Abstract
The North American Brain Tumor Consortium (NABTC) is a multi-institutional consortium with the primary objective of evaluating novel therapeutic strategies through early phase clinical trials. The NABTC has made substantial changes to the design and methodology of its trials since its inception in 1994. These changes reflect developments in technology, new types of therapies, and advances in our understanding of tumor biology and biological markers. We identify the challenges of early clinical assessment of therapeutic agents by reviewing the clinical trial effort of the NABTC and the evolution of the protocol template used to design trials. To better prioritize effort and allocation of patient resources and funding, we propose an integrated clinical trial design for the early assessment of efficacy of targeted therapies in neurooncology. This design would mandate tissue acquisition prior to therapeutic intervention with the drug, allowing prospective evaluation of its effects. It would also include a combined phase 0/I pharmacokinetic study to determine the safety and biologically optimal dose of the agent and to verify successful modulation of the target prior to initiating a larger, phase II efficacy study.
Keywords: brain tumor, clinical trials, North American Brain Tumor Consortium, targeted therapies
Over the last decade, increasing knowledge of the pathogenesis of glioma formation and progression has led to exploration of novel therapeutic strategies to improve patient outcome. Early phase I and II clinical trials are designed and conducted to assess the safety, toxicity, and efficacy of these strategies. To date, however, minimal antitumor activity has been demonstrated using the single-targeted-agent approach in neurooncology.1–3 Challenges in the evaluation of targeted agents have been previously identified,4–9 and in this review we focus on the North American Brain Tumor Consortium (NABTC) experience testing these agents to learn from this effort and to highlight areas for potential improvement in clinical trial design.
History of the NABTC: Goals, Institutions, and Infrastructure
The NABTC is a National Cancer Institute (NCI)–funded group effort with the primary objective of evaluating novel therapeutic strategies through early phase trials. The consortium was initially funded in 1994 and comprises 10 member institutions and one data management center (Table 1). Michael Prados, M.D., of the University of California, San Francisco, is the principal investigator for the grant supporting the consortium. A major initial challenge for the group was to establish a central operations office to oversee the regulatory process of activation and conduct of the clinical trials. Proposals to study novel agents are based on the availability of novel therapeutics provided by the Cancer Therapy Evaluation Program (CTEP) of the NCI. Chemotherapeutic agents are also provided by pharmaceutical companies if they are approved by CTEP.
Table 1.
North American Brain Tumor Consortium, 2007 member institutions
| University of California, San Francisco |
| University of California, Los Angeles |
| University of Texas M. D. Anderson Cancer Center |
| Pittsburgh Cancer Institute |
| University of Wisconsin, Madison |
| Dana Farber Cancer Institute |
| Memorial Sloan Kettering Cancer Center |
| Duke University |
| Neuro-Oncology Branch, National Cancer Institute/National Institute of Neurological Disorders and Stroke |
| University of Texas Health Science Center, San Antonio |
| Data Management Center M. D. Anderson Cancer Center |
Following a review of potential therapeutic strategies, consortium members prioritize study concepts. Individual institutional investigators are responsible for generating the protocol documents for activation. The NABTC performs phase I pharmacokinetic trials and pilot phase II studies in CNS tumors. A rotational system allocating sites for participation as phase I study slots become available facilitates accrual at all sites. Differences in patient eligibility criteria also allow for accrual to noncompeting studies. The pharmacology group at the University of Texas Health Science Center, San Antonio, under the leadership of John Kuhn, Pharm.D., has been critical for implementing the pharmacokinetic component of these studies. Over the last 12 years of funding, 21 trials have been completed and 11 are ongoing (Table 2). More than 1,000 patients, primarily with recurrent malignant glioma, have been accrued.
Table 2.
North American Brain Tumor Consortium therapeutic trials from 1994 to 2007
| Agent | Phase | Disease Status | Histology |
|---|---|---|---|
| Cytotoxic agents | |||
| Temozolomide and BCNU32,33 | II | Newly diagnosed and recurrent | Grade III |
| Temozolomide and irinotecan | I/II | Recurrent | HGG |
| Carboplatin and thymidine34 | II | Recurrent | HGG |
| Irinotecan35,36 | I/II | Recurrent | HGG |
| Targeted agents | |||
| OSI-77437 | I/II | Recurrent | HGG |
| ZD-183937 | I/II | Recurrent | HGG |
| R1157772 | I/II | Recurrent | HGG |
| CCI-7791,38 | I/II | Stable/recurrent | HGG |
| OSI-774/CCI-779 | I/II | Recurrent | HGG |
| Fenretinide39 | II | Recurrent | GBM |
| STI-571 | II | Recurrent | Meningioma |
| STI-5713 | I/II | Recurrent | HGG |
| Celengitidea | II | Recurrent | GBM |
| GW572016a | II | Recurrent | GBM |
| Depsipeptidea | I/II | Recurrent | HGG |
| R115777a | I/II | Newly diagnosed | GBM |
| Sorafenib and either erlotinib, tipifarnib, or temsirolimusa | I/II | Recurrent | GBM |
| VEGF-trapa | II | Recurrent | GBM |
| Pazopaniba | II | Recurrent | GBM |
| Cytotoxic and targeted agents | |||
| Temozolomide and cis-retinoic acid40 | II | Recurrent | HGG |
| Temozolomide and thalidomide41 | II | Recurrent | GBM |
| Temozolomide and ZD1839 | I | Recurrent | HGG |
| Temozolomide and vorinostata | I | Stable and recurrent | HGG |
| Temozolomide and rituximaba | II | Recurrent | CNS lymphoma |
| Other | |||
| O6-benzylguanine42 | I | Recurrent | HGG |
| Adenovirus-mediated wild-type p5343 | I | Recurrent | HGG |
| Poly-ICLC | II | Newly diagnosed | GBM |
| Poly-ICLC | II | Recurrent | Grade III |
| Phenylacetate44 | II | Recurrent | HGG |
| Phenylacetate45 | II | Recurrent | Primary brain tumor |
Abbreviations: BCNU, 1,3-bis(2-chloroethyl)-1-nitrosourea (carmustine); HGG, high-grade glioma; GBM, glioblastoma multiforme; VEGF, vascular endothelial growth factor; poly-ICLC, polyriboinosinic-polyribocytidylic acid-polylysine carboxymethylcellulose.
Current studies in the NABTC 2007 clinical trial portfolio.
Protocol Template Development
There were clear advantages to developing a protocol template for the consortium. The main objectives were to facilitate efficient design and development of studies and to standardize important elements such as eligibility criteria and assessment of outcome to create a historical database for the next generation of studies. Sections specific to the nature of the agent, the scientific background and rationale of the therapeutic approach, and expected toxicities were provided by the principal investigator responsible for the protocol. Biostatistical and pharmacology input is provided by the consortium’s core directors. Templates for phase I/II studies in newly diagnosed glioblastoma multiforme (GBM), recurrent malignant glioma, and recurrent meningioma were developed.
End Point Assessment
In 1999, Wong et al. described outcomes and progression for patients with recurrent glioma, which suggested that 6-month progression-free survival (6moPFS) was a more appropriate end point for these patients than clinical response.10 Following this development, the NABTC changed the end point for assessment of efficacy from response rate to 6moPFS on the protocol template in 2000. End points such as radiographic response and symptom assessment are often imprecise measures of tumor burden and drug efficacy.11,12 Progression status at 6 months can more directly define clinical benefit and is an important determinant of overall survival.13 While evaluation of efficacy might also take into consideration imaging and symptoms, progression-free survival (PFS) is strongly correlated to overall survival, and therefore an appropriate end point for determining the value of an experimental treatment. The biostatistical considerations associated with establishing 6moPFS as our primary efficacy end point were standardized in the protocol template. In the future, an end point that takes into consideration quality of life may also be appropriate when evaluating new therapies, but such an end point has yet to be validated.
Cytotoxic Therapies
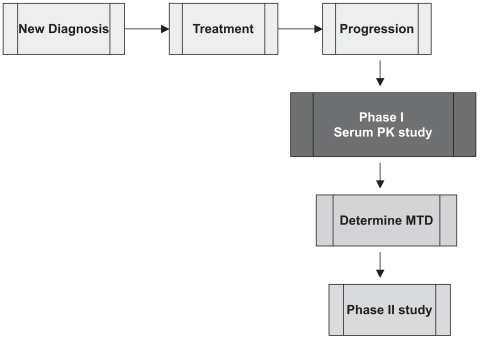
The standard approach for the early clinical evaluation of therapeutic agents for cancer involves the completion of a phase I study to assess dose-limiting toxicities and determine the maximal tolerated dose for phase II studies (Fig. 1). Pharmacokinetic studies are linked to these early studies to provide information on drug clearance and steady state characteristics. Between 1994 and 1998, phase II NABTC studies in brain tumor patients were performed using dosing schedules derived from previous phase I studies of cytotoxic agents in patients with other systemic solid tumors. However, the results of a phase II study of paclitaxel revealed that the toxicity profile seen in a population of patients with brain tumors was unexpectedly different from previous clinical experience in patients without brain tumors.14 Specifically, although a 30% rate of myelosuppression was expected, our patients rarely experienced this in the phase II study. This highlighted the potentially unique factors in this patient population with respect to the use of hepatic enzyme-inducing antiepileptic drugs (EIAEDs) such as phenytoin, carbamazepine, and phenobarbital. These agents alter the pharmacokinetics of therapeutic agents metabolized through the P450 cytochrome system. Based on this finding, a phase I/pharmacokinetic study of paclitaxel was performed in patients who were taking EIAEDs (designated group B), and the maximal tolerated dose was found to be 1.5 times the dose administered to patients who were not being treated with these agents (group A). Correlative pharmacokinetic studies demonstrated similar pharmacokinetic results at the respective maximum tolerated doses (MTDs) in the two groups.15 In addition, the toxicity profile was very different in the group B patients, for whom central neurotoxicity rather than standard myelosuppression was the dose-limiting toxicity.
Fig. 1.
Standard design of phase I toxicity study (to determine maximum tolerated dose [MTD] of drug) and phase II efficacy study in patients with recurrent malignant glioma. Abbreviation: PK, pharmacokinetic.
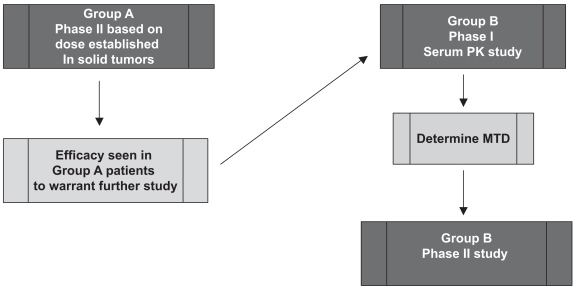
As a result of this finding and the known common metabolism of agents through the P450 cytochrome system, the first standardized template was developed allowing phase I/pharmacokinetic trials with concurrent dose escalations within the two groups (those patients taking EIAEDs and those not). This also afforded a comparison of the pharmacokinetic parameters between the two groups, which was critical for proceeding to the phase II studies. The availability of newer, non-P450-substrate antiepileptic agents such as levetiracetam allowed the NABTC to further modify its clinical trial design by first assessing efficacy in group A patients using established phase II doses prior to embarking on phase I/II evaluation in group B patients (Fig. 2). This protocol ensured that only if sufficient antitumor activity was seen in group A patients would the resources be committed to further study the appropriate dose in group B patients.
Fig. 2.
Phase I/II clinical trial design that takes into account use of P450 hepatic enzyme inducing antiepileptic drugs (EIAEDs). Only if sufficient antitumor activity is seen in patients not taking EIAEDs (group A) would resources be committed to further study of the appropriate dose in patients taking EIAEDs (group B). Abbreviations: PK, pharmacokinetic; MTD, maximum tolerated dose.
Molecularly Targeted Agents
Perhaps the most important factor that has necessitated review of the methodology and process of early evaluation of novel agents has been the availability of targeted therapies relevant to glioma and the rapid translation of these agents to the clinical arena. These therapies have been developed based on the knowledge of specific pathways known to affect glioma growth and invasion. Biological pathways dysregulated in tumors, but not in normal tissue, allow for rational selection of targets for modulation. There are many challenges in the evaluation of these agents in clinical research; key questions that should be addressed prior to initiating targeted studies are listed in Table 3.
Table 3.
Essential considerations for clinical evaluation of targeted therapies
| Is the target present? |
| Does the drug reach the target? |
| Is the target modulated? |
| Does hitting the target affect downstream signaling? |
| How many signals should be turned off? |
| Is there a biologic effect? |
| What is the biologically optimal dose and is it the same as the MTD? |
| What is the appropriate clinical end point to assess efficacy of the agent? |
| Can we select patients who are most likely to benefit from targeted therapy? |
| Can rational, multitarget approaches be determined based on mechanisms of drug resistance? |
| What is the role of multiple agents and multimodality approaches? |
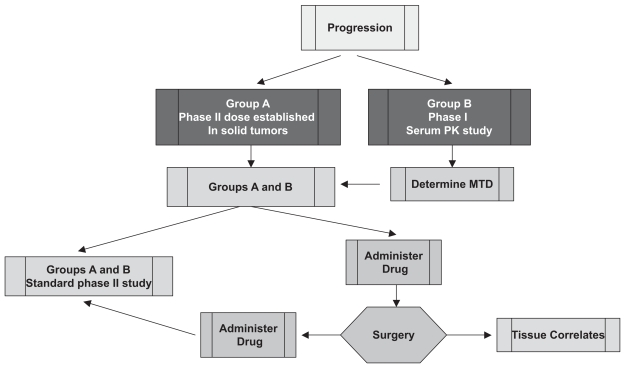
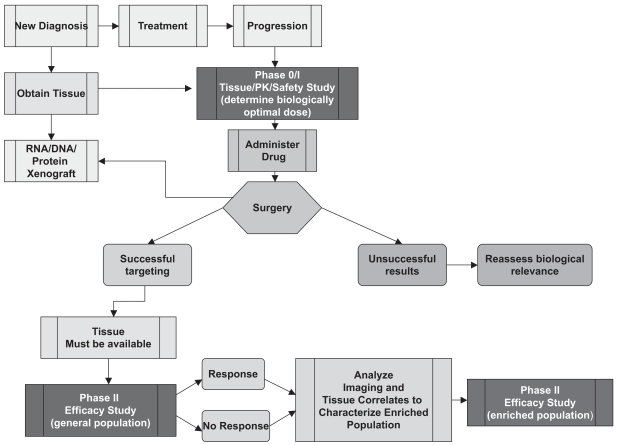
One of the primary questions is whether the drug reaches the tumor. To address this, the NABTC protocol template was changed to incorporate a small pilot arm of 10 patients with recurrent malignant glioma who required reoperation as part of their standard management. This served as an exploratory arm to determine the practicality of collecting data of this type, and the accrual of 10 patients was thought to be both feasible and sufficient to average out some patient variability. For this very select group of patients, the agent was administered preoperatively and at the time of surgery; tissue correlative studies assessing target inhibition and tissue pharmacokinetic studies were included (Fig. 3). This procedure provided preliminary data on tissue distribution and signaling pathway status pertinent to the agent administered.
Fig. 3.
Phase I/II design for recurrent malignant glioma taking into account use of hepatic enzyme-inducing antiepileptic agents, with pilot tissue correlate study for targeted agents. Abbreviations: PK, pharmacokinetic; MTD, maximum tolerated dose.
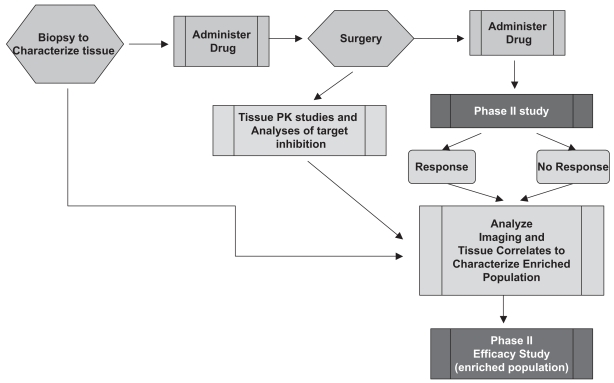
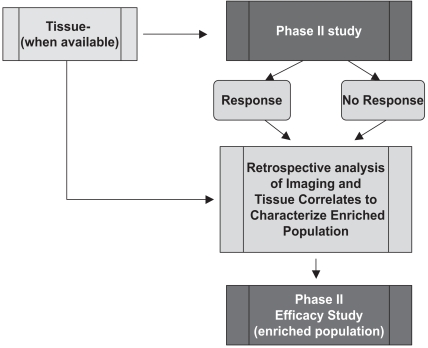
To fully address the utility of targeted therapies, particularly in trying to assess the biological effects of treatment and to identify the population of patients who may benefit most from the treatment, a more prospective approach is needed. This has been outlined by Lang et al.,16 and in ideal circumstances includes the acquisition of tissue to characterize baseline status of key signaling pathways before drug administration, followed by a short period of exposure to the agent, followed by another surgical procedure to evaluate the effects of the agent on the pathway of interest (Fig. 4). The advantages of this approach are the delineation of the pathway before and after treatment and the prospective acquisition of tissue that can be analyzed subsequently. However, there are many practical and ethical barriers to the conduct of these complex studies, including the requirement of two surgical procedures in a short time interval, one of which has no therapeutic intent, and the accompanying risks, as well as the high cost. In the past, biological and clinical correlations were made from retrospective analyses17,18 (Fig. 5). This approach has the disadvantages of providing incomplete data for some patients treated and inability to assess whether the target was modulated. The latter is of particular concern when the end point is PFS or overall survival rather than response, because it may be unclear whether improved outcome indicates that the therapy was more successful in a particular patient group or that tumors with a particular marker are inherently less aggressive. Also, selection of patients is not based on the pretreatment characteristics specific to the drug and additional time is necessary to conduct the retrospective analyses.
Fig. 4.
Ideal prospective analysis study of tissue correlates to identify populations of patients who may benefit from treatment with targeted agents. Abbreviation: PK, pharmacokinetic.
Fig. 5.
Retrospective analysis of tissue correlates to identify populations of patients who may benefit from treatment with targeted agents.
Lessons Learned
Several lessons have been learned from the completion of studies evaluating targeted agents. Minimal efficacy has been demonstrated for single agents tested in the brain tumor population to date. Knowledge of potential targets existing in glioma and the ease and tolerability of administration of the agents provided the rationale for evaluation in phase II studies without first addressing key aspects regarding the use of specific targeted therapies. Insufficient early studies have been performed to assess drug distribution (especially to the CNS), mechanism of action, and biological activity before proceeding with phase II studies. Furthermore, there has been a lack of prospective studies to ensure tissue analyses to select the population who may benefit and, instead, inefficient and usually incomplete analyses of most correlative studies have been performed as retrospective studies.
For newer NABTC trials implemented in 2006, prospective acquisition of tissue samples following drug administration is then followed by a standard phase II component. When possible, tissue for original diagnosis is also acquired. This approach improves our ability to link key aspects of the biological effects of the agent to clinical outcome in all patients. This approach has limitations, however, because relatively large numbers of patients are subjected to a therapeutic agent before its biological activity has been validated and because analyses of tissue correlates are performed retrospectively. Therefore, potentially important information regarding the agent’s mechanism of activity is not incorporated into the planning of the study.
The Challenge
Before committing patients and clinical trial resources to further studies of targeted therapies, the challenge is to improve our methods of investigating targeted agents. This has been the rationale in proposing an integrated phase 0/I/II correlative study protocol template for the NABTC (Fig. 6). Phase 0 trials generally evaluate pharmacokinetic and pharmacodynamic profiles of new drugs in a small group of patients prior to initiating classic phase I tolerability studies.19 These early studies can also examine the biological effects of targeted agents20 but cannot replace phase I dose-escalating or toxicity studies. Our new proposal consists of three distinct protocols, each with its own objective. The goal of this study design is to acquire the important translational information linked to clinical end points that would justify allocating resources to continued evaluation of a targeted agent. One of the major priorities of this approach is the acquisition of tissue for all patients at the time of initial diagnosis and at the time of recurrence when applicable. This is consistent with the glioma molecular diagnostic initiative led by Howard Fine, M.D., director of the Neuro-Oncology Branch of the National Institutes of Health.21 Tissue acquired at the time of initial surgery could be studied in laboratory models such as orthotopic xenograft models or stem cell cultures. These studies would include genomic characterization of individual tumors, to provide a tissue reference for future clinical trials and an ongoing resource for additional translational research. This approach is especially critical as more knowledge is gained about specific pathways and interrelationships of pathways. As basic science research has demonstrated, the interdependence of signaling pathways is extremely complex.
Fig. 6.
Integrated phase 0/I/II tissue correlate protocol designs for targeted therapies. The phase 0 protocol is used to determine the biologically optimal dose. The phase I protocol is a standard toxicity study. The phase II protocol is performed in a general population of patients with brain tumors to determine efficacy, and ultimately in an enriched population selected for their molecular characteristics. Abbreviation: PK, pharmacokinetic.
As it is well recognized that the MTD may not be the optimal biological dose for targeted agents, our design proposes the integration of an early phase study (phase 0) with the primary objective of determining the optimal biological dose as defined by “successful” targeting. This would require tissue acquisition from a limited number of patients who have had preoperative exposure to the agent. Only if the target is successfully modulated in the phase 0/I setting would a phase II study in a general patient population be performed. Otherwise, more preclinical studies would have to be performed before allocating further resources to study the agent. This early, small, tissue-based study has been a missing link in the prior evaluation of targeted therapies. However, it would not replace the standard phase I dose-escalation or toxicity assessment.
For phase II studies, mandating the availability of tissue for analysis of the presence of the target and other relevant biological markers would ensure that once the phase II efficacy study in the general brain tumor population has been completed, analyses of the biological markers in conjunction with clinical outcome would be performed to identify the population of patients most likely to benefit. Once this population is characterized, a phase II study of the preselected patient population enriched for the desired target would be planned to estimate the degree of activity of the agent. If the assays of biological activity and target modulation are robust enough to justify an enriched population for initial phase II study, the agent may not need to be tested in the general brain tumor population. In this case, following the phase 0/I protocol, the agent would proceed directly to a phase II study in the enriched population. To date this has not been possible, but it is where future trials should focus in order to avoid enrolling patients who are not expected to benefit.
Identifying and validating therapeutic targets require novel biomarkers and analytical tools, the development and application of which are not without their own challenges and have been thoroughly discussed elsewhere.22,23 It is likely that multiple oncogenes and pathways are activated or dysregulated before malignancies develop in the brain, and therefore the presumed target may not necessarily be responsible for efficacy of the drug. Development of clinically relevant animal models is needed to better understand the specific biological pathways and more precisely identify targets. It is also important to be able to measure whether the target was affected by the drug, the effect of target modulation on the pathway and relevant downstream components, and the clinical outcome.24 Methods of measuring the abnormal target and pathway modulation also need to be standardized in order to avoid potentially conflicting results, as has been seen in a number of retrospective genomic analyses.16,17 In addition, the statistical components should take into account the frequency of the target in the general population to avoid rejecting a drug that would require a large sample size to demonstrate efficacy. It is also important to note that identifying targets in tissue may require sophisticated or expensive molecular techniques, which would be unsuitable for screening large groups of patients prior to initiating clinical trials. Assays must be accessible and practical in order to be applicable to the clinical setting.
The rapid development of targeted agents has made it necessary to reevaluate clinical trial design in all fields of oncology, and other teams of researchers have reached similar conclusions with regard to more stringent studies of therapeutic agents in smaller populations of patients prior to initiating standard phase I studies.20,24–26 Kummar et al.20 outlined a proposal for incorporating “phase 0” trials that would utilize strong preclinical data, pharmacodynamic assays, and multiple biopsies to determine if a target has been modulated.
Many of these issues are relevant to clinical trials of gliomas; however, studies in brain tumor patients have unique limitations imposed by the challenges in tumor acquisition and the potential for brain injury from tumor sampling or treatment. Evidence of drug safety, from phase I pharmacokinetic studies of MTD or studies of the drug in solid tumors, is necessary prior to initiating a trial for brain tumors that requires tissue acquisition. For example, it must be determined that the drug will not inhibit wound healing or cause hemorrhage following surgical procedure. In addition, because of the increased risk of morbidity associated with serial biopsies, the development of surrogate markers of activity is much more critical in the design of future trials and resources must be allocated to developing such surrogates. Validated imaging markers would be particularly beneficial. MR spectroscopy imaging, diffusion- and perfusion-weighted imaging, and PET with novel imaging probes are promising tools that may be incorporated into future trials.
Challenges Specific to Antiangiogenic Agents in Neurooncology
Malignant gliomas rely on blood vessels for survival and growth, providing a strong rationale for antiangiogenic treatment strategies.27 There are specific challenges in assessing the efficacy of these agents in clinical trials. First, the integrated paradigm that includes tissue acquisition following administration of the agent to assess biological activity would not be feasible for studies of these agents. Inhibition of wound healing would be a major contraindication to the administration of antiangiogenic agents in the immediate preoperative period. Therefore, other methods of measuring of biological activity must be evaluated. Batchelor et al. have reported on elegant physiological imaging techniques, visualizing vascular perfusion and permeability characteristics, which were used to evaluate the activity of AZD2171.28 These techniques need to be validated, standardized, and assessed prospectively. The NABTC currently has two ongoing studies of vascular endothelial growth factor (VEGF)-trap and pazopanib, both of which target the VEGF-related pathway. Perfusion-weighted MRI scans of patients in these trials are being evaluated prospectively to assess the effects of treatment. However, until this technique has been validated as a surrogate of drug activity, the results of the imaging component of the study cannot be used to influence the design of the study in either determining eligibility criteria or assessing efficacy.
Second, appropriate end points to assess clinical benefit should be selected for these agents, including those related to response, survival, and quality of life. Clinical evaluation of bevacizumab and irinotecan demonstrated a high response rate and improvement in 6moPFS compared to prior strategies.29,30 Improvement in the volume of contrast enhancement, which in previous studies of cytotoxic agents has been assumed to indicate antitumor effect, may, in the case of antiangiogenic agents, represent restoration of the blood–brain barrier, which is a transient phenomenon for the patient. Although 6moPFS and response rate may be generally improved for patients treated with antiangiogenic therapies, overall survival may not differ from that for other treatments. Therefore, criteria for assessment of efficacy need to be revised for these agents, and 6moPFS must be revalidated as a clinically relevant surrogate of overall survival. Serial serum biomarkers, such as circulating endothelial cells and plasma basic fibroblast growth factor, may be surrogates of an agent’s activity and should also be validated in future studies. Transient improvement of edema, mass effect, and brain shift with concomitant improvement of clinical symptoms and reduction of the need for corticosteroids have been reported following treatment with antiangiogenic agents.28,29 Incorporation of standardized quality-of-life assessments are important for these studies and should be considered as secondary end points of clinical benefit.
Third, these agents can be associated with significant adverse effects, including hypertension, proteinuria, thromboembolic disease, and intracranial hemorrhage. It is therefore critical to try to identify the patient population that would benefit most from these treatments, thereby sparing ineffective, potentially toxic treatment for those unlikely to benefit. Although preoperative sampling as part of the clinical trial design of antiangiogenic agents may not be feasible, retrospective evaluation of tissue characteristics evaluating angiogenic markers may be important to assess benefit. Validated imaging surrogates, as explored by Chen et al.31 using fluorothymidine PET in patients treated with bevacizumab and irinotecan, or serum biomarkers may help in identifying the optimal patient population.
Finally, despite initial improvement in the imaging and clinical status of patients treated with antiangiogenic agents, tumors almost always recur. Strategies to circumvent the resistance mechanisms that result in progression of disease need to be evaluated, especially those that pertain to tumor invasion and cooption of normal brain vasculature. Early markers of progressive disease or lack of efficacy, when available, should be incorporated into the trial design. This is applicable for any new therapeutic agent and not limited to antiangiogenic therapies.
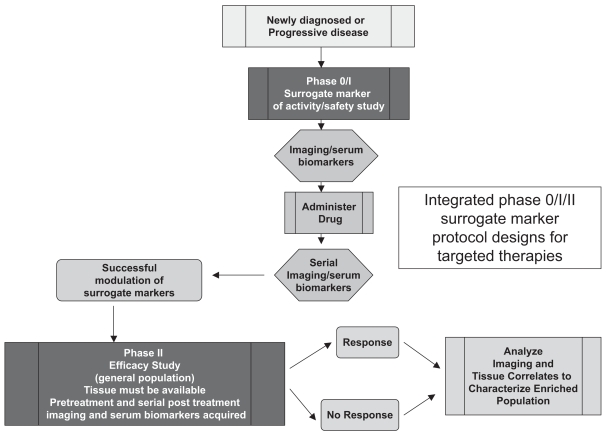
Fig. 7 outlines a potential trial design in which an imaging or serum biological surrogate of an agent’s activity is used, rather than direct measures of pathway modulation measured from tissue samples following exposure. The surrogate marker must have been validated as a measure of drug effect. The phase 0 trial evaluates a limited number of patients with pretreatment and serial posttreatment acquisitions of the surrogate marker to assess the success of the agent in having an effect on the marker. If this is demonstrated, then a phase II study is conducted that mandates the availability of tissue markers from a previous surgery as well as the planned pretreatment and serial acquisition of the surrogate markers. Standard assessment of efficacy is made and analyzed with the surrogate marker and tissue results to identify patients who may benefit and enable the selection of an enriched population for further study.
Fig. 7.
Integrated phase 0/I/II surrogate marker protocol designs for targeted therapies.
Key Elements to Ensure Success of the Integrated Approach
To ensure the success of this approach, some key elements would need to be in place. There must be a close interaction with basic scientists to understand and define pathways, targets, and the mechanism of action of the agent. Basic scientists need to be involved on an ongoing basis in the design and conduct of the clinical trial. This is critical for the definition of reliable, reproducible assays to identify targets and in the selection of biological end points that indicate successful effect of targeted therapy. Such assays are indispensable if information about the effect of the agent is to be accurately evaluated before studying the effects in the general brain tumor population. Involvement of a biostatistician to assist with determining the size of the phase 0/I study population will be critical. In addition, real-time assessment of the biological end points will need to be mandated to move the trial design forward efficiently. A multidisciplinary approach and infrastructure to ensure tissue acquisition and processing in all patients will need to be established, along with central tissue and animal cores to support continued research while clinical studies are ongoing. Noninvasive markers of activity (e.g., imaging, serum markers) need to be explored in addition to the known biological tissue markers.
Conclusions
Significant effort has been expended on the part of patients and clinical trial personnel to design and conduct early clinical trials in neurooncology through the NABTC. What we have learned has been critical in rethinking the design of trials involving targeted therapies. To gain the most information about these agents in an efficient manner, we propose an integrated clinical trial design for the early assessment of efficacy of targeted strategies. There will need to be close and ongoing interaction of basic scientists and clinicians to translate information learned both in the laboratory and at the bedside to effectively identify the patient population most likely to benefit and to assess efficacy. Further development of noninvasive biomarkers will be a key component of this effort. Identifying enriched populations will ensure that patients have quick access to the most effective strategy for their individual tumors, without being exposed to unnecessary treatments. With the budgetary constraints present in the current era, it is imperative that the prioritization of effort and allocation of resources allow for expeditious evaluation of these exciting agents.
Acknowledgments
We thank Ilona Garner, Department of Neurological Surgery, University of California San Francisco, for editorial support. Financial support was received through prime award CA 62399 from the National Institutes of Health to the North American Brain Tumor Consortium (NABTC) investigators and through the individual NABTC and General Clinical Research Center grants listed in the appendix.
Appendix.
Individual NABTC and General Clinical Research Center grants providing financial support for the research reported in this article
| Institution | Investigators | NABTC Grant | GCRC Grant |
|---|---|---|---|
| University of California, San Francisco | Michael D. Prados | CA 62422 | M01-RR00079 |
| Susan M. Chang* | CA 62399 | ||
| Kathleen R. Lamborn | |||
| University of Texas Health Science Center, San Antonio | John G. Kuhn* | CA 62426 | N/A |
| University of Texas M.D. Anderson Cancer Center | W.K. Alfred Yung | CA 62412 | CA 16672 |
| Mark R. Gilbert* | |||
| Dana-Farber Cancer Institute | Patrick Y. Wen* | CA 62407-13 | N/A |
| Neuro-Oncology Branch, NCI/NINDS | Howard A. Fine* | N/A | N/A |
| University of Wisconsin | Minesh Mehta* | CA 62421 | 1U11RR025011 |
| H. Ian Robbins | |||
| Memorial Sloan-Kettering Cancer Center | Lisa M. DeAngelis* | CA 105663-03 | N/A |
| Lauren E. Abrey | |||
| University of Pittsburgh | Frank Lieberman* | CA 62405 | M01-RR00056 |
| University of California, Los Angeles | Timothy F. Cloughesy* | CA 62399 | M01-RR0865 |
Abbreviations: NABTC, North American Brain Tumor Consortium; GCRC, General Clinical Research Center; N/A, not applicable; NCI, National Cancer Institute; NINDS, National Institute of Neurological Disorders and Stroke.
Principal investigators.
References
- 1.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 2.Cloughesy TF, Wen PY, Robins HI, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–3656. doi: 10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]
- 3.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 4.Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. Oncologist. 2002;7:401–409. doi: 10.1634/theoncologist.7-5-401. [DOI] [PubMed] [Google Scholar]
- 5.Ma BB, Britten CD, Siu LL. Clinical trial designs for targeted agents. Hematol Oncol Clin North Am. 2002;16:1287–1305. doi: 10.1016/s0889-8588(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 6.Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007;6:1909–1919. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- 7.Parulekar WR, Eisenhauer EA. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst. 2004;96:990–997. doi: 10.1093/jnci/djh182. [DOI] [PubMed] [Google Scholar]
- 8.Rowinsky EK. The pursuit of optimal outcomes in cancer therapy in a new age of rationally designed target-based anticancer agents. Drugs. 2000;60(suppl 1):1–14. doi: 10.2165/00003495-200060001-00001. [DOI] [PubMed] [Google Scholar]
- 9.Simon R, Maitournam A. Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res. 2004;10:6759–6763. doi: 10.1158/1078-0432.CCR-04-0496. [DOI] [PubMed] [Google Scholar]
- 10.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 11.Hess KR, Wong ET, Jaeckle KA, et al. Response and progression in recurrent malignant glioma. Neuro-Oncology. 1999;1:282–288. doi: 10.1215/15228517-1-4-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant R, Liang BC, Slattery J, et al. Chemotherapy response criteria in malignant glioma. Neurology. 1997;48:1336–1340. doi: 10.1212/wnl.48.5.1336. [DOI] [PubMed] [Google Scholar]
- 13.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-Oncology. 2008;10:162–70. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prados MD, Schold SC, Spence AM, et al. Phase II study of paclitaxel in patients with recurrent malignant glioma. J Clin Oncol. 1996;14:2316–2321. doi: 10.1200/JCO.1996.14.8.2316. [DOI] [PubMed] [Google Scholar]
- 15.Chang SM, Kuhn JG, Rizzo J, et al. Phase I study of paclitaxel in patients with recurrent malignant glioma: a North American Brain Tumor Consortium report. J Clin Oncol. 1998;16:2188–2194. doi: 10.1200/JCO.1998.16.6.2188. [DOI] [PubMed] [Google Scholar]
- 16.Lang FF, Gilbert MR, Puduvalli VK, et al. Toward better early-phase brain tumor clinical trials: a reappraisal of current methods and proposals for future strategies. Neuro-Oncology. 2002;4:268–277. doi: 10.1093/neuonc/4.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 18.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti S, Schellens JH. The impact of FDA and EMEA guidelines on drug development in relation to phase 0 trials. Br J Cancer. 2007;97:577–581. doi: 10.1038/sj.bjc.6603925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kummar S, Kinders R, Rubinstein L, et al. Compressing drug development timelines in oncology using phase “0” trials. Nat Rev Cancer. 2007;7:131–139. doi: 10.1038/nrc2066. [DOI] [PubMed] [Google Scholar]
- 21.Glioma Molecular Diagnostic Initiative. [Accessed October 29, 2007]; Available at http://home.ccr.cancer.gov/nob/mds/research.asp.
- 22.Lee JW, Figeys D, Vasilescu J. Biomarker assay translation from discovery to clinical studies in cancer drug development: quantification of emerging protein biomarkers. Adv Cancer Res. 2007;96:269–298. doi: 10.1016/S0065-230X(06)96010-2. [DOI] [PubMed] [Google Scholar]
- 23.Workman P. The opportunities and challenges of personalized genome-based molecular therapies for cancer: targets, technologies, and molecular chaperones. Cancer Chemother Pharmacol. 2003;52(suppl 1):S45–S56. doi: 10.1007/s00280-003-0593-0. [DOI] [PubMed] [Google Scholar]
- 24.Booth CM, Calvert AH, Giaccone G, et al. Design and conduct of phase II studies of targeted anticancer therapy: Recommendations from the task force on methodology for the development of innovative cancer therapies (MDICT) Eur J Cancer. 2008;44:25–29. doi: 10.1016/j.ejca.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Booth CM, Calvert AH, Giaccone G, et al. Endpoints and other considerations in phase I studies of targeted anticancer therapy: recommendations from the task force on Methodology for the Development of Innovative Cancer Therapies (MDICT) Eur J Cancer. 2008;44:19–24. doi: 10.1016/j.ejca.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 26.Leary A, Johnston SR. Small molecule signal transduction inhibitors for the treatment of solid tumors. Cancer Invest. 2007;25:347–365. doi: 10.1080/07357900701259694. [DOI] [PubMed] [Google Scholar]
- 27.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 28.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 30.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 32.Chang SM, Prados MD, Yung WK, et al. Phase II study of neoadjuvant 1, 3-bis (2-chloroethyl)-1-nitrosourea and temozolomide for newly diagnosed anaplastic glioma: a North American Brain Tumor Consortium Trial. Cancer. 2004;100:1712–1716. doi: 10.1002/cncr.20157. [DOI] [PubMed] [Google Scholar]
- 33.Prados MD, Yung WK, Fine HA, et al. Phase 2 study of BCNU and temozolomide for recurrent glioblastoma multiforme: North American Brain Tumor Consortium study. Neuro-Oncology. 2004;6:33–37. doi: 10.1215/S1152851703000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robins HI, Chang SM, Prados MD, et al. A phase II trial of thymidine and carboplatin for recurrent malignant glioma: a North American Brain Tumor Consortium Study. Neuro-Oncology. 2002;4:109–114. [PMC free article] [PubMed] [Google Scholar]
- 35.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro-Oncology. 2006;8:189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prados MD, Yung WK, Jaeckle KA, et al. Phase 1 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro-Oncology. 2004;6:44–54. doi: 10.1215/S1152851703000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01–03 and 00–01. Clin Cancer Res. 2005;11:7841–7850. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 38.Chang SM, Kuhn J, Wen P, et al. Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Invest New Drugs. 2004;22:427–435. doi: 10.1023/B:DRUG.0000036685.72140.03. [DOI] [PubMed] [Google Scholar]
- 39.Puduvalli VK, Yung WK, Hess KR, et al. Phase II study of fenretinide (NSC 374551) in adults with recurrent malignant gliomas: a North American Brain Tumor Consortium study. J Clin Oncol. 2004;22:4282–4289. doi: 10.1200/JCO.2004.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaeckle KA, Hess KR, Yung WK, et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2003;21:2305–2311. doi: 10.1200/JCO.2003.12.097. [DOI] [PubMed] [Google Scholar]
- 41.Groves MD, Puduvalli VK, Chang SM, et al. A North American Brain Tumor Consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81:271–277. doi: 10.1007/s11060-006-9225-y. [DOI] [PubMed] [Google Scholar]
- 42.Schold SC, Jr, Kokkinakis DM, Chang SM, et al. O6-benzylguanine suppression of O6-alkylguanine-DNA alkyltransferase in anaplastic gliomas. Neuro-Oncology. 2004;6:28–32. doi: 10.1215/S115285170300019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 44.Chang SM, Kuhn JG, Robins HI, et al. Phase II study of phenylacetate in patients with recurrent malignant glioma: a North American Brain Tumor Consortium report. J Clin Oncol. 1999;17:984–990. doi: 10.1200/JCO.1999.17.3.984. [DOI] [PubMed] [Google Scholar]
- 45.Chang SM, Kuhn JG, Ian Robins H, et al. A study of a different dose-intense infusion schedule of phenylacetate in patients with recurrent primary brain tumors consortium report. Invest New Drugs. 2003;21:429–433. doi: 10.1023/a:1026299118067. [DOI] [PubMed] [Google Scholar]