next studies were conducted for those seen between 1995 and 1999 by the Japanese Society for Therapeutic Radiology and Oncology (JASTRO) Lymphoma Study Group (JLSG) and the Chubu Radiation Oncology Group (CROG) separately.23,24 Recently, we collected new data on 131 patients seen between 2000 and 2004. In this study, we analyzed all these patients from the previous and recent surveys. Follow-up data were updated as far as possible also for the patients reported in the previous studies.
Materials and Methods
Subjects of all of the surveys were patients with histologically proven PCNSL who received radiation therapy. Patients who were suspected of having secondary CNS lymphoma were excluded at each institution. Those who did not complete planned radiotherapy were included. Clinical characteristics, treatment, and prognosis of each patient shown in the Results section were asked using a detailed questionnaire. Data on 466 patients had been collected from 62 institutions for patients seen between 1985 and 1994. For the period 1995–1999, a total of 142 patients were collected from 25 institutions with the two surveys conducted by JLSG and CROG; the results were published separately,23,24 but in the present study, the data from the two surveys were combined. Recently, data on 131 patients seen between 2000 and 2004 were collected from 17 institutions belonging to JLSG or CROG. Submission of the data was approved at each institution. Thus, a total of 739 patients with histologically proven PCNSL were the subject of this study. Results for HIV titer were negative in all patients who had had the examination, and no other patients were considered to have AIDS-related PCNSL. For the most recent survey, 76% of the institutions had also been surveyed for the period 1995–1999, and 68% of the institutions surveyed for the period 1995–1999 had been included in the survey for the period 1985–1994.
Extent of surgical resection had not been asked in the survey for 1985–1994, but it was done in the subsequent surveys. Other items were common to all surveys, but because of the nature of the survey, a number of the items were unanswered by the investigators. Various chemotherapy regimens had been used, but for convenience of analysis, they were categorized as high-dose (>1 g/m2) MTX-containing regimens or other regimens. Details of other chemotherapy regimens used during 1985–1999 were described previously;22–24 58% of non-MTX-containing regimens were cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP) or similar regimens.25 In the most recent period (2000–2004), 68% of non-MTX-containing regimens were CHOP or CHOP-based regimens and 18% were a dexamethasone-etoposide-ifosfamide-carboplatin (DeVIC) regimen. The remaining 14% were miscellaneous ones.
Differences in patient, tumor, and treatment characteristics between groups were examined by Fisher’s exact test or t-test. Survival rates were calculated from the date of starting radiotherapy using the Kaplan-Meier method, and differences in pairs of survival curves were examined by the log-rank test. Multivariate analysis of prognostic factors was carried out using the Cox proportional hazards model. In doing multivariate analysis, patients were divided into two groups, and all the parameters were entered as dichotomous variables. All statistical analyses were carried out using computer programs, StatView Version 5 (SAS Institute Inc., Cary, NC, USA) and HALWIN (Gendaisuugakusha, Kyoto, Japan).
Results
Table 1 shows patient and tumor characteristics in the three groups treated during the three periods. Several remarkable changes were noted. There were more female patients in the period 2000–2004 than in the preceding period; the male/female ratio was near unity in the most recent series. Also, the median patient age was higher in the period 2000–2004 than in the preceding period. The proportion of patients with multiple tumors increased to 55% in the most recent series, whereas it was 38% and 40% in the previous series. Other patient and tumor characteristics did not differ significantly between the two pairs of groups.
Table 1.
Patient and tumor characteristics
| Period (Year)
|
|||||
|---|---|---|---|---|---|
| Characteristic | 1985–1994 | 1995–1999 | 2000–2004 | p* | |
| Gender | Male (%) | 276/466 (59) | 96/142 (68) | 67/131 (51) | 0.077 |
| 0.0066 | |||||
| Age (years) | Median (range) | 60 (5–86) | 59 (15–93) | 65 (30–90) | 0.49 |
| 0.017 | |||||
| Performance status | 3, 4 (%) | 209/438 (48) | 55/138 (40) | 37/128 (29) | 0.12 |
| 0.071 | |||||
| Lactate dehydrogenase | High (%) | 103/267 (39) | 42/113 (37) | 32/121 (26) | 0.82 |
| 0.092 | |||||
| B symptom | Yes (%) | 33/418 (7.9) | 13/127 (10) | 6/122 (4.9) | 0.47 |
| 0.15 | |||||
| Phenotype | T cell (%) | 20/234 (8.5) | 6/115 (5.2) | 2/120 (1.7) | 0.29 |
| 0.16 | |||||
| Tumor number | Multiple (%) | 175/460 (38) | 56/140 (40) | 72/131 (55) | 0.69 |
| 0.015 | |||||
| Tumor size (cm) at diagnosis | Mean ± SD | 3.8 ± 1.4 | 3.8 ± 1.6 | 3.8 ± 1.2 | 0.71 |
| 0.96 | |||||
| CSF dissemination | Yes (%) | 56/422 (13) | 23/122 (19) | 20/126 (16) | 0.14 |
| 0.62 | |||||
Abbreviation: CSF, cerebrospinal fluid.
First and second p values are for comparison between 1985–1994 and 1995–1999 data and between 1995–1999 and 2000–2004 data, respectively. B symptom: fever (>38°C for 3 consecutive days), weight loss (>10% in 6 months), and/or drenching night sweats.
Table 2 shows changes in treatment. Radiotherapy characteristics were similar in all three groups. Nearly 90% or more of the patients were treated with whole-brain irradiation with or without focal boost, and the mean total and whole-brain doses were 47–49 Gy and 36–38 Gy, respectively. Whole-spinal irradiation was employed in less than 10% of the patients throughout the three periods. However, there were steady increases in the proportion of patients undergoing systemic chemotherapy. Particularly, MTX-containing regimens have been increasingly used (in 72% of patients undergoing chemotherapy in the most recent period).
Table 2.
Treatment characteristics
| Period (Year)
|
|||||
|---|---|---|---|---|---|
| Characteristic | 1985–1994 | 1995–1999 | 2000–2004 | p* | |
| Surgery | Biopsy (%) | — | 71/142 (50) | 83/131 (63) | — |
| 0.028 | |||||
| Radiotherapy course | Not completed | 25/466 (5.4) | 6/142 (4.2) | 5/131 (3.8) | 0.67 |
| 1.0 | |||||
| Brain radiation field | Partial brain (%) | 37/466 (7.9) | 12/142 (8.5) | 15/131 (11) | 0.86 |
| 0.42 | |||||
| Spinal radiation | Yes (%) | 37/445 (8.3) | 8/142 (5.6) | 4/131 (3.1) | 0.37 |
| 0.38 | |||||
| Total dose (Gy) | Mean ± SD | 48.4 ± 11.2 | 48.7 ± 10.8 | 47.0 ± 9.0 | 0.78 |
| 0.20 | |||||
| Whole-brain dose (Gy) | Mean ± SD | 35.6 ± 13.7 | 37.5 ± 8.0 | 36.4 ± 6.0 | 0.082 |
| 0.43 | |||||
| I.v. chemotherapy | Yes (%) | 212/420 (50) | 87/142 (61) | 99/131 (76) | 0.032 |
| 0.013 | |||||
| MTX-containing regimen | Yes (%) | 47/212 (22) | 27/87 (31) | 71/99 (72) | 0.14 |
| <0.0001 | |||||
| I.t. chemotherapy | Yes (%) | 42/415 (10) | 16/142 (11) | 8/131 (6.1) | 0.75 |
| 0.14 | |||||
Abbreviations: i.v., intravenous; MTX, methotrexate; i.t., intrathecal.
First and second p values are for comparison between 1985–1994 and 1995–1999 data and between 1995–1999 and 2000–2004 data, respectively.
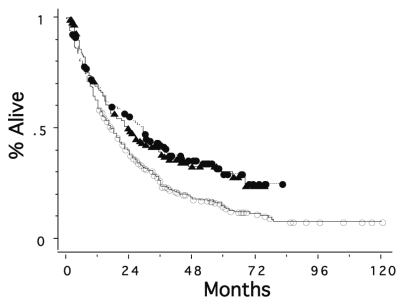
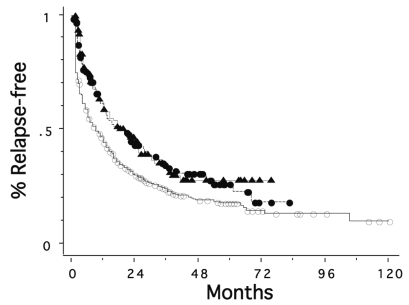
Figure 1 shows overall survival curves for the three groups. Patients treated between 1995 and 1999 and those treated between 2000 and 2004 had significantly better survival rates than those seen between 1985 and 1994 (p = 0.0004 and 0.0033, respectively); median survival time increased from 18 months to 29 and 24 months, respectively. The 5-year survival was 15%, 30%, and 30% for the periods 1985–1994, 1995–1999, and 2000–2004, respectively. Figure 2 shows relapse-free survival curves for the patients with known data on recurrence in the three periods. Relapse-free survival of the patients was also better in the more recent two periods than in the period 1985–1994 (p = 0.0020 and 0.0010, respectively). The median time to recurrence was 9, 18, and 20 months, and the 5-year relapse-free survival was 18%, 26%, and 28% for the periods 1985–1994, 1995–1999, and 2000–2004, respectively.
Fig. 1.
Survival curves for patients with primary CNS lymphoma seen in 1985–1994 (○, n = 466), in 1995–1999 (●, n = 142), and in 2000–2004 (▴, n = 131). The second and third groups had significantly better survival rates than the first group (p = 0.0004 and 0.0033, respectively).
Fig. 2.
Relapse-free survival curves for patients with primary CNS lymphoma seen in 1985–1994 (○, n = 408), in 1995–1999 (●, n = 137), and in 2000–2004 (▴, n = 127). The second and third groups had significantly better relapse-free survival rates than the first group (p = 0.0020 and 0.0010, respectively).
Table 3 summarizes survival data in the three groups according to patient- and tumor-related potential prognostic factors. In all the study periods, patients with age <60 years, WHO performance status (PS) of 0–2, or normal lactate dehydrogenase (LDH) level had significantly higher survival rates. Patients without B symptom or with a single tumor had better prognoses than those with B symptom or with multiple tumors, respectively, in the groups treated between 1985 and 1994 and between 1995 and 1999, but the trends were not seen in the most recent series.
Table 3.
Survival data according to patient or tumor-related potential prognostic factors
| 1985–1994
|
1995–1999
|
2000–2004
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prognostic Factor | n | MST (Months) | 5-YSR (%) | p | n | MST (Months) | 5-YSR (%) | p | n | MST (Months) | 5-YSR (%) | p | |
| Gender | Male | 276 | 17 | 17 | 0.92 | 96 | 30 | 31 | 0.23 | 67 | 17 | 29 | 0.40 |
| Female | 190 | 20 | 13 | 46 | 23 | 27 | 64 | 26.5 | 32 | ||||
| Age | <60 | 216 | 22 | 27 | <0.0001 | 75 | 39 | 40 | 0.0011 | 44 | 68 | 53 | 0.0001 |
| ≥60 | 250 | 14 | 5.2 | 67 | 16 | 17 | 87 | 17 | 18 | ||||
| Performance status | 0–2 | 229 | 24 | 20 | <0.0001 | 83 | 37 | 32 | 0.0024 | 91 | 26.5 | 40 | 0.0010 |
| 3, 4 | 209 | 12 | 10 | 55 | 12 | 27 | 37 | 13 | 5.8 | ||||
| B symptom | Yes | 33 | 10 | 0 | 0.030 | 13 | 13 | 15 | 0.0093 | 6 | 19 | 21 | 0.72 |
| No | 385 | 18 | 17 | 116 | 35 | 34 | 116 | 25 | 36 | ||||
| Lactate dehydrogenas | Normal | 164 | 22 | 26 | 0.0007 | 71 | 38 | 36 | 0.016 | 89 | 35 | 37 | 0.0024 |
| e | High | 103 | 14 | 5.7 | 42 | 16 | 25 | 32 | 13 | 15 | |||
| Tumor number | Single | 285 | 22 | 18 | 0.0012 | 84 | 39 | 36 | 0.026 | 59 | 24 | 38 | 0.72 |
| Multiple | 175 | 12 | 11 | 56 | 23 | 21 | 72 | 22 | 24 | ||||
| Tumor size (cm)* | ≤4 cm | 204 | 19 | 14 | 0.84 | 69 | 29 | 29 | 0.38 | 61 | 26.5 | 31 | 0.21 |
| >4 cm | 189 | 17 | 19 | 63 | 37 | 34 | 69 | 18.5 | 31 | ||||
| CSF dissemination | Yes | 56 | 10 | 14 | 0.039 | 23 | 50 | 33 | 0.50 | 20 | 32 | 37 | 0.74 |
| No | 366 | 19 | 16 | 99 | 29 | 32 | 106 | 24.5 | 31 | ||||
Abbreviations: MST, median survival time; 5-YSR, 5-year survival rate; CSF, cerebrospinal fluid.
Maximum tumor diameter at diagnosis.
To analyze the influence of treatment-related factors on outcome, patients who did not complete radiotherapy (receiving less than 30 Gy) and died soon were excluded. Table 4 shows survival data according to the treatment-related factors; no factors were found to be associated with improved prognosis throughout all three periods. In groups treated during 1995–1999 and during 2000–2004, patients receiving systemic chemotherapy had better survival rates than those treated with radiation alone, and those who received MTX-containing chemotherapy had better prognosis than those who did not. However, these phenomena were not observed in patients treated during the preceding decade. In the most recent series, patients receiving whole-brain doses less than 40 Gy (including those treated with partial-brain fields alone) did better than those receiving higher doses. No other treatment-related factors were found to be associated with prognosis in univariate analysis. Figure 3 shows survival curves for patients receiving or not receiving chemotherapy during the three periods. In the two more recent periods, patients receiving chemotherapy had better survival rates than those receiving radiation alone (p < 0.0001 and = 0.0006, respectively). Figure 4 shows survival curves according to the chemotherapy regimen (with or without high-dose MTX). Although there were no differences by the use of MTX, there was a trend toward improved survival in patients undergoing high-dose MTX-containing chemotherapy during the period 1995–1999 (p = 0.060).
Table 4.
Survival data according to treatment-related factors
| 1985–1994
|
1995–1999
|
2000–2004
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prognostic Factor | n | MST (Months) | 5-YSR (%) | p | n | MST (Months) | 5-YSR (%) | p | n | MST (Months) | 5-YSR (%) | p | |
| Surgical resection | Extensive | — | — | — | — | 32 | 28 | 32 | 0.99 | 21 | 23 | 27 | 0.45 |
| Nonextensive | — | — | — | 104 | 29 | 29 | 105 | 24 | 29 | ||||
| Radiation field | Whole brain | 405 | 19 | 15 | 0.72 | 126 | 29 | 29 | 0.72 | 111 | 22 | 26 | 0.17 |
| Partial brain | 34 | 16 | 17 | 10 | 35 | 38 | 15 | 37 | 47 | ||||
| Spinal radiation | Yes | 36 | 24 | 19 | 0.16 | 8 | — | 50* | 0.76 | 3 | — | 67 | 0.23 |
| No | 384 | 18 | 15 | 128 | 29 | 29 | 123 | 23 | 27 | ||||
| Total dose (Gy) | <50 | 134 | 18 | 17 | 0.97 | 35 | 30 | 34 | 0.79 | 45 | 26 | 35 | 0.71 |
| ≥50 | 305 | 8 | 16 | 101 | 29 | 29 | 81 | 23 | 27 | ||||
| Whole-brain dose (Gy) | <40 | 156 | 18 | 18 | 0.43 | 54 | 30 | 32 | 0.68 | 54 | 36 | 36 | 0.048 |
| ≥40 | 283 | 18 | 14 | 82 | 29 | 27 | 72 | 18.5 | 22 | ||||
| I.v. chemotherapy | Yes | 202 | 20 | 16 | 0.30 | 85 | 39 | 39 | <0.0001 | 95 | 26.5 | 39 | 0.0006 |
| No | 192 | 16 | 17 | 51 | 14 | 14 | 31 | 14.5 | 7.5 | ||||
| MTX-containing chemotherapy | Yes | 46 | 20 | 19 | 0.49 | 25 | NR | 54 | 0.0039 | 67 | 29 | 47 | 0.0016 |
| No | 348 | 18 | 16 | 111 | 25 | 24 | 59 | 16.5 | 12 | ||||
| I.t. chemotherapy | Yes | 39 | 16 | 20 | 0.78 | 15 | — | 58 | 0.13 | 7 | 24 | 43 | 0.52 |
| No | 350 | 19 | 16 | 114 | 27 | 26 | 119 | 23 | 27 | ||||
Abbreviations: MST, median survival time; 5-YSR, 5-year survival rate; i.v., intravenous; MTX, methotrexate; NR, not reached; i.t., intrathecal.
4-year survival rate.
Fig. 3.
Survival curves for patients with or without systemic chemotherapy. ○: chemotherapy (+) (n = 202, 85, and 95 for the three periods, respectively); ●: chemotherapy (−) (n = 192, 51, and 31 for the three periods, respectively). The difference was significant in the second and third groups (p = <0.0001 and 0.0006, respectively).
Fig. 4.
Survival curves according to chemotherapy regimens. ○: high-dose methotrexate-containing regimens (n = 46, 25, and 67 for the three periods, respectively); ●: other regimens (n = 156, 60, and 28 for the three periods, respectively). The p values were 0.66, 0.060, and 0.13, respectively, for the three periods.
To further analyze the effect of chemotherapy, patients seen during 1995–1999 and 2000–2004 were combined and those with ages >70 years and PS 3 or 4 were excluded in addition to those receiving radiation doses of less than 30 Gy, because these patients are often not indicated for intensive systemic chemotherapy. Figure 5 shows survival curves for patients with or without chemotherapy and according to the chemotherapy regimens. In this selected group of patients, those receiving systemic chemotherapy had markedly better survival rates than those receiving radiation alone (p = 0.0004), and those receiving MTX-containing chemotherapy had better survival than those receiving other regimens of chemotherapy (p = 0.049). However, in patients seen during 1995–2004 with ages >70 years and/or PS 3 or 4 who received radiation doses of 30 Gy or higher, those receiving systemic chemotherapy had better survival rates than those receiving radiation alone (median survival: 19.5 vs. 8.5 months; 5-year survival: 24% vs. 4.9%; p = 0.010), whereas those receiving MTX-containing chemotherapy and those receiving other regimens had similar prognosis (median survival: 22 vs. 16.5 months; 5-year survival: 32% vs. 20%; p = 0.57).
Fig. 5.
Survival curves for patients with or without chemotherapy and according to chemotherapy regimens in patients seen between 1995 and 2004 with WHO performance status of 0–2 and ages <70 years receiving radiation doses of 30 Gy or higher. Left panel, ○: chemotherapy (+) (n = 108); ●: chemotherapy (−) (n = 31); p = 0.0004. Right panel, ○: high-dose methotrexate-containing regimens (n = 56); ●: other regimens (n = 52); p = 0.049.
Multivariate analyses were carried out for potential prognostic factors that were significant in univariate analyses (Table 5). Analyses were carried out for patients seen during 1995–1999 and 2000–2004 and for all the patients combined. Multivariate analysis for patients during 1985–1994 was carried out previously.22 In the patient group seen between 1995 and 1999, lower age, better PS, absence of B symptom, and single tumor number were associated with better survival. In the group treated during 2000–2004, lower age, normal LDH level, and lower whole-brain dose were associated with better survival. When all patients were combined, age, tumor number, LDH level, and use of MTX-containing chemotherapy were significant factors.
Table 5.
Multivariate analyses for potential prognostic factors that were significant in univariate analysis
| 1995–1999 (n = 98)
|
2000–2004 (n = 114)
|
1985–2004 (n = 413)
|
||||
|---|---|---|---|---|---|---|
| Factor | p | Relative Risk | p | Relative Risk | p | Relative Risk |
| Age (<60 vs. ≥60 years) | 0.0028 | 0.40 (0.22–0.73)* | 0.0010 | 0.36 (0.20–0.66) | <0.0001 | 0.53 (0.41–0.68) |
| Performance status (0–2 vs. 3, 4) | 0.016 | 0.47 (0.26–0.87) | 0.34 | 0.78 (0.46–1.31) | 0.12 | 0.82 (0.64–1.06) |
| B symptom (− vs. +) | 0.010 | 0.33 (0.14–0.77) | — | — | 0.18 | 0.75 (0.49–1.15) |
| Lactate dehydrogenase (normal vs. high) | 0.24 | 0.71 (0.40–1.26) | 0.020 | 0.55 (0.33–0.91) | 0.0001 | 0.61 (0.47–0.78) |
| Tumor number (single vs. multiple) | 0.0025 | 0.41 (0.23–0.73) | — | — | 0.017 | 0.74 (0.57–0.95) |
| CSF dissemination (− vs. +) | — | — | — | — | 0.82 | 1.04 (0.72–1.51) |
| Whole-brain dose (<40 vs. ≥40 Gy) | — | — | 0.0019 | 0.42 (0.24–0.72) | 0.31 | 0.88 (0.68–1.13) |
| I.v. chemotherapy (− vs. +) | 0.87 | 1.05 (0.56–1.99) | 0.088 | 1.81 (0.92–3.56) | 0.69 | 1.06 (0.81–1.38) |
| MTX-containing chemotherapy (− vs. +) | 0.082 | 2.08 (0.91–4.74) | 0.52 | 1.23 (0.66–2.30) | 0.0014 | 1.82 (1.26–2.63) |
Abbreviation: CSF, cerebrospinal fluid; i.v., intravenous; MTX, methotrexate.
Figures in parentheses are 95% confidence intervals.
Discussion
PCNSL has been increasing and is becoming an important tumor in neuro-oncology. So, it was considered meaningful to survey data on PCNSL in our country every 5 years. Various changes have been noted with regard to patient and tumor characteristics. The reason for the decrease of the proportion of male patients to nearly 50% observed in this study is unknown, and the phenomenon is in contrast to that observed in other studies showing male preponderance.1,26 Further studies will clarify whether this trend is a universal one. The increase of aged patients may be due to recent better recognition of the disease; previously, aged patients might have remained undiagnosed, but with the recent establishment of managing PCNSL, the proportion of aged patients undergoing biopsy appeared to have increased. The recent increase in the incidence of multiple tumors is striking; it was as high as 55% in the most recent period, whereas it was between 30% and 40% in the two previous periods as well as in most previous reports.7,21,26,27 Probably, improvement in imaging modalities and techniques, including more frequent use of MRI, has contributed to the improved detection of small tumors.
Regarding treatment, attempts at resection of tumors have decreased, because it is now clear that surgical resection does not contribute to improved prognosis.3 This was also suggested in the present study. No major changes appeared to have occurred regarding radiotherapy. Shibamoto et al.28 suggested the possible use of partial-brain radiation for a solitary lesion, but the idea has not yet spread nationwide. To reduce radiation doses by using chemotherapy has not become popular in our country. Increased use of systemic chemotherapy, especially MTX-based regimens, appears to be a worldwide trend, and it was confirmed in the present study.
Prognosis of PCNSL patients has certainly improved during the last decade. The 5-year survival was 30% in two periods: 1995–1999 and 2000–2004. However, further improvement was not observed in the latter period as compared with the former period, despite the fact that more patients underwent MTX-containing chemotherapy. One reason for this observation may be the higher patient age in the newest period (median: 65 vs. 59 years). In addition, prognosis of patients undergoing MTX-containing chemotherapy appears to be poorer in the newest period than in the preceding period, suggesting that some patients who were not necessarily expected to benefit from MTX-containing chemotherapy were treated with the chemotherapy. Furthermore, patients who did not receive MTX-containing chemotherapy in the most recent period had poorer prognosis. This would suggest that many patients regarded as ineligible for MTX-containing chemotherapy were not in favorable conditions and had poor prognosis. As a result, the increase of patients undergoing MTX-containing chemotherapy in the most recent period did not lead to improved survival when all patients were analyzed.
Age, PS, and tumor multiplicity are well-known prognostic factors for PCNSL,22,23,27,29 and high LDH level and presence of B symptom or cerebrospinal fluid dissemination may also adversely affect prognosis.19,22,26,30 The present study with a large number of patients seen between 1985 and 2004 suggested that age and LDH level are the most important prognostic factors followed by tumor number. However, tumor multiplicity was not associated with prognosis in the most recent series; the increase in patients with multiple tumors might be a reason for this discrepancy.
Chemotherapy is being increasingly used in the treatment of PCNSL, as is some advocate deferred radiation therapy in elderly patients.2,16 Since the questionnaires were sent to radiation oncologists in the present study, all patients had received radiation. To our knowledge, however, very few patients are treated by chemotherapy alone in Japan, and results of the present study appear to represent the status of treatment for PCNSL in our country. The prognosis of the patients did not differ significantly by the radiation field and total dose. Partial-brain radiation was not associated with decreased survival. Moreover, patients receiving whole-brain doses lower than 40 Gy had better survival than those receiving higher doses; those treated with partial-brain fields are included in the former group. Reni et al.31 reported that whole-brain doses of 40 Gy or higher were associated with better prognosis, but later the same group found that they did not seem to improve prognosis.11 The result of the present study would suggest that the whole-brain dose may not be important, and it is not contradictory to the proposal made by Shibamoto et al.28 that partial-brain irradiation may be considered, especially in patients with a single lesion. With respect to the total radiation dose, Nelson et al.32 did not find improved survival with the use of 60 Gy versus 50 Gy. Comparing two series of prospective studies, however, Bessel et al.33 reported that a dose of 45 Gy appeared to be better than 30.6 Gy. In the present study, influence of total radiation dose was not clear when 50-Gy or higher doses were compared with lower doses. It seems difficult to draw any conclusions on optimal radiotherapy from retrospective studies; prospective studies should provide better answers.
There have been no randomized studies showing the benefit of chemotherapy as compared with radiation alone. A small randomized study failed to show the efficacy of CHOP chemotherapy when added to radiotherapy.34 In the present study, the effect of chemotherapy, especially MTX-containing chemotherapy, was suggested in patients treated between 1995 and 1999 and between 2000 and 2004. The effect of MTX-containing chemotherapy was supported by multivariate analysis of patients seen during the 20-year period. In addition, patients receiving MTX-based regimens had better prognosis than those receiving other regimens in the group seen during 1995–2004 with ages <70 years and PS 0–2. The discrepancy from the results of the preceding decade may be due to improvement of chemotherapy. Although full details of chemotherapy regimens were not necessarily reported in many patients, especially in the oldest survey, MTX-containing regimens in the oldest period appeared to be less intensive than those used in the more recent periods. Another reason may be improvement of management of patients undergoing chemotherapy. Before 1994, MTX-containing chemotherapy was not popular in our country and neuro-oncologists might not have been familiar with performing it. Shibamoto et al.30 recently reported an improved survival for patients undergoing radiotherapy alone in the 1990s with a 5-year survival of 18%, but in the present study, patients undergoing MTX-based chemotherapy and radiation had a 5-year survival of around 50% during 1995–2004. The lack of randomized trials regarding the effect of MTX-based regimens is a flaw in neuro-oncology, but at the present time, conducting a randomized study of radiation versus radiochemotherapy may not be possible in view of the results of the present as well as other phase II studies.
Acknowledgments
This work was supported in part by a research grant from JASTRO. The authors wish to thank Drs. Miwako Nomura, Jun-ichi Hiratsuka, and Yoshimi Horikawa for valuable help in collecting data. JASTRO and CROG authors’ affiliations are Kurume University (G.S., N.H.), Okayama University (M.T.), Kyoto University (N.A.), Chiba University (K.I.), Niigata University (E.T.), Kyushu University (K.N.), Hiroshima University (M.K.), Hamamatsu University (K.S.), Nagoya University (S.I.), Osaka City University (M.H.), Saga University (S.T.), Nagoya Medical Center (E.K.), and Mie University (N.I.).
References
- 1.Panageas KS, Elkin EB, DeAngelis LM, Ben-Porat L, Abrey LE. Trends in survival from primary central nervous system lymphoma, 1975–1999: a population-based analysis. Cancer. 2005;104:2466–2472. doi: 10.1002/cncr.21481. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis LM. Primary central nervous system lymphoma: a curable brain tumor. J Clin Oncol. 2003;21:4471–4473. doi: 10.1200/JCO.2003.08.900. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281–1288. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- 4.Nelson DF. Radiotherapy in the treatment of primary central nervous system lymphoma (PCNSL) J Neurooncol. 1999;43:241–247. doi: 10.1023/a:1006206602918. [DOI] [PubMed] [Google Scholar]
- 5.Bessell EM, Hoang-Xuan K, Ferreri AJ, Reni M. Primary central nervous system lymphoma: biological aspects and controversies in management. Eur J Cancer. 2007;43:1141–1152. doi: 10.1016/j.ejca.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Glass J, Gruber ML, Chef L, Hochberg FH. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg. 1994;81:188–195. doi: 10.3171/jns.1994.81.2.0188. [DOI] [PubMed] [Google Scholar]
- 7.Blay JY, Conroy T, Chevreau C, et al. High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. J Clin Oncol. 1998;16:864–871. doi: 10.1200/JCO.1998.16.3.864. [DOI] [PubMed] [Google Scholar]
- 8.Brada M, Hjiyiannakis D, Hines F, Traish D, Ashley S. Short intensive primary chemotherapy and radiotherapy in sporadic primary CNS lymphoma. Int J Radiat Oncol Biol Phys. 1998;40:1157–1162. doi: 10.1016/s0360-3016(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJM, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma: lessons from prospective trials. Ann Oncol. 2000;11:927–937. doi: 10.1023/a:1008376412784. [DOI] [PubMed] [Google Scholar]
- 10.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 11.Reni M, Ferreri AJ, Guha-Thakurta N, et al. Clinical relevance of consolidation radiotherapy and other main therapeutic issues in primary central nervous system lymphomas treated with upfront high-dose MTX. Int J Radiat Oncol Biol Phys. 2001;51:419–425. doi: 10.1016/s0360-3016(01)01639-x. [DOI] [PubMed] [Google Scholar]
- 12.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Bessel EM, Graus F, Lopez-Guillermo A, et al. Primary non-Hodgkin’s lymphoma of the CNS treated with CHOD/BVAM or BVAM chemotherapy before radiotherapy: long-term survival and prognostic factors. Int J Radiat Oncol Biol Phys. 2004;59:501–508. doi: 10.1016/j.ijrobp.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien PC, Roos DE, Pratt G, et al. Combined-modality therapy for primary central nervous system lymphoma: long-term data from a Phase II multicenter study (Trans-Tasman Radiation Oncology Group) Int J Radiat Oncol Biol Phys. 2006;64:408–413. doi: 10.1016/j.ijrobp.2005.07.958. [DOI] [PubMed] [Google Scholar]
- 15.Laack NN, Ballman KV, Brown PB, O’Neill BP. Whole-brain radiotherapy and high-dose methylprednisolone for elderly patients with primary central nervous system lymphoma: results of North Central Cancer Treatment Group (NCCTG) 96-73-51. Int J Radiat Oncol Biol Phys. 2006;65:1429–1439. doi: 10.1016/j.ijrobp.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 16.Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24:4570–4574. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 17.Kiewe P, Loddenkemper C, Anagnostopoulos I, Reinwald M, Thiel E, Korfel A. High-dose methotrexate is beneficial in parenchymal brain masses of uncertain origin suspicious for primary CNS lymphoma. Neuro-oncol. 2007;9:96–102. doi: 10.1215/15228517-2006-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrlinger U, Kuker W, Uhl M, et al. NOA-03 trial of high-dose methotrexate in primary central nervous system lymphoma: final report. Ann Neurol. 2005;57:843–847. doi: 10.1002/ana.20495. [DOI] [PubMed] [Google Scholar]
- 19.Shenkier TN, Voss N, Chhanabhai M, et al. The treatment of primary central nervous system lymphoma in 122 immunocompetent patients: a population-based study of successively treated cohorts from the British Colombia Cancer Agency. Cancer. 2005;103:1008–1017. doi: 10.1002/cncr.20868. [DOI] [PubMed] [Google Scholar]
- 20.Mathew BS, Carson KA, Grossman SA. Initial response to glucocorticoids: a potentially important prognostic factor in patients with primary CNS lymphoma. Cancer. 2006;106:383–387. doi: 10.1002/cncr.21583. [DOI] [PubMed] [Google Scholar]
- 21.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483–4488. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]
- 22.Hayabuchi N, Shibamoto Y, Onizuka Y, et al. Primary central nervous system lymphoma in Japan: a nationwide survey. Int J Radiat Oncol Biol Phys. 1999;44:265–272. doi: 10.1016/s0360-3016(98)00564-1. [DOI] [PubMed] [Google Scholar]
- 23.Shibamoto Y, Tsuchida E, Seki K, et al. Primary central nervous system lymphoma in Japan 1995–1999: changes from the preceding 10 years. J Cancer Res Clin Oncol. 2004;130:351–356. doi: 10.1007/s00432-004-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamura T, Ishiguchi T, Shibamoto Y, et al. Results of primary central nervous system lymphoma treated by radiation and chemotherapy: retrospective analysis of twelve institutions in the Tokai district in Japan, 1995–1999. Radiat Med. 2006;24:9–16. doi: 10.1007/BF02489983. [DOI] [PubMed] [Google Scholar]
- 25.Shibamoto Y, Sasai K, Oya N, Hiraoka M. Systemic chemotherapy with vincristine, cyclophosphamide, doxorubicin and prednisolone following radiotherapy for primary central nervous system lymphoma: a phase II study. J Neurooncol. 1999;42:161–167. doi: 10.1023/a:1006106530795. [DOI] [PubMed] [Google Scholar]
- 26.Ferreri AJM, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 27.Corry J, Smith JG, Wirth A, Quong G, Liew KH. Primary central nervous system lymphoma: age and performance status are more important than treatment modality. Int J Radiat Oncol Biol Phys. 1998;41:615–620. doi: 10.1016/s0360-3016(97)00571-3. [DOI] [PubMed] [Google Scholar]
- 28.Shibamoto Y, Hayabuchi N, Hiratsuka J, et al. Is whole-brain irradiation necessary for primary central nervous system lymphoma? Patterns of recurrence following partial-brain irradiation. Cancer. 2003;97:128–133. doi: 10.1002/cncr.11035. [DOI] [PubMed] [Google Scholar]
- 29.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 30.Shibamoto Y, Ogino H, Hasegawa M, et al. Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int J Radiat Oncol Biol Phys. 2005;62:809–813. doi: 10.1016/j.ijrobp.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 31.Reni M, Ferreri AJM, Garancini MP, Villa E. Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol. 1997;8:227–234. doi: 10.1023/a:1008201717089. [DOI] [PubMed] [Google Scholar]
- 32.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 33.Bessel EM, Lopez-Guillermo A, Villa S, et al. Importance of radiotherapy in the outcome of patients with primary CNS lymphoma: an analysis of the CHOD/BVAM regimen followed by two different radiotherapy treatments. J Clin Oncol. 2001;20:231–236. doi: 10.1200/JCO.2002.20.1.231. [DOI] [PubMed] [Google Scholar]
- 34.Mead GM, Bleehen NM, Gregor A, et al. A Medical Research Council randomized trial in patients with primary central non-Hodgkin’s lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer. 2000;89:1359–1370. [PubMed] [Google Scholar]