Abstract
Atrasentan is an oral selective endothelin-A receptor antagonist that may inhibit cell proliferation and interfere with angiogenesis during glioma growth. We conducted a dose-finding study to assess atrasentan’s safety and toxicity and to gather preliminary evidence of efficacy. Patients with recurrent malignant glioma received oral atrasentan at ⩾10 mg/day. We increased the dose among cohorts until the maximum tolerated dose (MTD) was defined. Patients were evaluated for response every 8 weeks and remained on the study until the tumor progressed or toxicities occurred. Twenty-five patients were enrolled, with a median age of 53 years (range, 25 – 70) and a median KPS of 90% (range, 60 – 100%). Twenty- two patients had glioblastoma multiforme (GBM), 2 had anaplastic astrocytoma, and 1 had an anaplastic oliogodendroglioma; 24 patients had received one prior chemotherapy regimen before being enrolled in the study. The most common atrasentan-related toxicities were grade 1 or 2 rhinitis, fatigue, and edema. One patient developed grade 3 hypoxia and peripheral edema at a dose of 90 mg/day. We observed no dose-limiting toxicities in an expanded cohort of 10 patients at 70 mg/day, which was declared the MTD. Two partial responses (8%) were seen in patients with GBM at the 70- and 90-mg/day dose levels, and 4 patients had stable disease before progressing. Nineteen patients have died, and median survival was 6.0 months (95% confidence interval, 4.2 – 9.5 months). We conclude that the MTD of daily oral atrasentan in patients with recurrent malignant glioma is 70 mg/day. Further study of atrasentan with radiation therapy and temozolomide in newly diagnosed GBM is warranted to evaluate the efficacy of this novel agent.
Keywords: anaplastic astrocytoma, antiangiogenesis, atrasentan, endothelin receptor antagonist, glioblastoma multiforme, malignant glioma
In the United States, primary malignant brain tumors are estimated to occur at an incidence of 7.4 per 100,000, with an estimated 18,500 new cases in 2005.1,2 Primary malignant brain tumors, the most common of which is glioblastoma multiforme (GBM) in adults, carry a grave prognosis.3 Surgery, steroids, and radiotherapy improve the outcome of disease in patients with these tumors.4 A recent study documented that temozolomide during and after radiation prolongs overall survival by approximately 2.5 months over radiotherapy alone in patients with GBM, with median survival of 14.6 months.5 Even with the best available therapy, only 25% of patients with this disease survive for 2 years.6,7 When GBM recurs, the efficacy of chemotherapy is limited, and median survival of patients is 6 months.8,9 Novel agents and approaches are needed to improve the outcome of this disease.
The endothelin (ET) axis, which includes three 21-amino-acid peptide ligands designated ET-1, ET-2, and ET-3 and two transmembrane G-coupled receptors, ETA and ETB, is emerging as an important regulator of vasomotor tone, tissue differentiation and development, cell proliferation, and hormone production. ET-1 appears to be a relevant growth factor in preclinical and clinical studies of many cancers, including cancer of the prostate, ovary, colon, cervix, breast, kidney, lung, and brain, as well as melanoma.10 These findings have led to a search for selective ETA antagonists as potential cytostatic agents. ET-1 is also involved in regulating cell proliferation; it has been shown to stimulate DNA synthesis and cell proliferation; and it is a mitogen for several different cell lines, including glioma, prostate, cervical, and ovarian cancer cells. These findings have propelled the development of several potent and selective ETA receptor antagonists, which have entered clinical oncology trials, contributing to our understanding of the relevance of the ET axis.11,12
To date, atrasentan has been investigated in 30 completed phase I, II, and III studies. These studies included 433 healthy volunteers, 1,154 subjects with prostate cancer, 61 subjects with other malignancies, 11 subjects with diabetic nephropathy, and 12 subjects with congestive heart failure. In the completed studies of subjects with prostate cancer, 1,124 subjects received at least 10 mg of atrasentan daily for a minimum of 3 months, 579 subjects received atrasentan for at least 6 months, 291 subjects received atrasentan for at least 12 months, 19 subjects received atrasentan for at least 24 months, and 4 subjects received atrasentan for at least 36 months. The pharmacodynamics of astrasentan demonstrate that it is orally bioavailable, readily absorbed with linear dose proportionality, and can be administered once daily. Plasma concentrations at doses of atrasentan ⩾2.5 mg exceeded the human ETA receptor Ki (0.034 nM) and corresponded to biologically active concentrations in human pharmacodynamic studies and in preclinical studies in vivo. In phase I studies involving both healthy subjects and subjects with cancer, atrasentan was well tolerated over a wide dose range.13 In healthy volunteers, the most commonly observed adverse events were headache, rhinitis, and peripheral edema. These adverse events are believed to be associated with the vasodilatory effects of atrasentan. Dosing in healthy volunteers was limited by headache at 30 mg. In subjects with cancer, the safety profile was consistent with that observed in healthy volunteers; however, subjects with cancer tolerated doses as high as 95 mg, and no maximum tolerated dose (MTD) was identified. Few National Cancer Institute (NCI) Cancer Toxicity Criteria grade 3 or 4 toxicities were observed in the studies involving subjects with cancer.
GBMs express high-affinity ETA receptors on vascular elements and high-affinity ETB receptors on non-vascular elements.14,15 Studies with human astrocytoma cell line U138MG demonstrated ETA receptor expression and suggest a possible role for ET in the regulation of angiogenesis of glioma growth by altering the blood-brain barrier or by acting as a growth factor. In 30 samples of malignant and low-grade gliomas, binding of transforming growth factor (TGF)-β and ET-1 to ETA correlated with malignant degeneration: 100% of GBMs, 40% of astrocytomas, and 20% of low-grade gliomas demonstrated binding to TGF-β and ET-1.16 In addition, ET may function synergistically with other factors to promote growth, angiogenesis, and malignant degeneration.
Atrasentan is a potent, orally bioavailable selective ET antagonist that binds to the ETA receptor.11 It reverses or blocks the effects of ET-1 on the ETA receptor, including its proliferative and angiogenic effects.17 This study was designed to describe the toxicities associated with the administration of atrasentan, to determine the MTD of this agent in patients with recurrent primary malignant glioma, and to obtain preliminary data regarding its therapeutic activity.
Materials and Methods
This study was conducted by the New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium, which is funded by NCI.18 Participating institutions included the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, the H. Lee Moffitt Cancer Center, Winship Cancer Institute at Emory University, Wake Forest University, the Cleveland Clinic, Henry Ford Hospital, the University of Alabama at Birmingham, and the University of Texas at San Antonio. The clinical research protocol was reviewed and approved by the Cancer Therapy Evaluation Program at NCI and by the institutional review boards of all participating institutions. Informed consent was obtained from each patient who participated in the research study. All patients eligible for this study were registered through the NABTT CNS Consortium’s Central Operations Office in Baltimore, MD, USA.
Patient Eligibility Criteria
Patients were eligible for the trial if they were over 18 years of age, able to give informed consent, and understood the investigational nature of this study and its potential risks and benefits. In addition, patients had to meet the following criteria: pathological confirmation of GBM, anaplastic astrocytoma, or anaplastic oligodendroglioma; progressive measurable disease on contrast-enhanced CT or MRI after treatment with radiation therapy; sufficient time for toxicities of previous therapies to have resolved (3 months since radiation, >6 weeks since last nitrosourea, or >3 weeks since other chemotherapy); KPS score of ⩾60%; life expectancy of >3 months; and adequate bone marrow (WBC >2,000/mm3 or absolute neutrophil count >1,500/mm3, platelets >100,000/mm3), renal (serum creatinine ⩽1.7 mg/dl), and hepatic function (total bilirubin ⩽1.5 mg/dl and aspartate aminotransferase/alanine aminotransferase ⩽4 times the upper limit of normal). Participating patients were required to have a Mini Mental Status Exam score of 15 or higher. Patients with the potential for pregnancy or paternity agreed to follow acceptable birth control methods to avoid conception. Women of childbearing potential had to have a negative pregnancy test result. Patients with uncontrolled hypertension, congestive heart failure, a New York Heart Association class-2 cardiovascular disability status, an active infectious process (including hepatitis and HIV), or medical or psychiatric problems unrelated to the malignancy that might jeopardize compliance or put them at undue risk were ineligible for this study. Patients who had received more than one chemotherapy regimen or prior Gliadel wafers or had a prior malignancy within 5 years, except curatively treated carcinoma in situ or basal cell carcinoma of the skin, were also ineligible.
Treatment Scheme
The first three planned dose levels were 10, 20, and 30 mg of oral atrasentan per day, and subsequent dose increments were by 20 mg (e.g., 50, 70 mg), up to a maximum of 150 mg. As we did not expect toxicity at the two lowest dose levels, only two patients were accrued to these cohorts. Cohorts of three patients were accrued to higher dose levels. Atrasentan was administered daily until disease progression or toxicity required the drug to be discontinued. We did not allow intrapatient dose escalations. We formally assessed toxicity on day 15, day 29, and every 4 weeks thereafter while patients were on study. All previously dispensed bottles and any unused study drug were collected at each visit, along with a medication diary to assess compliance.
Definition of MTD
For the purposes of this study, dose-limiting toxicity (DLT) was defined as any of the following treatment-related adverse events: (a) absolute neutrophil count ⩽500/μl for at least 3 days; (b) platelet count ⩽25,000/μl; (c) febrile neutropenia; (d) grade 3 or 4 nonhematologic toxicities, with the exception of nausea and vomiting without adequate antiemetic prophylaxis; and (e) a delay in starting a subsequent course of treatment of more than 7 days due to incomplete recovery from toxicity. The development of seizures, other neurological abnormalities, deep venous thrombosis, or pulmonary emboli were not dose-limiting considerations unless the investigator believed that the event was attributed to the study drug and not the underlying CNS malignancy.
Atrasentan is not a cytotoxic agent, and therefore the determination of the MTD did not follow the standard design for those agents, which targets a 30% – 33% toxicity rate. In this clinical trial we defined the MTD as the dose below the level that produced any DLT. We assigned no more than three patients to a given dose level until we had followed all patients for at least 4 weeks (one cycle), which permitted toxicity to be assessed. We continued to increase the dose until one patient experienced a DLT. We considered the dose preceding this dose level to be the MTD and subsequently expanded the cohort to a maximum of 10 patients. If one of these additional patients experienced a DLT, we planned to decrease the dose to the appropriate level and expand the cohort to a maximum of 10 patients at that dose. This de-escalation would continue until a cohort of 10 patients received a dose with no DLTs. This dose was defined as the MTD.
Toxicity Evaluations
We evaluated toxicities during each 4-week cycle and graded toxicity according to the NCI Common Toxicity Criteria, version 2.0. A complete blood count with differentials and platelet count was performed 7 days after initiating treatment and once a week thereafter, including within 3 days of starting each successive cycle of therapy. In the event of hematologic toxicity, as indicated by an absolute neutrophil count <1,500/μl or platelet count <100,000/μl, we repeated these tests at least every other day until recovery to normal values. Evaluations performed within 3 – 5 days before beginning every new treatment cycle included physical and neurological examinations; vital signs and performance status; complete blood count with differentials and platelet count; and a serum chemistry profile.
Response Assessment
All eligible patients who consented to this study had a baseline pretreatment MRI. CT scans were permitted if there was a medical contraindication to obtaining an MR scan. Scans were repeated before every other treatment cycle (e.g., before cycles 1, 3, 5). If a partial or complete response was noted, the scan was repeated in 1 month to confirm the response. Only confirmed responses were considered. The NABTT neuroradiologist reviewed all radiologic responses using NABTT response criteria.19
Statistical Considerations
The primary end point of this study was safety. We tallied the frequency of toxicities greater than grade 2. A secondary end point was to assess preliminary evidence of efficacy. We calculated patient survival time from start of treatment until death from any cause for overall survival and date of progression or death for progression-free survival (PFS). Survival times were censored at date of last follow-up. We estimated the survival distribution using the method of Kaplan and Meier,20 and calculated confidence intervals (CI) using standard methods. Analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) and STATA (version 8, College Station, TX, USA). All p values reported are two sided.
Results
Patient Characteristics
Twenty-five patients were enrolled in this study between July 2002 and May 2004, and their characteristics are summarized in Table 1. Twenty-four patients were white and one was Asian. Twenty-four patients had received one chemotherapy regimen (1 1,3-bis[2-chloroethyl]-1-nitrosourea; 1 procarbazine, lomustine, vincristine; 1 dalteparin; 2 CPT-11/temozolomide; 19 temozolomide), and 1 patient had received no chemotherapy.
Table 1.
Baseline demographic and clinical characteristics
| Characteristic | n (%) or Median (Range) |
|---|---|
| Age (years) | 52 (25 – 70) |
| Gender, male | 16 (64) |
| KPS | |
| 60% | 1 (4) |
| 70% or 80% | 11 (44) |
| 90% or 100% | 13 (52) |
| Histological diagnosis | |
| Glioblastoma multiforme | 22 (88) |
| Anaplastic astrocytoma | 2 (8) |
| Anaplastic oligodendroglioma | 1 (4) |
| Prior chemotherapy regimens | |
| 0 | 1 (4) |
| 1 | 24 (96) |
| Concomitant antiseizure drugs | |
| Enzyme inducing | 13 (52) |
| None or not enzyme inducing | 12 (48) |
Dose Escalation and Toxicities
We were able to evaluate only 23 patients for toxicity, as 2 patients developed early progression. The dose levels evaluated, the number of patients enrolled at each dose level, and the toxicities that were at least possibly related to atrasentan and occurred in at least 5% of the patients are summarized in Table 2. The most common adverse events were rhinitis, fatigue, and edema; these events occurred in at least 28% of the patients. The only DLT was a grade 3 hypoxia and peripheral edema in a patient after 18 days of atrasentan at 90 mg/day. The 70-mg/day cohort was expanded to 10 patients with no observed DLT, and this was declared the MTD. No myelosuppression was observed. Although the study groups were not stratified by P450 enzyme-inducing antiseizure drug (EIASD) use, there were near equal numbers of patients in each group, and we compared the frequency of toxicities across strata. We did not observe significant toxicities in either group. For the non-EIASD group (n = 12), there were 11 (92%) grade 2, 4 (33%) grade 3, and 2 (17%) grade 4 toxicities. In the EIASD group (n = 13), there were 12 (92%) grade 2, 10 (77%) grade 3, and 2 (15%) grade 4 toxicities.
Table 2.
All toxicities that occurred in at least 5% of patients and were related to atrasentan by dose level (n = 25)
| Dose Level (mg)
|
|||||||
|---|---|---|---|---|---|---|---|
| Toxicity | 10 (n = 2) | 20 (n = 2) | 30 (n = 4) | 50 (n = 3) | 70 (n = 11) | 90 (n = 3) | Overall Percentage of Patients |
| Nausea | 1 | 1 | 8% | ||||
| Vomiting | 1 | 1 | 8% | ||||
| Weight gain | 1 | 1 | 8% | ||||
| ANC | 1 | 1 | 8% | ||||
| Hypocalcemia | 2 | 8% | |||||
| Platelets | 1 | 1 | 8% | ||||
| SGPT | 1 | 1 | 8% | ||||
| Arthralgia | 1 | 1 | 1 | 12% | |||
| Constipation | 1 | 1 | 1 | 12% | |||
| Headache | 1 | 2 | 12% | ||||
| Low WBC | 2 | 1 | 12% | ||||
| Dyspnea | 1 | 2 | 1 | 16% | |||
| Dry mouth | 1 | 1 | 1 | 1 | 16% | ||
| Hemoglobin | 1 | 4 | 1 | 24% | |||
| Allergic rhinitis | 1 | 1 | 1 | 4 | 28% | ||
| Edema | 3 | 5 | 1 | 36% | |||
| Fatigue | 1 | 2 | 2 | 3 | 3 | 1 | 48% |
Abbreviations: ANC, absolute neutrophil count; SGPT, serum glutamic pyruvic transaminase; WBC, white blood count.
Antitumor Activity
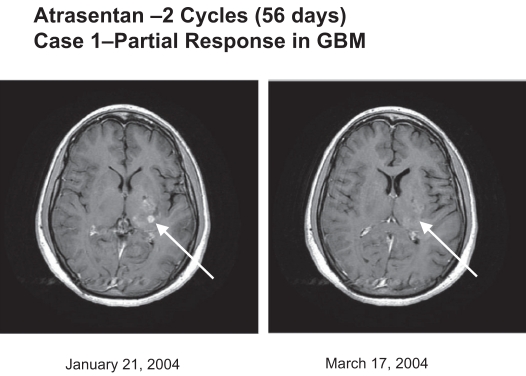
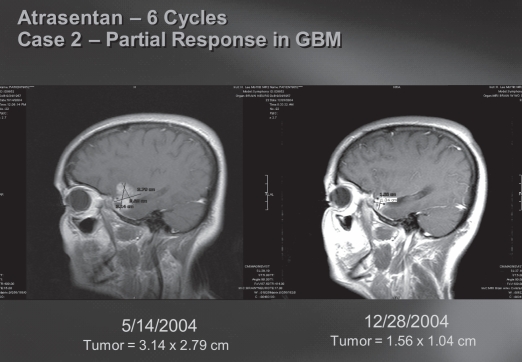
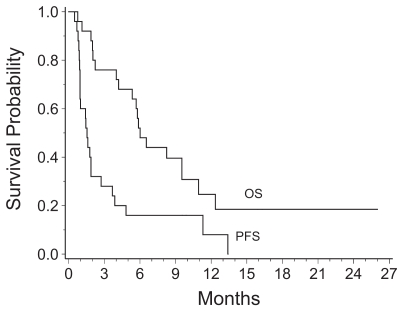
Two GBM patients (one at 90 mg/day and one at 70 mg/day dose level) had a partial response (8% response rate) for 111 and 356+ days, as illustrated in Figs. 1 and 2, and four additional GBM patients (16%) had stable disease for 117, 146, 343, and 407 days before progressing. One patient withdrew from the study before response evaluation. One patient treated at the 70 mg/day dose level has remained on study for more than 12 months. The other 23 patients have progressed, and 19 of the 25 (76%) patients have died. The Kaplan-Meier estimate of median overall survival was 6.0 months (95% CI, 4.2 – 9.5 months), and PFS was 1.5 months (95% CI, 1.0 – 2.7 months; Fig. 3). The 6-month PFS rate for the 22 patients with GBM was 18% (95 % CI, 5% – 40%).
Fig. 1.
Atrasentan (two cycles, 56 days): case 1, partial response in glioblastoma multiforme (GBM). Tumor regression was evidenced by MRI of the brain (axial T1 with gadolinium). A tumor (arrow) showed a partial response.
Fig. 2.
Atrasentan (six cycles): case 2, partial response in glioblastoma (GBM). Atrasentan-induced response in a patient with recurrent GBM after radiation and temozolomide by MRI of the brain (saggital T1 with gadolinium).
Fig. 3.
Kaplan-Meier survival curves for all patients enrolled in the phase I study. Survival was measured from the time of entry into the study. Abbreviations: OS, overall survival; PFS, progression-free survival.
Discussion
This study is the first evaluation of an ETA receptor antagonist in patients with glioma. The safety profile of atrasentan in these patients was consistent with that observed in healthy volunteers and prostate cancer patients, even at the higher doses in this study, and it reflects the physiologic antagonism of ETA receptors.13,21 The two patients with partial responses and several patients with prolonged stable disease reflect similar activity rates as those seen in relief of cancer pain and reduction in prostate-specific antigen and other tumor markers in prostate cancer.22 In addition, the 6-month PFS rate of 13% in patients with GBM is comparable to previous phase II studies of cytotoxic chemotherapy in recurrent GBM, but with less toxicity.8
The more frequently observed adverse effects of fatigue, rhinitis, and edema were attributable to the vasoactive pharmacology of ET receptor compounds. These effects were mild to moderate in intensity, reversible, and when necessary, readily controlled with symptomatic treatment, except in one patient at the 90 mg/day dose level, who developed hypoxia and peripheral edema as a DLT. Similar effects were previously observed in healthy subjects who received up to 40 mg of atrasentan once daily, and such effects have also been reported with administration of other ET antagonists.23,24 All cases of edema occurred in patients taking doses of ⩾50 mg of atrasentan. Although headaches have been reported in other studies of atrasentan and other ET antagonists, headache was not a significant problem in this brain tumor trial.25–27
There are several ways the ET axis could be of critical importance in the therapy of patients with malignant gliomas, aside from its impact on cell proliferation. ET-1 can be amplified by synergistic interactions with other growth factors, including epidermal growth factor, basic fibroblast growth factor, insulin, insulinlike growth factor, platelet-derived growth factor, TGF, and interleukin-6.28 The transcriptional up-regulation of vascular endothelial growth factor (VEGF) has been linked to a critical mediator of hypoxia signaling, the hypoxia-inducible factor-1α (HIF-1α). ET-1 promotes VEGF production through HIF-1α, and this mechanism might be responsible for increasing tumor angiogenesis. Degradation of HIF-1α was, in fact, reduced in ET-1-treated ovarian carcinoma cells under both hypoxic and normoxic conditions. After ET-1 stimulation, HIF-1α protein levels increase in the cells, and the HIF-1 transcription complex is formed and binds to the hypoxia-responsive element binding site. Therefore, ET-1 – induced HIF-1 accumulation activates all the signals necessary for a complete HIF-1 response.29 The HIF-1α – mediated transcription of VEGF by ET-1 under normoxic conditions points to a general mechanism through which oncogenes and growth factors might up-regulate VEGF and through which they could synergize with hypoxia during tumor growth. Addition of a specific ETA receptor antagonist blocked the ET-1 – induced up-regulation of VEGF expression and secretion as well as the ET-1 – induced activation of HIF-1 transcription complex. In tumor cells, ET-1 might be unregulated by hypoxia. Thus, under hypoxic conditions, ET-1 may potentiate the hypoxic stimulus by amplifying HIF-1α stability and VEGF production.30 Activation of the HIF-1 pathway, likely due to the effects of tumor tissue hypoxia, is a common feature of malignant gliomas.
The present study suggests that chronic oral administration of atrasentan, a potent and selective ETA receptor antagonist, is safe for patients with recurrent malignant gliomas. The MTD of daily oral atrasentan in patients with recurrent malignant glioma is 70 mg/day. The responses and stable disease noted in this study suggest that this noncytotoxic compound used as monotherapy in late-stage, recurrent GBM may have activity comparable to other cytotoxic regimens, with much less toxicity. Based on the results of this exploratory trial in recurrent GBM, a phase II study of atrasentan with radiation therapy and concurrent temozolomide is warranted to evaluate the efficacy of this agent in newly diagnosed GBM.
Acknowledgments
This study was performed by the New Approaches to Brain Tumor Therapy CNS Consortium, Baltimore, MD, USA, and supported in part by a grant from the National Cancer Institute, UO1 CA-62475, U01-CA62406 (NABTT) and Abbott Laboratories. Surasak Phuphanich was also supported in part as a Georgia Cancer Coalition Distinguished Cancer Scholar. He is currently the director of the Neuro-Oncology Program at the Cedars-Sinai Medical Center. We thank Todd J. Janus for assistance in original design of this study. This work was presented in part at the 9th annual meeting of the Society for Neuro-Oncology, Toronto, Canada, November 19 – 21, 2004, and the 41st annual meeting of the American Society of Clinical Oncology, Orlando, FL, May 14 – 17, 2005.
References
- 1.Central Brain Tumor Registry of the United States. Statistical report: primary brain tumors in the United States, 1998 – 2002. 2005 – 2006.
- 2.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985 – 1999. Neuro-Oncology. 2006;8:27 – 37. doi: 10.1215/S1522851705000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin VA, Leibel SA, Gutin PH. Neoplasms of the central nervous system. In: DeVita VT Jr, editor; Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th Ed. Philadelphia: Lippincott Williams & Wilkes; 2001. pp. 2100–2160. [Google Scholar]
- 4.DeAngelis LM, Burger PC, Green SB, Cairncross JG. Malignant glioma: who benefits from adjuvant chemotherapy? Ann Neurol. 1998;44:691–695. doi: 10.1002/ana.410440418. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, et al. European Organization for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987 – 996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323 – 1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 7.Levin VA, Kabra P. Effectiveness of the nitrosoureas as a function of their lipid solubility in the chemotherapy of experimental rat brain tumors. Cancer Chemother Rep. 1974;58:787 – 792. [PubMed] [Google Scholar]
- 8.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572 – 2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 9.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588 – 593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Natl Rev Cancer. 2003;3:110 – 116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 11.Opgenorth TJ, Adler AL, Calzadilla SV, et al. Pharmacological characterization of A-127722: an orally active and highly potent ETA-selective receptor antagonist. J Pharmacol Exp Ther. 1996;276:473 – 481. [PubMed] [Google Scholar]
- 12.Remuzzi G, Perico N, Benigni A. New therapeutics that antagonize endothelin: promises and frustrations. Natl Rev Drug Discov. 2002;1:986–1001. doi: 10.1038/nrd962. [DOI] [PubMed] [Google Scholar]
- 13.Zonnenberg BA, Groenewegen G, Janus TJ, et al. Phase I dose-escalation study of the safety and pharmacokinetics of atrasentan: an endothelin receptor antagonist for refractory prostate cancer. Clin Cancer Res. 2003;9:2965–2972. [PubMed] [Google Scholar]
- 14.Wu-Wong JR, Chiou W, Magnuson SR, Bianchi BR, Lin CW. Human astrocytoma U138MG cells express predominantly type-A endothelin receptor. Biochim Biophys Acta. 1996;1311:155 – 163. doi: 10.1016/0167-4889(95)00202-2. [DOI] [PubMed] [Google Scholar]
- 15.Harland SP, Kuc RE, Pickard JD, Davenport AP. Characterization of endothelin receptors in human brain cortex, gliomas, and meningiomas. J Cardiovasc Pharmacol. 1995;26(suppl 3):S408 – S411. [PubMed] [Google Scholar]
- 16.Tsutsumi K, Niwa M, Kitagawa N, et al. Enhanced expression of an endothelin ETA receptor in capillaries from human glioblastoma: a quantitative receptor autoradiographic analysis using a radioluminographic imaging plate system. J Neurochem. 1994;63:2240 – 2247. doi: 10.1046/j.1471-4159.1994.63062240.x. [DOI] [PubMed] [Google Scholar]
- 17.Stiles JD, Ostrow PT, Balos LL, et al. Correlation of endothelin-1 and transforming growth factor beta 1 with malignancy and vascularity in human gliomas. J Neuropathol Exp Neurol. 1997;56:435 – 439. doi: 10.1097/00005072-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Grossman SA, Fisher JD, Piantadosi S, Brem H. The new approaches to brain tumor therapy (NABTT) CNS consortium: organization, objectives, and activities. Cancer Control. 1998;5:107 – 114. doi: 10.1177/107327489800500201. [DOI] [PubMed] [Google Scholar]
- 19.Grossman SA, Alavi JB, Supko JG, et al. Efficacy and toxicity of the antisense oligonucleotide aprinocarsen directed against protein kinase C-alpha delivered as a 21-day continuous intravenous infusion in patients with recurrent high-grade astrocytomas. Neuro-Oncology. 2005;7:32 – 40. doi: 10.1215/S1152851703000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. 1958;53:457 – 481. [Google Scholar]
- 21.Ryan CW, Vogelzang NJ, Vokes EE, et al. Dose-ranging study of the safety and pharmacokinetics of atrasentan in patients with refractory malignancies. Clin Cancer Res. 2004;10:4406 – 4411. doi: 10.1158/1078-0432.CCR-04-0083. [DOI] [PubMed] [Google Scholar]
- 22.Carducci MA, Padley RJ, Breul J, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21:679 – 689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 23.Sutsch G, Kiowski W, Yan XW, et al. Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation. 1998;98:2262 – 2268. doi: 10.1161/01.cir.98.21.2262. [DOI] [PubMed] [Google Scholar]
- 24.Study Report M96-435. A phase I, double-blind, placebo-controlled, multiple dose study of the safety and tolerability of ABT-627. Abbott Laboratories; Chicago, IL, USA: 1998. [Google Scholar]
- 25.Verhaar MC, Grahn AY, Van Weerdt AW, et al. Pharmacokinetics and pharmacodynamic effects of ABT-627, an oral ETA selective endothelin antagonist, in humans. Br J Clin Pharmacol. 2000;49:562 – 573. doi: 10.1046/j.1365-2125.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dingemanse J, Clozel M, van Giersbergen PL. Pharmacokinetics and pharmacodynamics of tezosentan, an intravenous dual endothelin receptor antagonist, following chronic infusion in healthy subjects. Br J Clin Pharmacol. 2002;53:355 – 362. doi: 10.1046/j.1365-2125.2002.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan hypertension investigators. N Engl J Med. 1998;338:784 – 790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 28.Battistini B, Chailler P, D’Orleans-Juste P, Briere N, Sirois P. Growth regulatory properties of endothelins. Peptides. 1993;14:385 – 399. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- 29.Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem. 2002;277:27850 – 27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- 30.Gupta RA, Tejada LV, Tong BJ, et al. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003;63:906 – 911. [PubMed] [Google Scholar]