Abstract
Radiation therapy remains the only treatment that provides clinical benefit to children with diffuse brainstem tumors. Their median survival, however, rarely exceeds 9 months. The authors report a prospective trial of front-line chemotherapy aimed at delaying radiation until time of clinical progression. The aim was to investigate the possibility that radiotherapy would maintain its activity in children whose disease progressed after chemotherapy. Twenty-three patients took part in this protocol, the BSG 98 protocol, which consisted of frontline chemotherapy alternating hematotoxic and nonhematotoxic schedules. Each cycle included three courses delivered monthly; the first course was 1,3-bis(2-chloroethyl)-1-nitrosourea– cisplatin, and the second and third were high-dose methotrexate. Three patients underwent one cycle; 5 patients each, two and three cycles; and 10 patients, four cycles. Twenty of the 23 patients eventually received local radiation therapy. A historical cohort of 14 patients who received at least local radiation therapy served as controls. Four patients experienced severe iatrogenic infections, and 11 patients required platelet transfusions. Median survival increased significantly in patients participating in the protocol compared to that in the historical controls (17 months, 95% confidence interval [CI], 10–23 months, vs. 9 months, 95% CI, 8–10 months; p = 0.022), though hospitalization was prolonged (57 vs. 25 days, p = 0.001). Although frontline chemotherapy alternating hematotoxic and nonhematotoxic schedules significantly increases overall median survival, its cost from infection and hospitalization deserves honest discussion with the children and their parents.
Keywords: brainstem, chemotherapy, glioma, radiotherapy
From 10% to 15% of all pediatric tumors of the CNS arise in the brainstem, and 80% of these are diffuse intrinsic tumors. They clearly differ from focal, dorsally exophytic, and cervicomedullary tumors on many points. They are typically seen with rapidly progressing symptoms and signs comprising multiple erratic cranial nerve palsies, long track deficits, cerebellar symptoms, and/or raised intracranial pressure. The wide availability of MRI allows easy confirmation of diagnosis: the epicenter of the tumor sits in the pons, which is diffusely enlarged and abnormal, an area that is hypodense on T1-weighted images and hyperintense on T2-weighted images. Some tumors show central enhancement, usually with ringlike distribution that suggests necrosis, like that seen in high-grade gliomas. In typical cases, histological confirmation is not requested1 both because it is dangerous and because it uniformly shows grade III and IV gliomas. The natural history of these tumors has not changed since radiation therapy demonstrated its inconstant and always transient efficacy. Neurological deficits improve in more than 80% of cases, and steroids, often instituted at diagnosis, may be gradually withdrawn. Radiological follow-up does not help in this disease because any subtle modification of clinical symptoms is easily detected. Median time to disease progression is uniformly short (5–6 months), and median survival never exceeds 10–12 months. No more than 10% survive long term.
Because this disease behaves rather stereotypically, it is usually included in specific trials but not in trials concerning high-grade gliomas. Thus, considerable data since the 1980s show no improvement irrespective of modifications made to the radiotherapy schedule.2 Increasing total dose by hyperfractionation produced median survival of 9–12 months in several uni- and multicentric trials.3–9
Chemotherapy also failed to improve prognosis, whether given orally,10–12 intravenously at standard dose,13–22 intra-arterially,23 or at high dose with stem cell rescue.24–28 Similarly, clear improvement did not occur after chemotherapy given at time of progression.29 All these data have been revisited recently in a meta-analysis.30
Thus, innovative strategies are warranted. In protocols for brain tumors in infants, chemotherapy prolongs a period free of radiation31,32 to avoid neuropsychological sequelae in long-term survivors. In contrast, in tumors of the brainstem, because no cure is expected soon, one may only modestly propose to prolong life by postponing radiation therapy and instead prolonging chemotherapy as long as possible. Because literature regarding adult patients has shown significant advantage when nitrosourea-containing regimens were added to radiotherapy,33–35 and continuous infusion of 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) and cisplatin produced encouraging results in patients with naive glioblastomas,36 we designed a prospective trial of frontline chemotherapy that included nitrosoureas and cisplatin. To further enhance the activity of this protocol, we proposed to add tamoxifen37,38 during these courses. However, because the goal was to postpone radiation for a prolonged period, and because nitrosoureas are usually hematotoxic after four to six cycles, we had to find an effective nonhematotoxic compound that could be delivered between the cycles of nitrosourea. High-dose methotrexate was chosen because it satisfied these requirements.14,39 To further enhance and prolong the efficacy, we delivered tamoxifen during and hydroxyurea40 during and after the radiation therapy as maintenance.
Materials and Methods
From January 1998 through September 2004, every child admitted to the Centre Léon Bérard of Lyon for treatment of diffuse brainstem glioma (BSG) was invited to participate in the prospective BSG 98 protocol. Twenty-three patients were admitted to the protocol, and after written informed consent was obtained, chemotherapy was initiated as soon as central venous access was achieved.
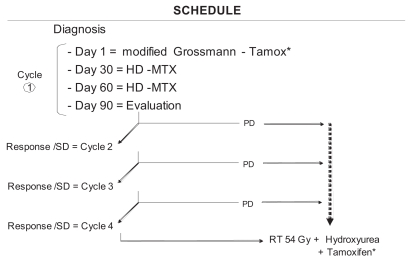
Fig. 1 summarizes the delivery strategy. Each cycle of chemotherapy consisted of three courses delivered at 30-day intervals. The first course comprised oral tamoxifen (100 mg/m2, day 1; 20 mg/m2, days 2–5) and continuous infusion of BCNU and cisplatin (both at 20 mg/m2, days 2–5). The second and third courses comprised high-dose methotrexate (12 g/m2) delivered as a 3-h infusion followed by a folinic rescue at 15 mg/m2 4 times a day as long as the serum level of methotrexate remained below 0.15 mmol (Table 1). These 3-month cycles were repeated until clinical deterioration was noticed. Steroid dose was reduced at each step according to clinical conditions.
Fig. 1.
Schedule of the BSG 98 prospective protocol. Abbreviations: Tamox, tamoxifen; HD-MTX, high-dose methotrexate; SD, stable disease; PD, progressive disease; RT, radiotherapy. Modified Grossmann refers to treatment of continuous infusion of BCNU and cisplatin based on Grossman et al.,36 as modified here (see “Materials and Methods”). *Tamoxifen was omitted in latest patients.
Table 1.
Drug dosing and schedule for the BSG 98 prospective protocol
| Day
|
|||||
|---|---|---|---|---|---|
| Treatment | 1 | 2 | 3 | 4 | 5 |
| Modified Grossman course 1a | |||||
| Tamoxifenb | 100 mg/m2 | 20 mg/m2 | 20 mg/m2 | 20 mg/m2 | 20 mg/m2 |
| BCNU 24-h continuous infusion | 40 mg/m2 | 40 mg/m2 | 40 mg/m2 | 40 mg/m2 | |
| CDDP 24-h continous infusion | 40 mg/m2 | 40 mg/m2 | 40 mg/m2 | 40 mg/m2 | |
| Courses 2 and 3: high-dose MTX | |||||
| MTX 3-h continuous infusion | 12 g/m2 | ||||
| Folinate rescue each 6 h | 15 mg/m2/6 h | 15 mg/m2/6 h | 15 mg/m2/6 h | ||
| Chemotherapy during and after RT | |||||
| Hydroxyurea | 20 mg/kg/day until progression | ||||
| Tamoxifenb | 20 mg/kg/day until during RT | ||||
Abbreviations: BCNU, bis(2-chloroethyl)-1-nitrosourea; CDDP, cisplatin; MTX, methotrexate; RT, radiotherapy.
Modified Grossmann refers to treatment of continuous infusion of BCNU and cisplatin based on Grossman et al., 36 as modified here (see “Materials and Methods”).
Tamoxifen was omitted in the latest patients.
Aware that transient deterioration is possible after either chemotherapy or radiotherapy, we only observed children during the first cycle and refrained from early irradiation when feasible. Later, at the first sign of clinical deterioration, the child underwent standard radiation therapy by two opposed portal fields; 54 Gy was delivered in daily 2-Gy fractions accompanied by oral administration of tamoxifen (20 mg/m2 during radiation therapy) and hydroxyurea (20 mg/kg/day orally until progression). Tamoxifen was deleted from pre- and postradiation protocols as soon as its lack of efficacy in treating BSGs was published.41 To constitute a group of historical controls, we retrospectively reviewed the data for all children with diffuse gliomas of the brainstem admitted to our institution during the previous 10 years; we identified 15 patients between April 1992 and December 2001 who were suitable for comparison. One patient was excluded from the historical control cohort because he had a genetic predisposition for multiple enchondromatosis, which we have found may confer an unusually long-term survival.42 Thus, two groups of patients were found: group A received radiation either directly at diagnosis or after frontline administration of procarbazine as part of a French Society of Pediatric Oncology (SFOP) phase II window study of frontline procarbazine,11 and group B received carboplatin before and during radiation therapy as part of an SFOP phase II study published elsewhere.15
The triangular test of Bellissant et al.43 was used to compare patients in the historical group and the current protocol. Results of the test correspond to the hypotheses H0 = p < 0.50 and H1 = p > 0.50, with p1 = 0.70 (= 0.10, = 0.05). The upper and lower boundaries are given by Z = S – Np0 and V = Np0 (1 – p0), where S represents progression-free survival and n the number of patients participating in the analysis. If the path crosses the upper boundary, the proposed strategy is concluded to be better; if it crosses the lower boundary, no apparent benefit for the proposed strategy is indicated.
Differences in characteristics between the two groups of patients were tested using Pearson’s chi square test or the nonparametric Mann-Whitney exact test where appropriate. Survival was defined as the time from (1) diagnosis or (2) radiation therapy until death by any cause. Survival was evaluated by Kaplan-Meier estimation, and differences between survival functions of the two groups were evaluated using the log-rank test. Significance was defined as p = 0.05, with 95% confidence intervals (CIs).
Results
Patient Characteristics and Treatment
Tables 2 and 3 summarize the characteristics of patients in the historical group (14 patients) and those in the current protocol (23 patients). The mean age at diagnosis was 6.3 ± 2.7 years in the historical group, lower than in our group (9.2 ± 4.9 years; p = 0.06); the ratio of boys to girls and the mean duration of symptoms before diagnosis were similar in the two groups. Diagnosis was obtained by MRI in all cases. Centralized retrospective review was possible in 21 of the 23 cases in the experimental arm. The percentage of invasion ranged from 50% to 100% (only two patients had 50% invasion; all others had at least 75%); involvement included peduncles in 12 cases, pons in all, bulbar region in all but one, and medulla oblongata in eight; the mean surface area of the largest invasion measured on two dimensions was 15.7 cm2 (range, 9–32 cm2). Biopsy and/or debulking was performed in 2 of the 14 patients in the historical group and 4 of the 23 patients in our group. In the latter cohort, the reasons for this surgery were as follows: two had biopsy prior to multidisciplinary advice, and two had excision of a necrotic mass that exerted mass effect. A shunt was inserted at diagnosis in one patient in each of the two groups, and later in the course of therapy in two patients in the historical group and six patients in the experimental protocol. One patient in the historical group underwent secondary debulking.
Table 2.
Characteristics of historical controls versus patients in current protocol
| Characteristic | Historical Group | Current Protocol | p-Value |
|---|---|---|---|
| Number of patients | 14 | 23 | |
| Mean age at diagnosis ± SD (years) | 6.3 ± 2.7 | 9.2 ± 4.9 | 0.056 |
| Male/female ratio | 4/10 | 10/13 | |
| Mean duration of symptoms ± SD (days) | 39.4 ± 24.5 | 63.9 ± 58.4 | 0.48 |
| Surgery | |||
| Biopsy ± debulking | 2 | 4 | |
| Primary shunt | 1 | 1 | |
| Secondary shunt | 2 | 6 | |
| Secondary surgery | 1 | 0 | |
| None | 8 | 12 | |
| Steroid withdrawala | 7/14 (50%) | 12/22 (55%) | 0.79 |
| Total stay in hospital ± SD (days) | 24.9 ± 22.0 | 57.3 ± 21.8 | 0.001 |
Data missing for one patient.
Table 3.
Diagnosis findings and outcome in experimental cohort
| Patient | Age (Years) | Sex | Delay Symptoms/Diagnosis (Days) | % Invasion | Surgery | Chemotherapy before RT (Number of Courses) | Steroid Withdrawn | Delay, Diagnosis to RT (Days) | RT Dose (Gy) | Delay, Diagnosis to Death (Months) | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | 42 | 90 | No | 4 GRT + 5 MTX | Y | 248 | 54 | 15 | DOD |
| 2 | 5 | F | 94 | 100 | Shunt II | 2 GRT | Y | 35 | 54 | 20 | DOD |
| 3 | 7 | F | 16 | 90 | No | 4 GRT + 1 MTX | N | 0 | 8 | DOD | |
| 4 | 4 | F | 27 | 75 | Biopsy | 4 GRT + 7 MTX | Y | 344 | 54 | 22 | DOD |
| 5 | 12 | M | 108 | 75 | Biopsy | 1 GRT | Y | 58 | 54 | 35 | DOD |
| 6 | 16 | F | 112 | 75 | No | 3 GR + 6 MTX | N | 256 | 54 | 13 | DOD |
| 7 | 7 | F | 11 | 75 | Biopsy | 4 GR + 8 MTX | Y | 318 | 54 | 18 | DOD |
| 8 | 14 | M | 66 | 90 | Ventriculo II | 4 GR + 6 MTX | Y | 296 | 54 | 27 | DOD |
| 9 | 7 | F | 50 | 75 | Shunt II | 2 GR + 4 MTX | N | 190 | 54 | 11 | DOD |
| 10 | 7 | M | 59 | 75 | Shunt II | 3 GR + 6 MTX | N | 293 | 54 | 17 | DOD |
| 11 | 4 | M | 15 | 90 | Shunt I | 4 GR + 8 MTX | Y | 351 | 54 | 21 | DOD |
| 12 | 15 | F | 95 | 75 | Shunt II | 4 GR + 7 MTX | N | 376 | 54 | 20 | DOD |
| 13 | 9 | F | 96 | 50 | No | 4 GR + 8 MTX | Y | 372 | 54 | 26 | DOD |
| 14 | 13 | F | 8 | No | 1 GR + 2 MTX | N | 103 | 54 | 6 | DTOX | |
| 15 | 17 | M | 184 | 50 | Biopsy | 2 GR + 4 MTX | N | 206 | 6 | 7 | DOD |
| 16 | 20 | M | 211 | 75 | No | 3 GR + 6 MTX | Y | 778 | 54 | 73 | DOD |
| 17 | 8 | M | 137 | 75 | No | 2 GR + 4 MTX | N | 215 | 30 | 13 | DOD |
| 18 | 10 | F | 11 | Shunt II | 3 GR + 5 MTX | Y | 0 | 9 | DOD | ||
| 19 | 10 | F | 9 | 75 | No | 4 GR + 6 MTX | N | 296 | 28 | 11 | DOD |
| 20 | 3 | M | 16 | 100 | No | 4 GR + 8 MTX | Y | 377 | 54 | 26 | DOD |
| 21 | 6 | F | 2 | 75 | No | 4 GR + 2 MTX | Y | 227 | 54 | 10 | DOD |
| 22 | 4 | M | 22 | 75 | No | 3 GR + 2 MTX | N | 147 | 42 | 8 | DOD |
| 23 | 4 | M | 55 | 100 | No | 1 GR + 2 MTX | N | 98 | 45 | 15 | DOD |
Abbreviations: RT, radiotherapy; F, Female; II, performed after diagnosis; GRT, Grossman-tamoxifen; MTX, high-dose methotrexate; Y, yes; M, male; DOD, dead of disease; N, no; GR, Grossman; Ventriculo, ventriculocisternostomy; I, performed at diagnosis; DTOX, dead of toxicity.
All patients in the experimental group received 1–12 courses of frontline chemotherapy (mean, 9 courses); the mean number of courses of tamoxifen–BCNU–cisplatin was 3 (range, 1–8 courses), and of high-dose methotrexate, 5 (range, 0–8 courses). Three patients received only one cycle: two suffered early progression, and one was advised by a second physician against chemotherapy. Ten patients discontinued chemotherapy after either two cycles (five patients) or three cycles (five patients): nine from clinical progression and one from protocol violation. Ten patients received four cycles. All of the 21 patients who ultimately received radiotherapy showed clinical progression; disease had stabilized in one patient and was still clinically responding in a second.
The complications related to chemotherapy included septicemia in four patients (gram negative, 3; Staphylococcus aureus, 1). Another patient suffered toxic death from gram-negative and Candida albicans septicemia. This 13-year-old girl became tetraparetic 1 month after diagnosis and suffered a lung abscess secondary to chronic swallowing deficiency. She received her last courses of chemotherapy 3 months and radiation therapy 2 months prior to the septicemia. Complete neutropenia developed from treatment with hydroxyurea, and massive doses of steroids left her chronically immuno-suppressed. Four other patients had episodes of fever of unknown origin. One of these also had an acute crisis from Plasmodium falciparum. One patient suffered herpes zoster infection. One patient had transient methotrexate intoxication and transient increased creatininemia (maximum, 140 mmol/l). Eleven patients required platelet transfusions (mean, 3 transfusions; range, 1–6), and six required red blood cell transfusions (mean, 1 transfusion; range, 1–4).
Altogether, 21 of the 23 patients received radiation therapy; the other two did not, one because of parental refusal (the child had durable clinical remission before progression of disease at cycle 4; his parents refused to risk a second sequence of improvement–deterioration) and one as a result of explosive clinical progression of disease. Seventeen patients received 54 Gy of radiation. Radiation therapy was interrupted because of poor neurological status in the remaining four patients, who had received, respectively, 6, 28, 30, and 42 Gy. Only 10 of the 23 patients received the planned postradiation therapy by hydroxyurea and tamoxifen because of compliance failure or protocol amendment (see above).
The historical control cohort included patients who received radiation directly (four patients), after participation in an SFOP study of a frontline window of procarbazine administration (four patients), or in an SFOP phase II study that administered carboplatin before and during radiation therapy (five patients). One further patient included in the historical cohort, admitted in 2001, received frontline radiotherapy because his parents refused participation in the experimental protocol.
Survival and Quality of Life
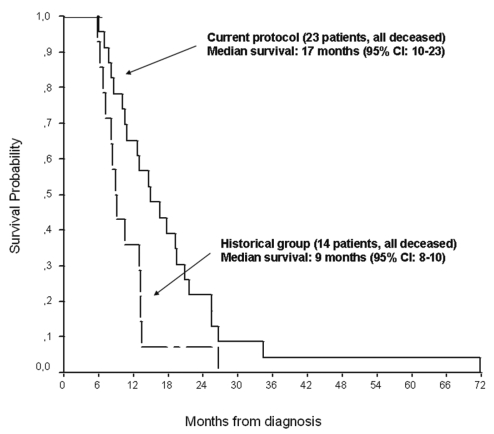
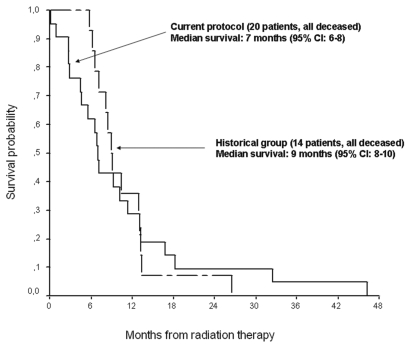
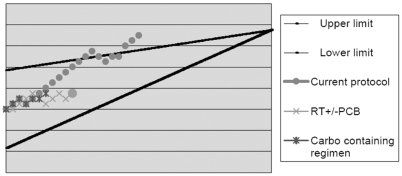
All patients ultimately died. In the experimental protocol, the median survival of 17 months (95% CI, 10–23 months) was significantly better than the 9 months in the historical group (95% CI, 8–10 months; p = 0.022) (Fig. 2). When survival is considered from time of radiotherapy in both groups, their representative curves superimpose (Fig. 3), suggesting that the 8-month difference in survival is due to chemotherapy. Only the line representing members of the experimental protocol crosses the superior boundary on the Bellisant triangle (Fig. 4), suggesting that this protocol is better than previous ones. Steroids could be at least transiently withdrawn in half of the patients of both groups (p = 0.79).
Fig. 2.
Overall survival of patients in the current protocol versus historical controls (p = 0.022, log-rank test). CI, confidence interval.
Fig. 3.
Overall survival of patients in the current protocol versus historical controls with time from radiation therapy as the starting point (p = not significant [NS], log-rank test). CI, confidence interval.
Fig. 4.
Triangular test of Bellissant et al.43 for patients in the current protocol versus historical controls. Abbreviations: RT, radiotherapy; PCB, procarbazine; Carbo, carboplatin.
For the patients included in the experimental arm, the mean length of hospitalization for chemotherapy and/or complications was 43 days (range, 10–81 days). For chemotherapy, complications, and palliative care, the mean length of hospitalization was 57.3 ± 21.8 days, significantly more than the 24.9 ± 22.0 days for patients in the historical group (p = 0.001). Altogether, the mean delay between diagnosis and radiotherapy initiation was 248 days (range, 35–778 days).
Discussion
The uniformly dismal prognosis, despite various therapeutic attempts, for children with BSGs warrants innovative strategies. We wanted to investigate the possibility that radiotherapy would maintain its activity in children whose disease progressed after chemotherapy.
In the adult literature, two meta-analyses showed a modest but significant advantage when regimens including nitrosourea were added to radiotherapy.33,35 Moreover, when the protocol was designed, Grossman et al.36 had recently reported only 5% of progression in patients with naive glioblastomas who received continuous infusion of BCNU and cisplatin. This suggested that chemotherapy might be safely administered prior to radiation, and this preadministration served as the backbone of the present trial. However, the hematological and potential renal, pulmonary, and audiological toxicities of this treatment, when delivered regularly, precluded its use as an elective treatment.
To maintain the chemotherapeutic pressure, the search for an alternative nonhematotoxic regimen suggested high-dose methotrexate as a good candidate. Some studies have reported encouraging results in children with gliomas.14,39 Delivered prior to radiotherapy, this drug does not expose the patient to the risk of leukoencephalopathy that it would if delivered after radiation. Courses of high-dose methotrexate may be delivered weekly. The unusually long delay between courses herein was a compromise between efficacy and quality of life.
Tamoxifen was shown to have an antiproliferative effect in glioma cells in vitro.37,38 This may be explained by the drug’s inhibitory effect on the protein kinase C activity of glioma cells.37 Because tamoxifen is highly protein bound, we could not use it in combination with high-dose methotrexate, and it was proposed for use only during infusion of BCNU-cisplatin and during radiation therapy. A recent study has failed to show its activity in patients with gliomas,44 and its use was thus discontinued in the latest patients.
The administration of hydroxyurea after radiation therapy was advocated because of its demonstrated activity in adult series of gliomas.40 Moreover, the possible antiangiogenic role of continuous low-dose oral administration of chemotherapy was recently pointed out with etoposide or vinblastine. We had hypothesized a similar effect with prolonged oral administration of hydroxyurea. It is impossible to conclude in this series whether the drug contributed to prolonged survival, but this is unlikely because most patients refused to take it.
The strategy proposed in the current study was similar to that used in babies to try to delay the deleterious effect of radiation therapy on young brains.31,32 However, the goal of this delay was different from that in babies; the question was whether radiation therapy would retain its activity in patients whose disease was progressing under chemotherapy. We set a maximal duration of chemotherapy at 1 year, and this goal was reached in half of our patients. Our study showed that the median time from radiation therapy to death (7 months) did not differ significantly from that expected from use of frontline radiotherapy, as reported in the literature, nor did it vary from what we found in our historical group. This suggests that frontline chemotherapy does not decrease the efficacy of radiation therapy delivered at time of relapse. The ultimate goal, to increase the number of long-time survivors, was not reached.
There is no selection bias in the population presented. All demographic and clinical data correspond to those usually reported in the literature: mean age 9 years with no patient younger than 3 years, involvement of cranial nerves in all but one patient, and standard centrally reviewed MRI in all but two patients. The mean duration of symptoms (60 days) may be considered longer than usual: however, among the three patients who had more than 120 days’ duration of symptoms, all had typical MRI findings, and only one had an unusually long survival; all published series have such rare long survivors. To our knowledge, the results presented here are the best among such groups of patients, though it may be suggested modestly that chemotherapy increases overall survival by 8 months. However, in terms of toxicity, the costs associated with the need for a central line and prolonged hospitalization may not be worthwhile and should at least be discussed with the parents. One such case serves as an important reminder that repetition of sequences of remission and progression of disease may adversely affect the well-being of parents and may not apparently benefit the children. The case involved a child’s durable clinical remission before progression of disease at cycle 4. Having seen their child improve after chemotherapy and then deteriorate, his parents refused to risk a second sequence of improvement– deterioration.
This series suggests that standard chemotherapy that alternates hematotoxic and nonhematotoxic courses prior to radiotherapy may significantly, but modestly, improve median survival. The cost from infection and hospitalization deserves honest discussion with the children and their parents. In such a cohort, steps to improving survival include omission of drugs from the treatment regimen that have not been demonstrated to be useful, such as tamoxifen and hydroxyurea; optimizing drug delivery by modifying duration of methotrexate injection and folinic rescue; and because some efficacy from association with biological modifiers, such as retinoic acid, has been suggested in adults with high-grade gliomas,45 addition of some form of antiangiogenic and/or biological modifier during chemotherapy. Most likely, if cure is ever obtained in such disease, it will come from a multidisciplinary approach that uses additive components. There is still a large place for research.
Acknowledgments
We are grateful to Rosalyn Uhrig for technical editing, to David Perol for finalizing statistical analysis, and to J. Popescu for typing the manuscript. This work was presented at the Fourteenth International Conference on Brain Tumor Research and Treatment in Asheville, North Carolina, USA, May 27–30, 2001, and at the International Symposium on Pediatric Neuro-Oncology in London, UK, June 9–12, 2002.
References
- 1.Epstein F, McCleary EL. Intrinsic brain-stem tumors of childhood: surgical indications. J Neurosurg. 1986;64:11–15. doi: 10.3171/jns.1986.64.1.0011. [DOI] [PubMed] [Google Scholar]
- 2.Mandell L, Kadota R, Douglas EC, et al. Is it time to rethink the role of hyperfractionated radiotherapy in the management of children with newly-diagnosed brainstem glioma? [abstract] Int J Radiat Oncol Biol Phys. 1997;39(suppl):2143. doi: 10.1016/s0360-3016(98)00501-x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman CR, Krischer JP, Sanford RA, et al. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1993;27:197–206. doi: 10.1016/0360-3016(93)90228-n. [DOI] [PubMed] [Google Scholar]
- 4.Freeman CR, Bourgouin PM, Sanford RA, Cohen ME, Friedman HS, Kun LE. Long term survivors of childhood brain stem gliomas treated with hyperfractionated radiotherapy: clinical characteristics and treatment related toxicities. The Pediatric Oncology Group. Cancer. 1996;77:555–562. doi: 10.1002/(SICI)1097-0142(19960201)77:3<555::AID-CNCR19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Freeman CR, Kepner J, Kun LE, et al. A detrimental effect of a combined chemotherapy-radiotherapy approach in children with diffuse intrinsic brain stem gliomas? Int J Radiat Oncol Biol Phys. 2000;47:561–564. doi: 10.1016/s0360-3016(00)00471-5. [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Allen JC, Goldwein JL, et al. Hyperfractionated radiotherapy for children with brainstem gliomas: a pilot study using 7,200 cGy. Ann Neurol. 1990;27:167–173. doi: 10.1002/ana.410270212. [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Children’s Cancer Group phase I/II trial. Cancer. 1993;72:1414–1421. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Children’s Cancer Group phase I/II trial. Cancer. 1994;74:1827–1834. doi: 10.1002/1097-0142(19940915)74:6<1827::aid-cncr2820740628>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Prados MD, Wara WM, Edwards MS, Larson DA, Lamborn K, Levin VA. The treatment of brain stem and thalamic gliomas with 78 Gy of hyperfractionated radiation therapy. Int J Radiat Oncol Biol Phys. 1995;32:85–91. doi: 10.1016/0360-3016(95)00563-E. [DOI] [PubMed] [Google Scholar]
- 10.Broniscer A, Iacono L, Chintagumpala M, et al. Role of temozolomide after radiotherapy for newly diagnosed diffuse brainstem glioma in children: results of a multiinstitutional study (SJHG-98) Cancer. 2005;103:133–139. doi: 10.1002/cncr.20741. [DOI] [PubMed] [Google Scholar]
- 11.Gentet JC, Frappaz D, Pautard B. Procarbazine in children with malignant brain stem glioma [abstract] Med Pediatr Oncol. 1999;33:205. [Google Scholar]
- 12.Wolff JEE, Molenkamp G, Lemmer A. Oral chemotherapy for children with glioblastoma and brain stem tumors [abstract] Med Pediatr Oncol. 1997;29:413. [Google Scholar]
- 13.Allen JC, Siffert J, Donahue B. A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer. 1999;86:1064–1069. doi: 10.1002/(sici)1097-0142(19990915)86:6<1064::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Allen JC, Walker R, Rosen G. Preradiation high-dose intravenous methotrexate with leucovorin rescue for untreated primary childhood brain tumors. J Clin Oncol. 1988;6:649–653. doi: 10.1200/JCO.1988.6.4.649. [DOI] [PubMed] [Google Scholar]
- 15.Doz F, Neuenschwander S, Bouffet E, et al. Carboplatin before and during irradiation for malignant brainstem tumor : a study by the Société Française d’Oncologie. Eur J Cancer. 2002;38:815–819. doi: 10.1016/s0959-8049(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 16.Jenkin RD, Boesel C, Ertel I, et al. Brain-stem tumors in childhood: a prospective randomized trial of irradiation with and without adjuvant CCNU, VCR, and prednisone. A report of the Childrens Cancer Study Group. J Neurosurg. 1987;66:227–233. doi: 10.3171/jns.1987.66.2.0227. [DOI] [PubMed] [Google Scholar]
- 17.Kadota RP, Mandell LR, Fontanesi J, et al. Hyperfractionated irradiation and concurrent cisplatin in brain stem tumors: a Pediatric Oncology Group pilot study (9139) Pediatr Neurosurg. 1994;20:221–225. doi: 10.1159/000120794. [DOI] [PubMed] [Google Scholar]
- 18.Kretschmar CS, Tarbell NJ, Barnes PD, Krischer JP, Burger PC, Kun L. Pre-irradiation chemotherapy and hyperfractionated radiation therapy 66 Gy for children with brain stem tumors. A phase II study of the Pediatric Oncology Group, Protocol 8833. Cancer. 1993;72:1404–1413. doi: 10.1002/1097-0142(19930815)72:4<1404::aid-cncr2820720441>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Levin VA, Edwards MS, Wara WM, Allen J, Ortega J, Vestnys P. 5-Fluorouracil and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) followed by hydroxyurea, misonidazole, and irradiation for brain stem gliomas: a pilot study of the Brain Tumor Research Center and the Childrens Cancer Group. Neurosurgery. 1984;14:679–681. doi: 10.1227/00006123-198406000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Pakisch B, Urban C, Slavc I, et al. Hyperfractionated radiotherapy and polychemotherapy in brain stem tumors in children. Childs Nerv Syst. 1992;8:215–218. doi: 10.1007/BF00262849. [DOI] [PubMed] [Google Scholar]
- 21.Pendergrass TW, Milstein JM, Geyer JR, et al. Eight drugs in one day chemotherapy for brain tumors: experience in 107 children and rationale for preradiation chemotherapy. J Clin Oncol. 1987;5:1221–1231. doi: 10.1200/JCO.1987.5.8.1221. [DOI] [PubMed] [Google Scholar]
- 22.Walter AW, Gajjar A, Ochs JS, et al. Carboplatin and etoposide with hyperfractionated radiotherapy in children with newly diagnosed diffuse pontine gliomas: a phase I/II study. Med Pediatr Oncol. 1998;30:28–33. doi: 10.1002/(sici)1096-911x(199801)30:1<28::aid-mpo9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara T, Ogawa T, Irie K, Tsuchida T, Nagao S, Ohkawa M. Intra-arterial chemotherapy for brain stem glioma: report of four cases. Neuroradiology. 1994;36:74–79. doi: 10.1007/BF00599203. [DOI] [PubMed] [Google Scholar]
- 24.Bouffet E, Khelfaoui F, Philip I, Biron P, Brunat-Mentigny M, Philip T. High-dose carmustine for high-grade gliomas in childhood. Cancer Chemother Pharmacol. 1997;39:376–379. doi: 10.1007/s002800050586. [DOI] [PubMed] [Google Scholar]
- 25.Dunkel I, Garvin J, Goldman S. High dose chemotherapy with autologous bone marrow rescue for children with diffuse pontine brainstem tumors. Children’s Cancer Group. J Neurosci. 1998;37:67–73. doi: 10.1023/a:1005874508975. [DOI] [PubMed] [Google Scholar]
- 26.Jakacki R, Jamison C, Mathews V. Dose-intensification of procarbazine, CCNU, vincristine with peripheral blood stem cell support in young patients with gliomas. Med Pediatr Oncol. 1998;31:683–690. doi: 10.1002/(sici)1096-911x(199812)31:6<483::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Kalifa C, Hartman O, Vassal G. High dose busulphan and thiotepa following radiation therapy in childhood malignant brainstem glioma [abstract] Pediatr Neurosurg. 1994;21:214. [Google Scholar]
- 28.Kedar A, Maria BL, Graham-Pole J, et al. High-dose chemotherapy with marrow reinfusion and hyperfractionated irradiation for children with high-risk brain tumors. Med Pediatr Oncol. 1994;23:428–436. doi: 10.1002/mpo.2950230507. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger LJ, Sinniah D, Siegel SE, et al. Combination chemotherapy with cyclophosphamide, vincristine, procarbazine, and prednisone (COPP) in children with brain tumors. J Neurooncol. 1985;3:263–269. doi: 10.1007/BF00165188. [DOI] [PubMed] [Google Scholar]
- 30.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 31.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 32.Grill J, Sainte-Rose C, Jouvet A, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6:573–580. doi: 10.1016/S1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 33.Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Frappaz D, Chinot O, Bataillard A, et al. Summary version of the standards, options and recommendations for the management of adult patients with intracranial glioma (2002) Br J Cancer. 2003;89(suppl 1):S73–S83. doi: 10.1038/sj.bjc.6601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 36.Grossman SA, Wharam M, Sheidler V, et al. Phase II study of continuous infusion carmustine and cisplatin followed by cranial irradiation in adults with newly diagnosed high-grade astrocytoma. J Clin Oncol. 1997;15:2596–2603. doi: 10.1200/JCO.1997.15.7.2596. [DOI] [PubMed] [Google Scholar]
- 37.Baltuch GH, Couldwell WT, Villemure JG, Yong VW. Protein kinase C inhibitors suppress cell growth in established and low-passage glioma cell lines: a comparison between staurosporine and tamoxifen. Neurosurgery. 1993;33:495–501. doi: 10.1227/00006123-199309000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Pollack IF, Randall MS, Kristofik MP, et al. Effect of tamoxifen on DNA synthesis and proliferation of human malignant glioma lines in vitro. Cancer Res. 1990;50:7134–7138. [PubMed] [Google Scholar]
- 39.Djerassi I, Kim JS, Reggev A. Response of astrocytoma to high-dose methotrexate with citrovorum factor rescue. Cancer. 1985;55:2741–2747. doi: 10.1002/1097-0142(19850615)55:12<2741::aid-cncr2820551202>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Levin VA, Wilson CB, Davis R, Wara WM, Pischer TL, Irwin L. A phase III comparison of BCNU, hydroxyurea, and radiation therapy to BCNU and radiation therapy for treatment of primary malignant gliomas. J Neurosurg. 1979;51:526–532. doi: 10.3171/jns.1979.51.4.0526. [DOI] [PubMed] [Google Scholar]
- 41.Broniscer A, Leite CC, Lanchote VL, Machado TM, Cristofani LM. Radiation therapy and high-dose tamoxifen in the treatment of patients with diffuse brainstem gliomas: results of a Brazilian cooperative study. Brainstem Glioma Cooperative Group. J Clin Oncol. 2000;18:1246–1253. doi: 10.1200/JCO.2000.18.6.1246. [DOI] [PubMed] [Google Scholar]
- 42.Frappaz D, Ricci AC, Kohler R, Bret P, Mottolese C. Diffuse brain stem tumor in an adolescent with multiple enchondromatosis (Ollier’s disease) Childs Nerv Syst. 1999;15:222–225. doi: 10.1007/s003810050377. [DOI] [PubMed] [Google Scholar]
- 43.Bellissant E, Benichou J, Chastang C. Application of the triangular test to phase II cancer clinical trials. Stat Med. 1990;9:907–917. doi: 10.1002/sim.4780090807. [DOI] [PubMed] [Google Scholar]
- 44.Puchner MJ, Herrmann HD, Berger J, Cristante L. Surgery, tamoxifen, carboplatin, and radiotherapy in the treatment of newly diagnosed glioblastoma patients. J Neurooncol. 2000;49:147–155. doi: 10.1023/a:1026533016912. [DOI] [PubMed] [Google Scholar]
- 45.See SJ, Levin VA, Yung WK, Hess KR, Groves MD. 13-Cis-retinoic acid in the treatment of recurrent toma multiforme. Neuro-Oncology. 2004;6:253–258. doi: 10.1215/S1152851703000607. [DOI] [PMC free article] [PubMed] [Google Scholar]