Abstract
People with neurofibromatosis 1 (NF1) have multiple benign neurofibromas and a 10% lifetime risk of developing malignant peripheral nerve sheath tumors (MPNSTs). Most MPNSTs develop from benign plexiform neurofibromas, so the burden of benign tumors may be a risk factor for developing MPNST. We studied 13 NF1 patients with MPNSTs and 26 age- and sex-matched controls (NF1 patients who did not have MPNSTs) with detailed clinical examinations and whole-body MRI to characterize their body burden of internal benign neurofibromas. Internal plexiform neurofibromas were identified in 22 (56%) of the 39 NF1 patients studied. All six of the NF1 patients with MPNSTs under 30 years of age had neurofibromas visualized on whole-body MRI, compared to only 3 of 11 matched NF1 controls under age 30 (p < 0. 05). Both the median number of plexiform neurofibromas (p < 0.05) and the median neurofibroma volume (p < 0.01) on whole-body MRI were significantly greater among MPNST patients younger than 30 years of age than among controls. No significant differences in whole-body MRI findings were observed between NF1 patients with MPNSTs and controls who were 30 years of age or older. Whole-body MRI of NF1 patients allows assessment of the burden of internal neurofibromas, most of which are not apparent on physical examination. Whole-body imaging of young NF1 patients may allow those at highest risk for developing MPNST to be identified early in life. Close surveillance of these high-risk patients may permit earlier diagnosis and more effective treatment of MPNSTs that develop.
Keywords: malignant peripheral nerve sheath tumor, MRI, neurofibromas, neurofibromatosis 1
Neurofibromatosis 1 (NF1) is an autosomal-dominant disease caused by mutations of the NF1 tumor-suppressor gene.1–3 The cardinal feature of NF1, and the one that gives the disease its name, is multiple cutaneous neurofibromas. NF1 patients may also develop subcutaneous neurofibromas, nodular plexiform neurofibromas, and diffuse plexiform neurofibromas.4,5 Nodular and diffuse plexiform neurofibromas may occur anywhere in the body, but many are internal and therefore not apparent on physical examination. At least 40% of adults with NF1 have internal plexiform neurofibromas that can be seen on CT or MRI examination, although most of these tumors are asymptomatic.6,7
People with NF1 also have a risk of developing connective tissue malignancy that is more than 100 times greater than expected8; most of these soft tissue sarcomas are malignant peripheral nerve sheath tumors (MPNSTs). The lifetime risk of MPNST among NF1 patients has been estimated to be about 10%.9 Most MPNSTs in people with NF1 are diagnosed in adolescence or early adulthood rather than in late adulthood,9 as occurs in the general population.10
The risk of developing MPNST appears to be higher in some NF1 patients than in others. People with NF1 whose pathogenic mutation is a deletion of the whole NF1 gene are thought to have a 16%–25% chance of developing MPNSTs.11 Other factors that may be associated with a higher risk of developing MPNST in people with NF1 are the occurrence of neurofibromatous neuropathy,12,13 exposure to therapeutic radiation,9 previous occurrence of MPNST,13,14 or the occurrence of MPNST in a relative with NF1.15–17
Tucker et al.18 found an association of MPNSTs with the presence of subcutaneous neurofibromas in a clinical study of 464 probands with NF1 (odds ratio = 2.8, 95% confidence interval 1.1–6.9). One hundred forty-one individuals included in the Tucker et al. study had routine chest radiographs or abdominal ultrasound examinations to screen for internal tumors. A very strong association was observed between the presence of internal neurofibromas and MPNSTs in these patients (odds ratio = 18.1, 95% confidence interval 4.6–73.4, after adjustment for age and subcutaneous neurofibromas). The imaging methods used in the Tucker et al. study provided information about only part of the total body burden of benign tumors in patients who developed MPNSTs, and in many of these cases the malignancy developed in an area other than the one that had been imaged.18
To evaluate the relationship of the total body burden of internal neurofibromas to MPNSTs more fully, we performed whole-body MRI on 13 NF1 patients with MPNSTs and 26 controls matched for age and sex who had NF1 but did not have MPNSTs. We found an association between the presence of MPNSTs and higher body burdens of benign internal neurofibromas on MRI among young NF1 patients.
Materials and Methods
Patients
Patients with NF1 diagnosed by the NIH Diagnostic Criteria19,20 and followed in the University Hospital Eppendorf Neurofibromatosis Clinic during 2004 and 2005 were invited to participate in an imaging study to define their body burden of benign neurofibromas. Fourteen of the NF1 patients seen during this time had histologically confirmed MPNSTs, and all 14 agreed to participate. During the same period, 77 NF1 patients older than 2 years of age who were not known or suspected to have MPNSTs were invited to take part in the study, and 66 agreed to do so.
All patients were screened for large deletions of the NF1 locus by testing for heterozygosity of six intragenic microsatellite markers and one 3′ flanking microsatellite marker as previously described.21 Deletions identified by marker screening were confirmed by fluorescence in situ hybridization.22 Ten of the 91 patients who volunteered for our study had NF1 whole-gene deletions as their constitutional mutations. These 10 subjects (1 with a MPNST and 9 without MPNSTs) were eliminated from our study because constitutional whole-gene deletions of NF1 are associated with unusually large benign tumor burdens and an increased risk of MPNST.11,23,24
We matched two NF1 patients without MPNSTs as controls to each of the 13 remaining NF1 patients with MPNSTs by sex and age ±3 years. If more than two matching controls were available for a particular MPNST patient, random numbers were electronically assigned to each subject, and the two matching controls whose random numbers were closest to that of the MPNST patient were selected. All participants provided informed consent to take part in the study, which was approved by the institutional human experimentation committee.
Each patient was evaluated clinically by one of the authors (V-F.M), who has extensive experience in caring for patients with NF1. Complete medical history and physical examination were performed with particular attention to features of NF1, and the numbers of cutaneous, subcutaneous, and externally visible plexiform neurofibromas in each patient were counted (if <1000) or estimated (if ⩾1000). Cutaneous and subcutaneous neurofibromas were differentiated by the fact that cutaneous tumors move with the skin, whereas the skin can be moved over the top of subcutaneous tumors.
MRI and Estimation of Whole-Body Tumor Volume
The whole-body MRI protocol utilized a 1.5 tesla magnet, integrated body coil, and sequences with and without intravenous contrast. The subject was imaged in supine position from head to knee in four steps (head, thorax, abdomen, and legs) in accordance with the maximum range of table movement (Fig. 1). The head was imaged with contrast using T1 and T2 spin echo sequences and T1 sequences with and without spectral fat saturation. The body was imaged without contrast using T1 gradient echo sequences and T2 STIR (short T1 inversion recovery) technique in the axial and coronal plane and with contrast using T1 gradient echo sequences with spectral fat saturation in the axial plane. Slice thickness was 5–10 mm with no skips between slices.
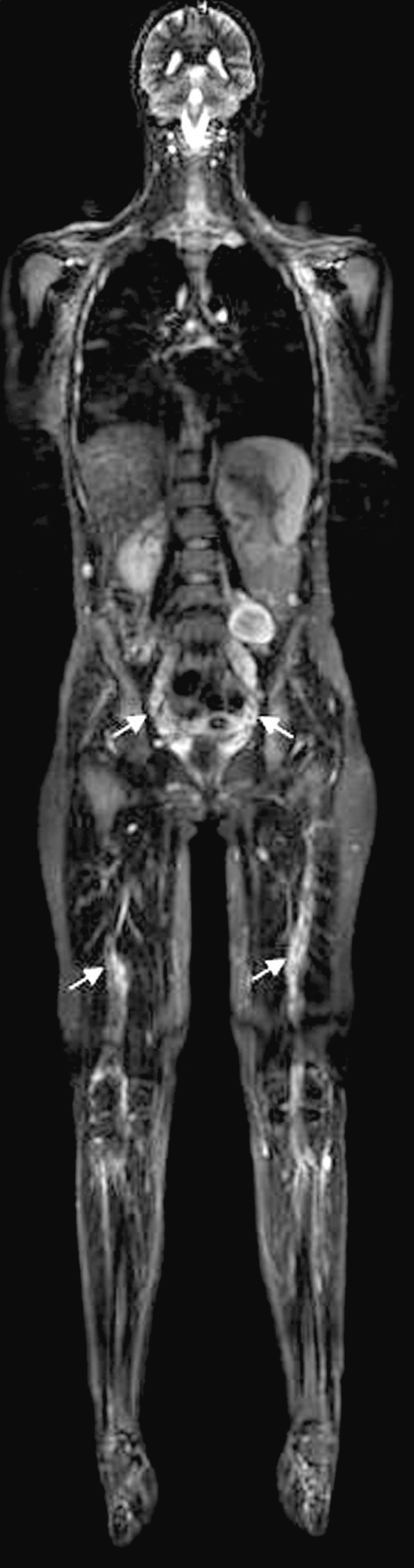
Fig. 1.
Coronal STIR (short T1 inversion recovery) image from whole-body MRI of an asymptomatic 27-year-old female with neurofibromatosis 1. Note deep nodular plexiform neurofibromas of lumbosacral region and both sciatic nerves (arrows).
MRI analysis was performed by a physician trained in image analysis of NF1-associated tumors. The analysis was done without any other information regarding the patients, all of whom were unknown to the reader. Plexiform neurofibroma volume was determined using a previously described method on the MEDx software platform.25 The method is based on:
Contrast, defined by intensity in the tumor (high signal intensity) compared with the surrounding tissue (low signal intensity),
Intensity gradient, defining the outside border (margin) of the lesion, and
Size of the lesion: plexiform neurofibromas are usually substantial in size, and small, isolated areas of high signal intensity can be ignored because their contribution to the total plexiform neurofibroma volume is insignificant.
The method used for this automated volumetric analysis is sensitive (it detects volume changes as small as 10%), reproducible (coefficient of variation 0.6%–5.6%), and it produces results similar to manual tumor tracings (R = 0.999). When automated tumor volume measurement was not feasible, the reason was recorded and manual tumor tracings with the MEDx software drawing tool were used to define tumor volume as previously described.25 Using this method, we could reliably measure plexiform neurofibromas that were ⩾3 cm in greatest diameter. The volumes of all plexiform neurofibromas that were ⩾3 cm in greatest diameter were summed to get the total body plexiform neurofibroma volume. Volumetric analysis was not done on lesions that were <3 cm in greatest diameter.
The resolution of the whole-body scan allowed for only limited evaluation of spinal neurofibromas. When visible, spinal neurofibromas were classified by location (cervical, thoracic, or lumbar spine) and size. The volume of spinal neurofibromas.>3 cm in greatest diameter was calculated and included in the total plexiform neurofibroma volume.
Statistical Analyses
The distributions of age, tumor numbers, and tumor size were highly skewed, so comparisons of these variables between the NF1 patients with MPNSTs and matched control subjects were made using the nonparametric Mann-Whitney U test. Frequencies of occurrence of tumors of various kinds identified on MRI were compared between groups of NF1 patients using Fisher’s exact test for 2 × 2 comparisons and Pearson’s χ2 test when there were multiple categories.
Results
We studied 13 patients with NF1 and MPNSTs and 26 age- and sex-matched controls (NF1 patients who were not known to have or suspected of having MPNSTs). Detailed clinical examination and whole-body MRI were performed on each subject. Clinical features of the patients with MPNSTs are summarized in the Supplementary Table. The numbers of cutaneous neurofibromas and external plexiform neurofibromas among the NF1 patients with MPNSTs and controls were similar, but the median number of subcutaneous neurofibromas was significantly greater among NF1 patients with MPNSTs than among controls (Table 1). The presence of internal neurofibromas, median number of measurable internal neurofibromas, and total volume of internal neurofibromas on whole-body MRI did not differ significantly between NF1 patients with MPNSTs and controls without MPNSTs overall (Table 2).
Table 1.
Comparison of NF1 patients with MPNSTs and matched control NF1 patients without MPNSTs
| Group | NF1 Cases with MPNSTs | NF1 Controls without MPNSTs |
|---|---|---|
| Total number | 13 | 26 |
| Median age, years (range) | 30 (3–62) | 30.5 (2–63) |
| Number of males: females | 6:7 | 12:14 |
| Median number of externally visible plexiform neurofibromas (range) | 1 (0–10) | 1 (0–5) |
| Median number of subcutaneous neurofibromas (range) | 40* (0–2000) | 13.5* (0–1000) |
| Median number of cutaneous neurofibromas (range) | 10 (0–5000) | 12 (0–1000) |
Abbreviations: NF1, neurofibromatosis 1; MPNSTs, malignant peripheral nerve sheath tumors.
p = 0.018 (Mann-Whitney U test).
Table 2.
Comparison of whole-body MRI findings in NF1 patients with MPNSTs and matched control NF1 patients without MPNSTs
| Group | NF1 with MPNSTs | NF1 without MPNSTsa |
|---|---|---|
| Total number | 13 | 26 |
| Number of patients with internal neurofibromas on MRI (%) | 9 (69) | 13 (50) |
| Median number of internal neurofibromas.>3 cm (range) | 1 (0–4) | 0.5 (0–9) |
| Median internal neurofibroma volume, ml (range) | 427 (0–2252) | 5 (0–7030) |
| Number with spinal neurofibromas (%) | 9 (69) | 12 (46) |
Abbreviations: NF1, neurofibromatosis 1; MPNSTs, malignant peripheral nerve sheath tumors.
None of these differences is statistically significant.
Many NF1 patients who develop MPNSTs do so at an extraordinarily young age26,27: the median age of NF1 patients with MPNSTs in this study was only 30 years. In comparison, the median age of diagnosis of MPNST in people who do not have NF1 is 62 years.9 Rapid growth of plexiform neurofibromas in NF1 patients may occur in children but is unusual in adults,4,5 so we wondered whether the burden of plexiform neurofibromas seen on whole-body MRI might be associated with the development of MPNSTs in younger NF1 patients. We therefore compared NF1 patients with MPNSTs whose age was less than the median in this study (30 years) to NF1 controls without MPNSTs who were younger than 30 years old.
All 6 of the NF1 patients with MPNSTs younger than 30 years of age had neurofibromas visualized on whole-body MRI, and both the median number of internal neurofibromas and the median internal neurofibroma volume were significantly greater among these patients than among NF1 controls younger than 30 years of age (Table 3). No significant differences in whole-body MRI findings were observed between NF1 patients with MPNSTs and controls without MPNSTs who were older than 30 (Table 3).
Table 3.
Comparison of whole-body MRI findings in NF1 patients with MPNSTs younger or older than the median age and matched control NF1 patients without MPNSTs
| Age <30 Years
|
Age >30 Years
|
|||
|---|---|---|---|---|
| Group | NF1 with MPNSTs | NF1 without MPNSTs | NF1 with MPNSTs | NF1 without MPNSTs |
| Total number | 6 | 11 | 7 | 15 |
| Median number of externally visible plexiform neurofibromas (range) | 0.5 (0–3) | 1 (0–3) | 1 (0–10) | 1 (0–5) |
| Median number of subcutaneous neurofibromas (range) | 112.5* (0–2000) | 2* (0–30) | 40 (10–300) | 25 (0–1000) |
| Median number of cutaneous neurofibromas (range) | 0 (0–400) | 4 (0–300) | 300 (0–5000) | 30 (0–1000) |
| Number of patients with internal neurofibromas on MRI (%) | 6‡ (100) | 3‡ (27) | 3 (43) | 10 (67) |
| Median number of internal neurofibromas >3 cm (range) | 1† (1–4) | 0† (0–3) | 0 (0–3) | 1 (0–9) |
| Median internal neurofibroma volume, ml (range) | 460** (8–2252) | 0** (0–5588) | 0 (0–825) | 115 (0–7030) |
| Number with spinal neurofibromas (%) | 4 (67) | 6 (54) | 5 (71) | 6 (40) |
Abbreviations: NF1, neurofibromatosis 1; MPNSTs, malignant peripheral nerve sheath tumors.
p = 0.048 by Mann-Whitney U test;
p = 0.027 by Mann-Whitney U test;
p = 0.009 by Fisher’s exact test;
p = 0.037 by Mann-Whitney U test.
Discussion
Neurofibromas can occur anywhere in the body in people with NF1, and at least 40% of affected adults have neurofibromas internally, although most are not apparent on physical examination.6,7 Using whole-body MRI, we found internal plexiform neurofibromas in 13 (50%) of 26 NF1 patients without MPNSTs. This figure underestimates the proportion of NF1 patients who have internal plexiform neurofibromas because lesions,<3 cm in greatest diameter, which include many spinal tumors, were excluded.
We found an association between the median number of subcutaneous neurofibromas in NF1 patients and the occurrence of MPNSTs overall (Table 1). In contrast, we found no difference in the median number of cutaneous neurofibromas or of external visible plexiform neurofibromas between the NF1 patients with MPNSTs and their matched controls without MPNSTs (Table 1). Our findings are similar to those of Tucker et al.,18 who studied 476 French NF1 patients, 25 of whom had MPNSTs. Tucker et al. found that the presence of subcutaneous neurofibromas, but not the presence of superficial plexiform neurofibromas or the number of cutaneous neurofibromas, was associated with the occurrence of MPNSTs. Our findings are also consistent with a cohort study performed by the same investigators in which subcutaneous, but not cutaneous, neurofibromas were associated with a higher risk of death among adults with NF1.28
The association of MPNSTs with subcutaneous, but not cutaneous, neurofibromas may reflect differences in pathogenesis of these two types of benign peripheral nerve sheath tumors. Cutaneous neurofibromas develop from the terminal branches of peripheral nerves distal to the perineurial sheath.5 Subcutanteous neurofibromas, in contrast, develop within a peripheral nerve and are circumscribed by the perineurium.5 Older adults with NF1 may have thousands of cutaneous neurofibromas, but malignant degeneration of these tumors is exceedingly rare, if it occurs at all. Discrete subcutaneous neurofibromas do not often become malignant, but they may, and subcutaneous plexiform neurofibromas carry a relatively high risk of malignant transformation. Some internal plexiform neurofibromas, such as those of major spinal nerves or nerve roots, also develop intraneurally and carry a relatively high risk of malignant transformation.5 The similarities in pathogenesis and natural history of these internal and subcutaneous neurofibromas and the differences in comparison to cutaneous tumors may explain the association we observed between MPNSTs and the occurrence of subcutaneous but not cutaneous neurofibromas.18
Tucker et al.18 found a strong association between the occurrence of MPNSTs and the presence of internal neurofibromas. We did not find such an association overall, but this may reflect the smaller number of subjects in our study. The most striking associations we observed are between MPNSTs in NF1 patients younger than 30 years of age and the presence of internal neurofibromas, median number of internal neurofibromas, and median total volume of neurofibromas on MRI in comparison to NF1 controls (Table 3). The occurrence of these statistically significant associations despite the small number of MPNST patients in this younger subset is remarkable. Independent confirmation of our findings is necessary.
Good medical care for all NF1 patients requires long-term clinical follow-up,20,29–31 but we do not know the natural history of internal neurofibromas in NF1 patients of various ages. The only reported series of serial MRI examinations in NF1 patients with plexiform neurofibromas included only young patients, most of whom had been selected for therapeutic trials because of inoperable or progressive tumors.25 These data do not provide an appropriate basis for recommendations regarding the use of longitudinal MRI for follow-up of NF1 patients found to have internal tumors on whole-body MRI. Therefore, we cannot make evidence-based recommendations regarding the use of MRI in the routine follow-up of NF1 patients with internal plexiform neurofibromas at the present time.
Inhomogeneous appearance and patchy contrast enhancement of an NF1-associated tumor on MRI32,33 can help distinguish benign and malignant peripheral nerve sheath tumors in people with NF1, but histopatho-logical examination is necessary for definitive diagnosis of MPNSTs. We cannot be certain that some of the internal tumors that we considered to be neurofibromas are not early malignancies except by long-term clinical follow-up, which is ongoing for all of our patients.
Growth of neurofibromas can vary over time, and a recent longitudinal volumetric MRI study of plexiform neurofibromas in 49 NF1 patients younger than 26 years of age found that growth was fastest in the youngest patients.25 This period of rapid growth early in life may also be a time during which malignant transformation of benign peripheral nerve sheath tumors occurs in NF1 patients. MPNSTs generally have a poor prognosis, but the prognosis is even worse in people with NF1.9,39 Our findings raise the possibility that routine whole-body imaging of young NF1 patients may permit identification of those at highest risk for developing MPNSTs early in life. Closer clinical monitoring and serial MRI examinations for changes in the appearance or growth of internal tumors may allow earlier diagnosis and more effective treatment of MPNSTs in these high-risk patients. Positron emission tomography may also help differentiate benign and malignant peripheral nerve sheath tumors in NF1 patients.34–38 Longitudinal studies are needed to assess the value of MRI for early detection and serial monitoring of internal plexiform neurofibromas on the outcome of MPNSTs that develop in people with NF1. Instituting such a policy would require a major change from current standards of clinical care.20,29–31
References
- 1.Korf B. Clinical features and pathobiology of neurofibromatosis 1. J Child Neurol. 2002;17:573–577. 602–604, 646–651. doi: 10.1177/088307380201700806. [DOI] [PubMed] [Google Scholar]
- 2.Thomson S, Fishbein L, Wallace M. NF1 mutations and molecular testing. J Child Neurol. 2002;17:555–561. 571–572, 646–651. doi: 10.1177/088307380201700803. [DOI] [PubMed] [Google Scholar]
- 3.Viskochil D. Genetics of neurofibromatosis 1 and the NF1 gene. J Child Neurol. 2002;17:562–572. 646–651. doi: 10.1177/088307380201700804. [DOI] [PubMed] [Google Scholar]
- 4.Friedman JM, Riccardi VM. Clinical and epidemiological features. In: Friedman JM, Gutmann DH, MacCollin M, Riccardi VM, editors. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. 3rd ed. Baltimore, MD: Johns Hopkins University Press; 1999. pp. 29–86. [Google Scholar]
- 5.Riccardi V. The genetic predisposition to and histogenesis of neurofibromas and neurofibrosarcoma in neurofibromatosis type 1. Neurosurg Focus. 2007;22(6):E3. doi: 10.3171/foc.2007.22.6.4. [DOI] [PubMed] [Google Scholar]
- 6.Thakkar SD, Feigen U, Mautner VF. Spinal tumours in neurofibromatosis type 1: an MRI study of frequency, multiplicity and variety. Neuroradiology. 1999;41:625–629. doi: 10.1007/s002340050814. [DOI] [PubMed] [Google Scholar]
- 7.Tonsgard J, Kwak S, Short M, Dachman A. CT imaging in adults with neurofibromatosis-1: frequent asymptomatic plexiform lesions. Neurology. 1998;50:1755–1760. doi: 10.1212/wnl.50.6.1755. [DOI] [PubMed] [Google Scholar]
- 8.Walker L, Thompson D, Easton D, et al. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer. 2006;95:233–238. doi: 10.1038/sj.bjc.6603227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.De Raedt T, Brems H, Wolkenstein P, et al. Elevated risk for MPNST in NF1 microdeletion patients. Am J Hum Genet. 2003;72:1288–1292. doi: 10.1086/374821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drouet A, Wolkenstein P, Lefaucheur J, et al. Neurofibromatosis 1- associated neuropathies: a reappraisal. Brain. 2004;127:1993–2009. doi: 10.1093/brain/awh234. [DOI] [PubMed] [Google Scholar]
- 13.Ferner R, Hughes R, Hall S, Upadhyaya M, Johnson M. Neurofibromatous neuropathy in neurofibromatosis 1 (NF1) J Med Genet. 2004;41:837–841. doi: 10.1136/jmg.2004.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doorn P, Molenaar W, Buter J, Hoekstra H. Malignant peripheral nerve sheath tumors in patients with and without neurofibromatosis. Eur J Surg Oncol. 1995;21:78–82. doi: 10.1016/s0748-7983(05)80073-3. [DOI] [PubMed] [Google Scholar]
- 15.Dales R, McEver V, Quispe G, Davies R. Update on biologic behavior and surgical implications of neurofibromatosis and neurofibrosarcoma. Surg Gynecol Obstet. 1983;156:636–640. [PubMed] [Google Scholar]
- 16.Poyhonen M, Niemela S, Herva R. Risk of malignancy and death in neurofibromatosis. Arch Pathol Lab Med. 1997;121:139–143. [PubMed] [Google Scholar]
- 17.Shearer P, Parham D, Kovnar E, et al. Neurofibromatosis type I and malignancy: review of 32 pediatric cases treated at a single institution. Med Ped Oncol. 1994;22:78–83. doi: 10.1002/mpo.2950220203. [DOI] [PubMed] [Google Scholar]
- 18.Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman J. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology. 2005;65:205–211. doi: 10.1212/01.wnl.0000168830.79997.13. [DOI] [PubMed] [Google Scholar]
- 19.Neurofibromatosis: conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- 20.Gutmann D, Aylsworth A, Carey J, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51–57. [PubMed] [Google Scholar]
- 21.Kluwe L, Siebert R, Gesk S, et al. Screening 500 unselected neurofibromatosis 1 patients for deletions of the NF1 gene. Hum Mutat. 2004;23:111–116. doi: 10.1002/humu.10299. [DOI] [PubMed] [Google Scholar]
- 22.Kehrer-Sawatzki H, Kluwe L, Sandig C, et al. High frequency of mosaicism among patients with neurofibromatosis type 1 (NF1) with microdeletions caused by somatic recombination of the JJAZ1 gene. Am J Hum Genet. 2004;75:410–423. doi: 10.1086/423624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehrer-Sawatzki H, Kluwe L, Fünsterer C, Mautner V. Extensively high load of internal tumors determined by whole body MRI scanning in a patient with neurofibromatosis type 1 and a non-LCR-mediated 2-Mb deletion in 17q11.2. Hum Genet. 2005;116:466–475. doi: 10.1007/s00439-005-1265-4. [DOI] [PubMed] [Google Scholar]
- 24.Kluwe L, Friedrich R, Peiper M, Friedman J, Mautner V. Constitutional NF1 mutations in neurofibromatosis 1 patients with malignant peripheral nerve sheath tumors. Hum Mutat. 2003;22:420. doi: 10.1002/humu.9193. [DOI] [PubMed] [Google Scholar]
- 25.Dombi E, Solomon J, Gillespie AJ, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68:643–647. doi: 10.1212/01.wnl.0000250332.89420.e6. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich R, Hartmann M, Mautner V. Malignant peripheral nerve sheath tumors (MPNST) in NF1-affected children. Anticancer Res. 2007;27:1957–1960. [PubMed] [Google Scholar]
- 27.Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68:1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosrotehrani K, Bastuji-Garin S, Riccardi VM, Birch P, Friedman JM, Wolkenstein P. Subcutaneous neurofibromas are associated with mortality in neurofibromatosis 1: a cohort study of 703 patients. Am J Med Genet A. 2005;132:49–53. doi: 10.1002/ajmg.a.30394. [DOI] [PubMed] [Google Scholar]
- 29.Ferner R. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 30.Pinson S, Creange A, Barbarot S, et al. Neurofibromatosis 1: recommendations for management. Arch Pediatr. 2002;9:49–60. doi: 10.1016/s0929-693x(01)00695-9. [DOI] [PubMed] [Google Scholar]
- 31.Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:2–7. doi: 10.1016/j.spen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich R, Kluwe L, Funsterer C, Mautner V. Malignant peripheral nerve sheath tumors (MPNST) in neurofibromatosis type 1 (NF1): diagnostic findings on magnetic resonance images and mutation analysis of the NF1 gene. Anticancer Res. 2005;25(3A):1699–1702. [PubMed] [Google Scholar]
- 33.Fuchs B, Spinner R, Rock M. Malignant peripheral nerve sheath tumors: an update. J Surg Orthop Adv. 2005;14:168–174. [PubMed] [Google Scholar]
- 34.Bensaid B, Giammarile F, Mognetti T, et al. Utility of 18 FDG positon emission tomography in detection of sarcomatous transformation in neurofibromatosis type 1. Ann Dermatol Venereol. 2007;134:735–741. doi: 10.1016/s0151-9638(07)92528-4. [DOI] [PubMed] [Google Scholar]
- 35.Bredella M, Torriani M, Hornicek F, et al. Value of PET in the assessment of patients with neurofibromatosis type 1. Am J Roentgenol. 2007;189:928–935. doi: 10.2214/AJR.07.2060. [DOI] [PubMed] [Google Scholar]
- 36.Brenner W, Friedrich R, Gawad K, et al. Prognostic relevance of FDG PET in patients with neurofibromatosis type-1 and malignant peripheral nerve sheath tumours. Eur J Nucl Med Mol Imaging. 2006;33:428–432. doi: 10.1007/s00259-005-0030-1. [DOI] [PubMed] [Google Scholar]
- 37.Ferner R, Golding J, Smith M, et al. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol. 2007;19:390–394. doi: 10.1093/annonc/mdm450. [DOI] [PubMed] [Google Scholar]
- 38.Ferner R, Lucas J, O’Doherty M, et al. Evaluation of (18) fluorodeoxyglucose positron emission tomography ((18)FDG PET) in the detection of malignant peripheral nerve sheath tumours arising from within plexiform neurofibromas in neurofibromatosis 1. J Neurol Neurosurg Psychiatr. 2000;68:353–357. doi: 10.1136/jnnp.68.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta G, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Focus. 2007;22(6):E12. doi: 10.3171/foc.2007.22.6.13. [DOI] [PubMed] [Google Scholar]