Abstract
The objective of this study was to identify differentially expressed and prognostically important genes in pediatric medulloblastoma and pediatric ependymoma by Affymetrix microarray analysis. Among the most discriminative genes, three members of the SOX transcription factor family were differentially expressed. Both SOX4 and SOX11 were significantly overexpressed in medulloblastoma (median, 11-fold and 5-fold, respectively) compared with ependymoma and normal cerebellum. SOX9 had greater expression in ependymoma (median, 16-fold) compared with normal cerebellum and medulloblastoma (p < 0.001 for all comparisons). The differential expression of the SOX genes was confirmed at the protein level by immunohistochemical analysis. Survival analysis of the most discriminative probe sets for each subgroup showed that 35 and 13 probe sets were predictive of survival in patients with medulloblastoma and ependymoma, respectively. There was a trend toward better survival with increasing SOX4 expression in medulloblastoma. SOX9 expression was predictive for favorable outcome in ependymoma. The mRNA levels of BCAT1, a mediator of amino acid breakdown, were higher (median, 15-fold) in medulloblastoma patients with metastases compared with those without metastasized disease (p < 0.01). However, the correlation between BCAT1 expression and metastatic medulloblastoma could not be confirmed at the protein level. The potential prognostic effect of the genes associated with outcome should be evaluated in ongoing studies using larger groups of patients. Furthermore, our findings support further analysis of the functional properties of the selected genes, especially SOX4 and BCAT1 for medulloblastoma and SOX9 for ependymoma, to evaluate the use of these genes as potential tumor markers, prognostic markers, and drug targets in pediatric brain tumors.
Keywords: BCAT1, ependymoma, gene expression profiling, medulloblastoma, SOX genes
Tumors of the CNS are the second most common malignancy of childhood and are generally associated with a worse prognosis than many other pediatric malignancies.1 Medulloblastoma and ependymoma are frequently observed malignant brain tumors in children.2 Medulloblastomas arise in the infratentorial posterior fossa and have a tendency to metastasize within the CNS.3,4 Ependymomas most frequently occur in the posterior fossa or spinal cord.3,5
Most currently known prognostic factors for pediatric brain tumors are based on clinical and histological criteria, such as age, extent of tumor resection, and histological grade. However, insight into molecular abnormalities (genetic and/or posttranscriptional) underlying these tumors is limited. Hence, information on markers that can be used to characterize these tumors better and potentially serve as new targets for therapy is lacking. Studies based on molecular analyses and gene expression profiling are now evolving and may provide possible clues about pathogenesis and prognostic factors in pediatric brain tumors.6–8
In this study, we sought to identify aberrantly expressed and prognostically important genes in pediatric medulloblastoma and ependymoma. By analyzing microarray data, we identified genes that may provide new insights into the biological behavior of these brain tumors. We validated the aberrant expression of these genes on the protein level by immunohistochemical analysis in a larger group of pediatric brain tumor samples. Further characterization may clarify whether these genes can serve as new tumor markers, prognostic factors, or therapeutic targets in pediatric brain tumors.
Materials and Methods
Study Population and Samples
In this study we analyzed 40 fresh-frozen tumor samples from 25 newly diagnosed medulloblastomas (19 without and 6 with radiological visible leptomeningeal metastases), 2 medulloblastomas at relapse (1 without and 1 with radiological visible leptomeningeal metastases), 11 newly diagnosed ependymomas (4 cellular ependymomas and 7 anaplastic ependymomas), and 2 ependymomas at relapse (1 cellular ependymoma and 1 anaplastic ependymoma). Most of these tumor samples were also analyzed by two-dimensional difference in gel analysis, which resulted in the identification of stathmin, annexin A1, and calcyphosine as differentially expressed proteins in medulloblastoma and ependymoma.9 Mean patient age at the time of tumor sample collection was 7.0 years (range, 0.98–14.87 years) for the medulloblastoma patients and 5.6 years (range, 0.66–15.57 years) for the ependymoma patients (p > 0.05). All samples were collected at the Erasmus MC Sophia Children’s Hospital, University Medical Center Rotterdam (Rotterdam, The Netherlands) between 1990 and 2004. Each patient or patient’s relatives gave informed consent prior to enrollment. After surgery, tumor samples were immediately placed in liquid nitrogen and stored at −80°C until processing. Control cerebellar tissue was obtained postmortem from five adult patients without a history of brain tumor. Before RNA extraction, 4–μm cryosections were prepared from the same tissue blocks as used for RNA extraction and stained with hematoxylin and eosin to confirm tumor histology.
Paraffin-embedded tissue for immunohistochemical validation of the differentially expressed genes was available for 23 (19 nonmetastatic and 4 metastatic) of the 27 medulloblastomas and 10 (3 cellular ependymomas and 7 anaplastic ependymomas) of the 13 ependymomas studied by microarray. To enlarge the number of patients for these immunohistochemical validation experiments, we also included paraffin-embedded material from patients with newly diagnosed medulloblastoma and ependymoma from whom no fresh-frozen tissue was available for microarray analysis. Additionally, paraffin-embedded tissues from normal cerebellum, normal cortex, normal ependyma, and normal plexus choroideus were included as normal controls.
Microarray
RNA extraction, labeling, and hybridization
Total RNA was isolated by homogenizing tissue samples in Trizol reagent (Invitrogen, Breda, The Netherlands) using a tissue homogenizer (B. Braun, Spangenberg, Germany). RNA was isolated according to the manufacturer’s protocol with minor modifications.10 RNA integrity was checked using the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). Production of biotinylated antisense complementary RNA (cRNA) and hybridization of the labeled cRNA to Human Genome U133 (HGU133) plus 2.0 GeneChip oligonucleotide microarrays (Affymetrix, Santa Clara, CA, USA) were performed according to the manufacturer’s protocols. All arrays had a 3′ to 5′ GAPDH ratio of <3.0.
Statistical analysis of microarray data
We analyzed expression data using R version 2.4.0 (http://www.r-project.org/). Raw data were normalized using the variance stabilization procedure (vsn).11 Expression profiles of medulloblastoma, ependymoma, and cerebellum were statistically compared using the Wilcoxon test and corrected for multiple testing errors by applying the false discovery rate (FDR) described by Benjamini et al.12 (MULTTEST package). An FDR of <1% was considered statistically significant, meaning that less than 1% of the significant genes are false positives. We calculated the fold up-regulation or down-regulation using the formula e(vsn value A−vsn value B), in which A and B are the groups to be compared.
We estimated survival probabilities by the Kaplan-Meier method using the SPSS software package, version 11.0 (SPSS Inc., Chicago, IL, USA). Overall survival was defined as time from diagnosis until death or last contact. Event-free survival was defined as time from diagnosis until disease progression, relapse, second malignancy, death, or last contact. We performed a Cox regression analysis using SPSS 11.0 to identify genes whose expression was predictive for survival. For each gene, we calculated a hazard ratio and its 95% confidence interval. An effect was considered statistically significant if p was ⩽0.05. A hazard ratio of <1 indicated a favorable influence on survival, and a hazard ratio of >1 indicated an unfavorable influence on survival.
Functional Gene Ontology Analysis
Functional properties of the differentially expressed genes were analyzed by Ontologizer, an XML-based Java application (www.charite.de/ch/medgen/ontologizer),13 using annotations from the Gene Ontology (GO) database (www.geneontology.org). Probe sets with the same gene symbol were counted as one. Overrepresented genes in brain tumor samples were identified by comparing the percentage of genes in each annotation from the total number of genes on the Affymetrix HGU133 plus 2.0 microarray to the percentage of genes in each annotation from the selected subsets of genes using the parent–child method implemented in Ontologizer.13 The parent–child method was preferred to the term-to-term method, because it takes into account the dependencies between different GO annotations. Overrepresentation of a GO annotation was thus measured with respect to the presence of its parental terms in the selected set of genes, instead of measuring individual GO terms. To correct for multiple testing errors, we used the Westfall-Young correction.14 An FDR of <1% was considered statistically significant.
Immunohistochemistry
For the selected discriminative genes, data were validated at the protein level by immunohistochemical analysis. For this purpose, we collected formalin-fixed paraffin-embedded tissues for the medulloblastoma, ependymoma, control cerebella, cortex, normal ependyma, and plexus choroideus. Tissue sections (4 μm) were deparaffinized and rehydrated through a graded xylene-ethanol series and incubated for 30 min in 3% hydrogen peroxide in phosphate-buffered saline (PBS) to inhibit endogenous peroxidases. Antigen retrieval was performed by boiling the slides for 15 min in 0.01 mol/liter citric acid (pH 6.0). Six percent goat serum (Vector Laboratories, Burlingame, CA, USA) in PBS was used as a protein block for 1 h at room temperature. Tissue sections were incubated with antibodies against SOX4 (1:500 in 1% goat serum; Sigma Aldrich, Zwijndrecht, The Netherlands), SOX9 (1:2,000 in 1% goat serum; U.S. Biological, Swampscott, MA, USA), SOX11 (1:50 in 1% goat serum; Sigma Aldrich), and BCAT1 (1:200 in 1% goat serum; BD Biosciences, Alphen aan den Rijn, The Netherlands) at 4°C overnight. The sections were then incubated with biotinylated goat antirabbit IgG (Vector Laboratories) at a dilution of 1:1,000 in PBS for 1 h at room temperature. This was followed by a 1–h incubation with avidin-biotin peroxidase complex (1:400 dilution; Vectastain ABC kit, Vector Laboratories). Staining was performed using 0.5 mg/ml 3,3′-diaminobenzidine, 0.03% (vol/vol) hydrogen peroxide in 30 mM imidazole, 1 mM EDTA (pH 7.0). Tissue sections were slightly counterstained with hematoxylin, dehydrated, and mounted. As a negative control, the primary antibodies were omitted.
Immunohistochemistry was scored according to the percentage of cells that were positively stained in each slide: weak, 0%–25% of the tumor cells stained positive; moderate, 25%–50%; strong, 50%–75%; and very strong, 75%–100%. Differences in the results for immunohistochemistry for the analyzed subgroups were tested for significance using the chi-square test in SPSS 11.0.
Results
Microarray Gene Expression Analysis
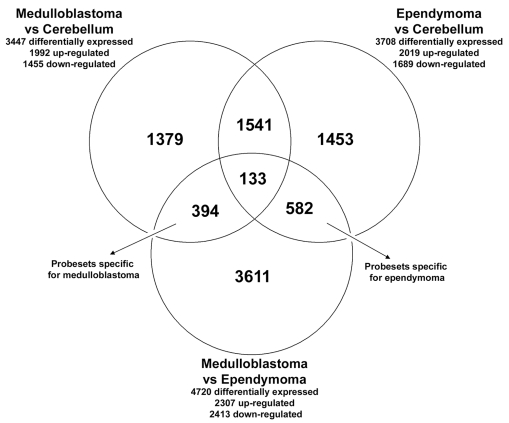
Gene expression profiles were generated for 27 medulloblastoma, 13 ependymoma, and 5 control cerebellum samples. A comparison between medulloblastoma and normal cerebellum samples showed that 3,447 probe sets were differentially expressed, with an FDR of <1%. Between ependymoma and normal cerebellum samples, we found 3,708 differentially expressed probe sets, and between medulloblastoma and ependymoma, 4,720 differentially expressed probe sets, with an FDR of <1% (Fig. 1).
Fig. 1.
Venn diagram indicating the number of differentially expressed probe sets from the comparisons of medulloblastoma, ependymoma, and normal cerebellum. All probe sets that were differentially expressed with a false discovery rate <1% between medulloblastoma, ependymoma, and control cerebellum were used to create a Venn diagram: 394 probe sets were most discriminative for medulloblastoma, being differentially expressed both between medulloblastoma and cerebellum and between medulloblastoma and ependymoma; 582 probe sets were found to be most discriminative for ependymoma, being significantly different between ependymoma and cerebellum and between ependymoma and medulloblastoma; 1,541 were differentially expressed in both medulloblastoma and ependymoma when compared with cerebellum, but they were not found to discriminate between medulloblastoma and ependymoma; 133 probe sets were differentially expressed among all subgroups.
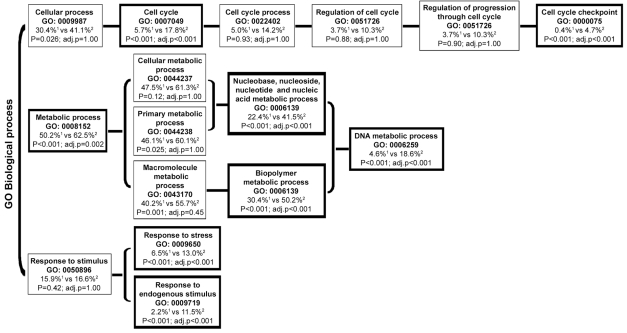
We found that 394 probe sets were most discriminative for medulloblastoma, because these were significantly differentially expressed both between medulloblastoma and cerebellum and between medulloblastoma and ependymoma (Fig. 1). Functional analysis (GO) revealed that the GO annotations involved in the regulation of the cell cycle, DNA replication, and response to stimuli were overrepresented in this set of 394 differentially expressed probe sets (corresponding to 253 genes with annotations in the GO database) (Fig. 2). The number of most discriminative probe sets for ependymoma was 582 (Fig. 1). Functional GO analysis did not reveal any significant overrepresentation of GO annotations in the 582 probe sets specific for ependymoma. We found that 1,541 probe sets were aberrantly expressed in both medulloblastoma and ependymoma compared with normal cerebellum, but the expression of these did not differ significantly between medulloblastoma and ependymoma (Fig. 1). Thus, these probe sets are likely to be related to the presence of a tumor but not specifically discriminative for either medulloblastoma or ependymoma. Of all probe sets, 133 probe sets were statistically differentially expressed among all subgroups (medulloblastoma, ependymoma, and cerebellum) (Fig. 1).
Fig. 2.
Functional Gene Ontology database (GO) analysis. The figure displays the GO annotations that were overrepresented with an FDR <1% within the 394 probe sets that were discriminative for medulloblastoma. The 394 probe sets corresponded to 253 genes for which a biological GO annotation was available. Functional categories that are proportionally overrepresented in the sets of selected probe sets compared with all probe sets present on the array are shown in boxes with bold outlining. 1: Percentage of genes from the total number of genes present on the Affymetrix Human Genome U133 plus 2.0 microarrays per GO annotation. 2: Percentage of genes from the selected differentially expressed genes in medulloblastoma per GO annotation. adj.p, p-value after correction for multiple testing.
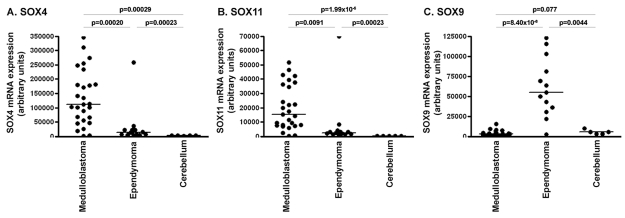
As previously found by others,15,16 OTX2 was differentially expressed in medulloblastoma with the highest fold-change ratio (median, 158-fold) compared with cerebellum. Several members of the SOX gene family were differentially expressed among medulloblastoma, ependymoma, and control cerebellum. SOX4 expression was higher in medulloblastoma (median, 16-fold) compared with normal cerebellum and in medulloblastoma (median, 8-fold) compared with ependymoma (Fig. 3A). SOX11 was highly overexpressed in both brain tumor types but especially in medulloblastoma, with a median 84-fold increase in medulloblastoma and median 21-fold increase in ependymoma compared with normal cerebellum (Fig. 3B). SOX9 was significantly overexpressed in ependymoma, with a median 10-fold increase in ependymoma compared with normal cerebellum and median 17-fold increase in ependymoma compared with medulloblastoma (Fig. 3C) (p < 0.001 for all comparisons).
Fig. 3.
Differential expression of SOX4, SOX11, and SOX9 in medulloblastoma, ependymoma, and control cerebellum: variance stabilization procedure normalized mRNA expression levels of SOX4 (A), SOX11 (B), and SOX9 (C) in medulloblastoma, ependymoma, and cerebellum obtained by Affymetrix Human Genome U133 plus 2.0 microarray analysis. SOX4 and SOX11 were significantly overexpressed in medulloblastoma, whereas SOX9 was significantly overexpressed in ependymoma.
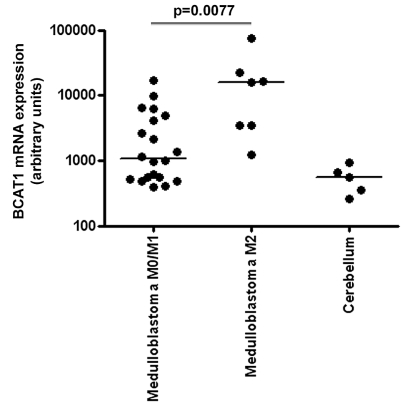
No significant probe sets were found that could explain differences between low- and high-grade ependymoma or between medulloblastoma with and without radiological metastases at diagnosis. The lack of significant probe sets after multiple testing may be due to the limited sample size. To obtain an impression of potentially metastasis-related probe sets in medulloblastoma, we also investigated which probe sets were significantly differentially expressed at p < 0.01, without correction for multiple testing, and had a fold-change ratio higher than 5. Remarkably, 3 of 241 probe sets with p < 0.01 encoded the BCAT1 gene. This gene had a median 15-fold increased expression in medulloblastoma patients with radiological metastases than in medulloblastoma patients without radiological metastases (Fig. 4). Between low- and high-grade ependymoma, no probe sets were differentially expressed (p < 0.01) with a fold-change ratio higher than 5.
Fig. 4.
Differential BCAT1 expression in metastatic medulloblastoma: variance stabilization procedure normalized mRNA expression levels of BCAT1 in medulloblastoma without (M0/M1) and with (M2) radiological leptomeningeal metastases at diagnosis obtained by Affymetrix Human Genome U133 plus 2.0 microarray analysis.
Survival Analysis
Overall survival and event-free survival for medulloblastoma patients were 31.7 ± 9.0% and 29.6 ± 9.0%, respectively. For patients with ependymoma, overall survival was 9.7 ± 9.2% and event-free survival was 8.3 ± 8.0%. In patients with medulloblastoma, increasing age was significantly associated with unfavorable survival (p = 0.0040; hazard ratio = 0.82). Metastatic disease was predictive only for unfavorable event-free survival and not for overall survival. For patients with ependymoma, increasing age was not associated with unfavorable outcome.
Using the most discriminative probe sets for either medulloblastoma or ependymoma (394 and 582, respectively, as described above), we performed a Cox regression analysis to identify the probe sets whose expression was predictive for outcome. In patients with medulloblastoma, 14 probe sets were significantly correlated with a favorable overall survival (hazard ratio <1.0), and 21 probe sets were predictive of unfavorable overall survival (hazard ratio >1.0; Table 1). In patients with ependymoma, six probe sets were associated with favorable overall survival, and seven probe sets were indicative of unfavorable outcome (Table 2).
Table 1.
Probe sets predictive for overall survival in patients with medulloblastoma
| Probe ID | Gene Name | Gene Symbol | Chromosomal Location | p Value | Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| 236852_at | F-box protein 43 | FBXO43 | 8q22.2 | 0.030 | 0.096 (0.012–0.80) |
| 209884_s_at | Solute carrier family 4, sodium bicarbonate cotransporter, member 7 | SLC4A7 | 3p22 | 0.045 | 0.33 (0.11–0.97) |
| 206142_at | Zinc finger protein 135 | ZNF135 | 19q13.4 | 0.022 | 0.36 (0.15–0.87) |
| 1554101_a_at | Transmembrane and tetratricopeptide repeat containing 4 | TMTC4 | 13q32.3 | 0.022 | 0.38 (0.16–0.87) |
| 225982_at | Upstream binding transcription factor, RNA polymerase I | UBTF | 17q21.3 | 0.024 | 0.39 (0.18–0.89) |
| 235422_at | Full-length cDNA clone CS0DB008YK14 of Neuroblastoma Cot 10-normalized of Homo sapiens | — | — | 0.036 | 0.41 (0.17–0.94) |
| 242736_at | — | — | — | 0.048 | 0.43 (0.19–0.99) |
| 244387_at | Transcribed locus | — | — | 0.0020 | 0.46 (0.28–0.75) |
| 204061_at | Protein kinase, X-linked | PRKX | Xp22.3 | 0.028 | 0.52 (0.29–0.93) |
| 223627_at | Ring finger and KH domain containing 3 | RKHD3 | 15q25.2 | 0.045 | 0.54 (0.30–0.99) |
| 219740_at | Vasohibin 2 | VASH2 | 1q32.3 | 0.045 | 0.57 (0.33–0.99) |
| 230596_at | CDNA FLJ39261 fis, clone OCBBF2009391 | — | — | 0.0060 | 0.60 (0.42–0.87) |
| 208497_x_at | Neurogenin 1 | NEUROG1 | 5q23-q31 | 0.0050 | 0.64 (0.47–0.88) |
| 237007_at | — | — | — | 0.028 | 0.66 (0.46–0.96) |
| 227911_at | Rho GTPase activating protein 28 | ARHGAP28 | 18p11.31 | 0.052 | 1.62 (1.00–2.63) |
| 228109_at | Ras protein-specific guanine nucleotide- releasing factor 2 | RASGRF2 | 5q13 | 0.015 | 1.63 (1.10–2.41) |
| 213056_at | FERM domain containing 4B | FRMD4B | 3p14.1 | 0.0010 | 1.81 (1.29–2.54) |
| 204023_at | Replication factor C (activator 1) 4, 37kDa | RFC4 | 3q27 | 0.036 | 2.24 (1.06–4.77) |
| 205393_s_at | CHK1 checkpoint homolog (S. pombe) | CHEK1 | 11q24-q24 | 0.051 | 2.36 (1.00–5.56) |
| 205394_at | CHK1 checkpoint homolog (S. pombe) | CHEK1 | 11q24-q24 | 0.043 | 2.36 (1.028–5.42) |
| 204407_at | Transcription termination factor, RNA polymerase II | TTF2 | 1p22 | 0.029 | 2.80 (1.11–7.05) |
| 201833_at | Histone deacetylase 2 | HDAC2 | 6q21 | 0.036 | 2.89 (1.07–7.79) |
| 234863_x_at | F-box protein 5 | FBXO5 | 6q25-q26 | 0.017 | 3.39 (1.24–9.23) |
| 211450_s_at | MutS homolog 6 (E. coli) | MSH6 | 2p16 | 0.011 | 3.66 (1.34–10.01) |
| 203207_s_at | Mitochondrial fission regulator 1 | MTFR1 | 8q13.1 | 0.0050 | 3.69 (1.48–9.17) |
| 230503_at | Transcribed locus | — | — | 0.011 | 3.89 (1.36–11.17) |
| 234992_x_at | Epithelial cell transforming sequence 2 oncogene | ECT2 | 3q26.1-q26.2 | 0.030 | 3.92 (1.15–13.44) |
| 235295_at | Transcribed locus | — | — | 0.023 | 3.92 (1.20–12.79) |
| 227928_at | Chromosome 12 open reading frame 48 | C12orf48 | 12q23.2 | 0.027 | 4.33 (1.18–15.86) |
| 216559_x_at | Heterogeneous nuclear ribonucleoprotein A1 | HNRPA1 | 10q11.22 | 0.010 | 4.72 (1.44–15.41) |
| 223225_s_at | SEH1-like (S. cerevisiae) | SEH1L | 18p11.21 | 0.021 | 6.49 (1.33–31.68) |
| 218433_at | Pantothenate kinase 3 | PANK3 | 5q34 | 0.024 | 12.48 (1.40–111.51) |
| 236030_at | REST corepressor 2 | RCOR2 | 11q13.1 | 0.0010 | 12.73 (2.78–58.20) |
| 231380_at | Chromosome 8 open reading frame 34 | C8orf34 | 8q13 | 0.018 | 14.1 (1.58–125–87) |
| 205953_at | Leucine-rich repeats and immunoglobulin- like domains 2 | LRIG2 | 1p13.1 | 0.033 | 35.0 (1.32–927.42) |
Table 2.
Probe sets predictive for overall survival in patients with ependymoma
| Probe ID | Gene Name | Gene Symbol | Chromosomal Location | p Value | Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| 243929_at | — | — | — | 0.022 | 0.0010 (0.00010–0.29) |
| 238479_at | Full length insert cDNA clone ZC34E11 | — | — | 0.024 | 0.0040 (0.00010–0.47) |
| 227313_at | Protein Associated with Tlr4 | MGC40499 | 7q22.1 | 0.040 | 0.024 (0.0010–0.85) |
| 233223_at | CDNA FLJ20843 fis, clone ADKA01954 | — | — | 0.025 | 0.052 (0.0040–0.69) |
| 201324_at | Epithelial membrane protein 1 | EMP1 | 12p12.3 | 0.047 | 0.26 (0.068–0.98) |
| 201426_s_at | Vimentin | VIM | 10p13 | 0.040 | 0.42 (0.18–0.96) |
| 215321_at | Rap2-binding protein 9 | RPIB9 | 7q21.12 | 0.027 | 2.26 (1.10–4.67) |
| 217478_s_at | Major histocompatibility complex, class II, DM alpha | HLA-DMA | 6p21.3 | 0.048 | 3.53 (1.01–12.39) |
| 229823_at | Transcribed locus | — | — | 0.046 | 10.81 (1.04–112.08) |
| 225792_at | Hook homolog 1 (Drosophila) | HOOK1 | 1p32.1 | 0.039 | 11.72 (1.14–121.01) |
| 211742_s_at | Ecotropic viral integration site 2B | EVI2B | 17q11.2 | 0.030 | 37.41 (1.43–979.18) |
| 203998_s_at | Synaptotagmin I | SYT1 | 12cen-q21 | 0.032 | 550.73 (1.743–174045.00) |
| 206137_at | Regulating synaptic membrane exocytosis 2 | RIMS2 | 8q22.3 | 0.0080 | 1508.40 (6.73–338057.40) |
We also evaluated the aberrantly expressed genes chosen for further analysis (SOX4, SOX9, SOX11, and BCAT1) for their predictive effect on survival. There was a trend toward favorable prognosis with increasing SOX4 expression levels in patients with medulloblastoma (p = 0.065; hazard ratio = 0.78). Expression of SOX9, SOX11, or BCAT1 was not significantly associated with the outcome of medulloblastoma. SOX9 expression was significantly associated with favorable overall survival in patients with ependymoma (p = 0.027; hazard ratio = 0.44).
Validation of Potential Tumor Markers by Immunohistochemistry
The striking difference in mRNA expression of the three SOX genes among medulloblastoma, ependymoma, and cerebellum, and the potential prognostic effect of SOX4 and SOX9 expression, prompted us to validate these genes with a different technique in an extended set of patient samples.
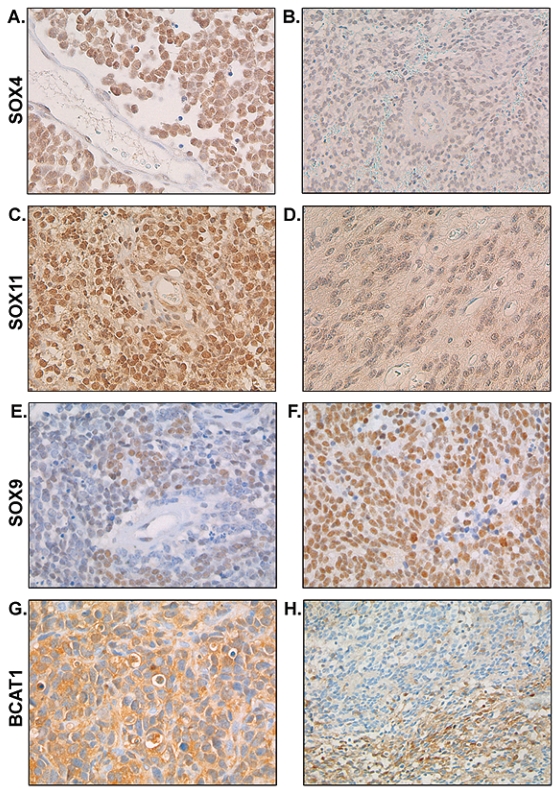
Immunohistochemistry confirmed the differential expression of SOX4 and SOX11, showing stronger SOX4 and SOX11 staining in medulloblastoma compared with ependymoma (Table 3; p < 0.001 for both SOX4 and SOX11). We observed strong to very strong SOX4 and SOX11 nuclear positivity in almost 75% of the medulloblastomas. Representative images of intense SOX4 and SOX11 staining in medulloblastomas are shown in Fig. 5, A and C, respectively. SOX4 staining was weak to moderate in all ependymoma cases except one. SOX11 expression was limited (<50% of the cells) in more than 75% of the ependymomas. Representative images of SOX4 and SOX11 staining in ependymomas are shown in Fig. 5, B and D, respectively. SOX4 and SOX11 positivity was not observed in normal cerebellum, cortex, normal ependyma, or plexus choroideus.
Table 3.
Immunohistochemical results for SOX4, SOX11, SOX9, and BCAT1 immunoreactivity in medulloblastoma, ependymoma, and control tissues
| Gene Expression | n | 0%–25% Weak | 25%–50% Moderate | 50%–75% Strong | 75%–100% Very Strong |
|---|---|---|---|---|---|
| SOX4 | |||||
| Medulloblastoma | 29 | 7% | 21% | 31% | 41% |
| Nonmetastatic | 25 | 8% | 24% | 28% | 36% |
| Metastasis | 4 | 0% | 0% | 50% | 50% |
| Ependymoma | 15 | 87% | 13% | 0% | 0% |
| Cellulare | 7 | 86% | 14% | 0% | 0% |
| Anaplastic | 8 | 88% | 13% | 0% | 0% |
| Cerebellum | 5 | 100% | 0% | 0% | 0% |
| Cortex | 5 | 100% | 0% | 0% | 0% |
| Normal ependyma | 5 | 100% | 0% | 0% | 0% |
| Plexus choroideus | 5 | 100% | 0% | 0% | 0% |
| SOX11 | |||||
| Medulloblastoma | 30 | 7% | 10% | 30% | 53% |
| Nonmetastatic | 24 | 8% | 8% | 29% | 54% |
| Metastasis | 6 | 0% | 17% | 33% | 50% |
| Ependymoma | 16 | 56% | 19% | 19% | 6% |
| Cellulare | 7 | 57% | 29% | 0% | 14% |
| Anaplastic | 9 | 56% | 11% | 33% | 0% |
| Cerebellum | 5 | 100% | 0% | 0% | 0% |
| Cortex | 5 | 100% | 0% | 0% | 0% |
| Normal ependyma | 5 | 100% | 0% | 0% | 0% |
| Plexus choroideus | 5 | 100% | 0% | 0% | 0% |
| SOX9 | |||||
| Medulloblastoma | 30 | 63% | 30% | 7% | 0% |
| Nonmetastatic | 25 | 60% | 32% | 8% | 0% |
| Metastasis | 5 | 80% | 20% | 0% | 0% |
| Ependymoma | 16 | 13% | 13% | 31% | 44% |
| Cellulare | 7 | 0% | 14% | 14% | 71% |
| Anaplastic | 9 | 22% | 11% | 44% | 22% |
| Cerebellum | 5 | 100% | 0% | 0% | 0% |
| Cortex | 5 | 100% | 0% | 0% | 0% |
| Normal ependyma | 5 | 100% | 0% | 0% | 0% |
| Plexus choroideus | 5 | 100% | 0% | 0% | 0% |
| BCAT1 | |||||
| Medulloblastoma | 34 | 62% | 12% | 18% | 9% |
| Nonmetastatic | 28 | 64% | 11% | 21% | 4% |
| Metastasis | 6 | 50% | 17% | 0% | 33% |
| Ependymoma | 5 | 100% | 0% | 0% | 0% |
| Cerebellum | 5 | 100% | 0% | 0% | 0% |
| Cortex | 5 | 100% | 0% | 0% | 0% |
| Normal ependyma | 5 | 100% | 0% | 0% | 0% |
| Plexus choroideus | 5 | 100% | 0% | 0% | 0% |
Fig. 5.
Immunohistochemistry for SOX4, SOX11, SOX9, and BCAT1 in medulloblastoma and ependymoma. Immunohistochemistry confirmed the overexpression of SOX4 in medulloblastoma (A) compared with ependymoma (B). SOX11 protein expression was also shown to be higher in medulloblastoma (C) compared with ependymoma (D). In contrast, SOX9 expression was confirmed to be higher in ependymoma (F) compared with medulloblastoma (E). (G and H) Representative images of strong homogeneous cytoplasmic staining of BCAT1 and focal BCAT1 staining, respectively, in predominantly metastasized medulloblastoma.
The high expression of SOX9 in ependymomas was also confirmed by immunohistochemistry. SOX9 staining was nuclear. SOX9 nuclear positivity was strong to very strong in approximately 75% of the ependymomas (Table 3, Fig. 5F) and weak to moderate in more than 90% of the medulloblastomas (p < 0.001; Table 3, Fig. 5E). Cerebellum, cortex, normal ependyma, and plexus choroideus were negative for SOX9.
Expression of BCAT1, which was differentially expressed at the mRNA level between medulloblastoma with and without radiological metastases at diagnosis, was also studied by immunohistochemical analysis (Table 3). At the protein level, the difference in BCAT1 expression between metastatic and nonmetastatic medulloblastoma was not evident (Table 3). Three medulloblastomas showed very strong homogeneous cytoplasmic BCAT1 staining (Fig. 5G). Two of these had radiological metastases at diagnosis; one patient did not have radiological metastases, although tumor cells were found in the diagnostic cerebrospinal fluid of this case. Six medulloblastomas also showed very strong staining, but only within distinct areas of the tumor (50%–75% of the cells) (Fig. 5H). None of these patients had radiologically visible metastases at diagnosis, and one of these patients had tumor cells in the diagnostic cerebrospinal fluid. Four medulloblastomas showed intense BCAT1 staining in less than 50% of the cells (one of these cases had radiological visible metastases at diagnosis, and in another case tumor cells were found in the diagnostic cerebrospinal fluid). In the other medulloblastomas, ependymomas, cerebellum, cortex, normal ependyma, and plexus choroideus, BCAT1 positivity was absent.
Discussion
In this study we compared gene expression profiles of 27 pediatric medulloblastomas, 13 pediatric ependymomas, and 5 normal cerebella. Because at least parts of all medulloblastomas are thought to arise from the external granular layer of the cerebellum, cerebellum was used as a normal control. For each tumor subgroup, we identified probe sets that were most discriminative and that could be used for functional (GO) and survival analysis. Functional analysis (GO) of the most discriminative probe sets for medulloblastoma mainly showed overrepresentation of genes involved in regulation of the cell cycle, replication, and response to stimuli, but among them there were also many transcription factors. For example, expression of OTX2 was found to be 140-fold higher in medulloblastoma than in normal cerebellum, which validates earlier findings using serial analysis of gene expression (SAGE).15
In addition to OTX2, several members of the SOX gene transcription factor family were differentially expressed in either medulloblastoma or ependymoma. SOX4 and SOX11 were highly overexpressed in medulloblastoma, whereas SOX9 was highly overexpressed in ependymoma. The differential mRNA expression of SOX4, SOX11, and SOX9 was confirmed at the protein level by immunohistochemistry. In addition, survival analysis showed that both SOX4 and SOX9 were predictive of outcome in medulloblastoma and ependymoma, respectively. The SOX proteins compose a family of more than 20 transcription factors characterized by the presence of a high-mobility-group (HMG) DNA-binding domain.17 SOX4 and SOX11 belong to subgroup C of the SOX gene family. SOX4 is known to play a role in normal embryonic development of, for example, the heart, CNS, lungs, and thymus.18–20 SOX4 has been shown to be overexpressed in various malignancies,21–23 including medulloblastoma.16,24 Interestingly, SOX4 is one of the most frequently targeted genes by retroviral insertional mutagenesis.25,26 Down-regulation of SOX4 expression in prostate cancer cell lines resulted in a strong decrease in cell viability and a corresponding increase in apoptosis.23 However, SOX4 induction has also been shown to impair cell viability and induce apoptosis.27 The contradictory effect of SOX4 expression on apoptosis in different cell types suggests that SOX4, like c-Myc, has both anti- and proapoptotic activities. The balance between anti- and proapoptotic signals induced by SOX4 expression might thus be tissue specific and dependent on external signals. Therefore, and because the biological role of high SOX4 expression in medulloblastoma is unknown, further research should focus on the functional characteristics of SOX4, for example, by modulating SOX4 expression by RNA interference. In our study, SOX4 expression was associated with a more favorable outcome in medulloblastoma. Because these findings are in contrast with those of Neben et al.,28 who found SOX4 to be a poor prognostic factor in medulloblastoma, prospective studies with large numbers of patients should be conducted to determine the exact influence of SOX4 on the outcome in medulloblastoma.
SOX11 is expressed in neural precursors throughout the neuroepithelium and is also expressed in areas of the brain in which neurons undergo differentiation during later stages of neural development.29 SOX11 was found to be overexpressed in fetal brain tissue and malignant gliomas.30 Expression of SOX11 in malignant glioma may be the result of a dedifferentiation process during tumorigenesis, because SOX11 is normally down-regulated after maturation of the brain.30 Because we observed medulloblastoma to be characterized by a high expression level of SOX11, our data may underline the embryonal origin of these tumors.
In contrast to SOX4 and SOX11, SOX9 mRNA and protein expression levels were up-regulated in ependymoma. SOX9 belongs to the subgroup of SOX E genes, which control different aspects of differentiation of astrocytes, oligodendrocytes, and Schwann cells.31 Outside the CNS, SOX9 is required for chondrogenesis and the development of the male gonads.32 SOX9 is likely to be of importance in ependymomas, because, in concordance with our results, strong nuclear SOX9 expression has also been described in pediatric and adult high-grade neuroepithelial tumors.33 Moreover, data from the study of Modena et al.34 (see their supplementary table 3 at pierotti.group.ifom-ieo-campus.it/suppl/epd.html) also confirm SOX9 overexpression in ependymoma, showing an approximately 6-fold higher SOX9 expression in ependymomas compared with other tissues in the study. In the intestinal epithelium, SOX9 was found to be expressed in a pattern characteristic of Wnt targets.35 Overexpression of SOX9 in cultured colon carcinoma cells resulted in decreased expression of the tumor- suppressor gene CDX2, suggesting SOX9 to be a potential important contributor to cancer progression.35 In contrast, SOX9 expression was significantly associated with a more favorable outcome in ependymoma in our study. Because SOX9 does not seem to result in potentiation of disease progression of ependymoma, as was suggested with colon carcinoma, larger prospective studies should determine the exact role of SOX9 as a prognostic factor in ependymoma.
The presence of radiological leptomeningeal metastases at diagnosis is an important adverse prognostic factor in medulloblastoma patients.36 We found BCAT1 mRNA expression levels to be median 15-fold increased in medulloblastoma with radiological leptomeningeal metastases. BCAT1 is a cytosolic branched-chain amino acid aminotransferase reported to be involved in the control of the cell cycle by suppressing the G1-to-S transition of normal cells.37 BCAT1 is involved in various malignancies. The mouse homologue of BCAT1 has shown to be amplified and overexpressed in a teratocarcinoma cell line.38 Retroviral transduction of fetal rat brain cells with SV40 large T-antigen induced tumors with characteristic features of medulloblastoma that showed amplification of BCAT1.39,40 In colorectal adenocarcinomas, high BCAT1 expression was associated with a greater tendency to metastasize and a decreased disease-free survival rate.41 Because BCAT1 was found to be a direct target for myc activation in oncogenesis,37,42 the overexpression of BCAT1 in malignancies might be a result of myc activation. In our study, BCAT1 mRNA expression was not significantly correlated with c-, N-, or L-myc mRNA expression (data not shown), which suggests that other factors are responsible for the up-regulation of BCAT1 expression in medulloblastoma, as was also suggested for other neuronal tumors.43
Evaluation of BCAT1 expression in the data set of Thompson et al.7 did not show a significant difference in expression between medulloblastomas with and those without evident metastasized disease (⩾M2 stage). In our study, the correlation between BCAT1 expression and metastatic disease in medulloblastoma was not as clear at the protein level, because several nonmetastatic medulloblastomas also showed strong BCAT1 immunoreactivity. The lack of correlation between mRNA and protein expression levels can be caused by the instability of mRNA or protein, high turnover of protein, or translational repression by microRNAs.44 Moreover, the fact that the pattern of BCAT1 staining was mainly focal and not homogeneous (Fig. 5H) might also explain why positive BCAT1 immunohistochemistry did not correlate with high BCAT1 mRNA expression. Because BCAT1 positivity has been shown to be very variable within the tumor, overall BCAT1 expression as detected by microarray analysis might depend on which part of the tumor is analyzed. The focal pattern of staining might suggest the presence of a subset of BCAT1–positive tumor cells in some medulloblastomas that may have a higher tendency to metastasize. It would be interesting to specifically study BCAT1 expression in the cells already metastasized within the CNS. Because these isolated cells were not available, we evaluated BCAT1 protein expression (Western blot) in the cerebrospinal fluid of several medulloblastoma patients with metastasized disease and a number of control patients. Interestingly, BCAT1 protein expression was very low in the cerebrospinal fluid of all control patients and high in the cerebrospinal fluid of the medulloblastoma patients (data not shown). Therefore, BCAT1 might still be an interesting new marker for metastasis, and more extensive studies should be performed. In addition, because BCAT1 can be inhibited by gabapentin,45 its use as a therapeutic target should also be investigated.
In addition to evaluating the differential expression of genes in medulloblastoma and ependymoma, we performed a Cox regression analysis to determine which genes were predictive of survival in these tumors. Interestingly, several of these genes have already been shown to be of importance in the biology of malignancies and may well be new interesting therapeutic targets in pediatric brain tumors. In medulloblastoma, one of the genes associated with adverse outcome was CHEK1, which codes for a serine/threonine kinase implicated in the DNA damage checkpoint response and the replication checkpoint.46 Inhibition of CHEK1 has been used to sensitize tumor cells to DNA antimetabolite chemo-therapeutic drugs.47 Because increasing CHEK1 expression resulted in worse overall survival for patients with medulloblastoma, inhibition of CHEK1 expression might also result in higher chemosensitivity of these tumors and consequently higher survival rates.
HDAC2, a histone deacetylase negatively influencing prognosis in medulloblastoma in our study, was overexpressed in colorectal cancer and was required to maintain the transformed phenotype of colon carcinoma cells.48 In addition, HDAC2 was found to be capable of regulating p53 activity by inhibiting p53 DNA binding.49
Interestingly, ECT2, another poor prognostic gene for medulloblastoma in our study, was indicative of an unfavorable outcome in glioma.50
A gene associated with more favorable prognosis in medulloblastoma was NEUROG1, a basic helix-loop-helix transcription factor that is important during neurogenesis. NEUROG1 has been suggested to be a marker for a subgroup of medulloblastomas deriving from progenitor cells of the cerebellar ventricular zone.51 NEUROG1 may thus be of interest in the search for the cells of origin of medulloblastoma. In contrast to our data, Rostomily et al.52 found a correlation between NEUROG1 expression and the presence of distant metastases in medulloblastoma. However, they evaluated NEUROG1 expression in only three medulloblastoma patients without distant metastases.
In conclusion, this study shows that gene expression profiling, using Affymetrix HGU133 plus 2.0 microarrays, is a tool to identify genes that are aberrantly expressed and of prognostic importance in pediatric brain tumors. SOX4 and SOX9, which are overexpressed in medulloblastoma and ependymoma, respectively, also had an influence on outcome. Prospective studies involving a larger number of patients will be needed to confirm the prognostic effect of the genes identified in our study, and analysis of the functional characteristics of the selected genes should reveal the possibilities of using these genes as new tumor markers and therapeutic targets in pediatric medulloblastoma and ependymoma.
References
- 1.Bleyer WA. Epidemiologic impact of children with brain tumors. Childs Nerv Syst. 1999;15(11–12):758–763. doi: 10.1007/s003810050467. [DOI] [PubMed] [Google Scholar]
- 2.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–229. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ. Brain tumors in children. Arch Neurol. 1999;56(4):421– 425. doi: 10.1001/archneur.56.4.421. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald TJ, Rood BR, Santi MR, et al. Advances in the diagnosis, molecular genetics, and treatment of pediatric embryonal CNS tumors. Oncologist. 2003;8(2):174–186. doi: 10.1634/theoncologist.8-2-174. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ. New insights into childhood ependymoma. Curr Neurol Neurosci Rep. 2005;5(2):107–109. doi: 10.1007/s11910-005-0007-2. [DOI] [PubMed] [Google Scholar]
- 6.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 7.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 8.Lukashova VZI, Kneitz S, Monoranu CM, et al. Ependymoma gene expression profiles associated with histological subtype, proliferation, and patient survival. Acta Neuropathol (Berl) 2007;113(3):325–337. doi: 10.1007/s00401-006-0190-5. [DOI] [PubMed] [Google Scholar]
- 9.De Bont JM, den Boer ML, Kros JM, et al. Identification of novel biomarkers in pediatric primitive neuroectodermal tumors and ependymomas by proteome-wide analysis. J Neuropathol Exp Neurol. 2007;66(6):505–516. doi: 10.1097/01.jnen.0000240475.35414.c3. [DOI] [PubMed] [Google Scholar]
- 10.Stam RW, den Boer ML, Meijerink JP, et al. Differential mRNA expression of Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL gene-rearranged infant acute lymphoblastic leukemia. Blood. 2003;101(4):1270–1276. doi: 10.1182/blood-2002-05-1600. [DOI] [PubMed] [Google Scholar]
- 11.Huber W, Von Heydebreck A, Sultmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18(Suppl 1):S96–S104. doi: 10.1093/bioinformatics/18.suppl_1.s96. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 13.Robinson PN, Wollstein A, Bohme U, Beattie B. Ontologizing gene-expression microarray data: characterizing clusters with Gene Ontology. Bioinformatics. 2004;20(6):979–981. doi: 10.1093/bioinformatics/bth040. [DOI] [PubMed] [Google Scholar]
- 14.Westfall P, Young S. Resampling-Based Multiple Testing. New York: Wiley; 1993. [Google Scholar]
- 15.Michiels EM, Oussoren E, Van Groenigen M, et al. Genes differentially expressed in medulloblastoma and fetal brain. Physiol Genomics. 1999;1(2):83–91. doi: 10.1152/physiolgenomics.1999.1.2.83. [DOI] [PubMed] [Google Scholar]
- 16.Yokota N, Mainprize TG, Taylor MD, et al. Identification of differentially expressed and developmentally regulated genes in medulloblastoma using suppression subtraction hybridization. Oncogene. 2004;23(19):3444–3453. doi: 10.1038/sj.onc.1207475. [DOI] [PubMed] [Google Scholar]
- 17.Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27(6):1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16(4):182–187. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- 19.Cheung M, Abu-Elmagd M, Clevers H, Scotting PJ. Roles of Sox4 in central nervous system development. Brain Res Mol Brain Res. 2000;79(1–2):180–191. doi: 10.1016/s0169-328x(00)00109-1. [DOI] [PubMed] [Google Scholar]
- 20.Schilham MW, Oosterwegel MA, Moerer P, et al. Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature. 1996;380(6576):711–714. doi: 10.1038/380711a0. [DOI] [PubMed] [Google Scholar]
- 21.Aaboe M, Birkenkamp-Demtroder K, Wiuf C, et al. SOX4 expression in bladder carcinoma: clinical aspects and in vitro functional characterization. Cancer Res. 2006;66(7):3434–3442. doi: 10.1158/0008-5472.CAN-05-3456. [DOI] [PubMed] [Google Scholar]
- 22.Frierson HF, Jr, El-Naggar AK, Welsh JB, et al. Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol. 2002;161(4):1315–1323. doi: 10.1016/S0002-9440(10)64408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Ramachandran S, Ali Seyed M, et al. Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006;66(8):4011–4019. doi: 10.1158/0008-5472.CAN-05-3055. [DOI] [PubMed] [Google Scholar]
- 24.Lee CJ, Appleby VJ, Orme AT, Chan WI, Scotting PJ. Differential expression of SOX4 and SOX11 in medulloblastoma. J Neurooncol. 2002;57(3):201–214. doi: 10.1023/a:1015773818302. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Shen H, Akagi K, et al. New genes involved in cancer identified by retroviral tagging. Nat Genet. 2002;32(1):166–174. doi: 10.1038/ng949. [DOI] [PubMed] [Google Scholar]
- 26.Du Y, Spence SE, Jenkins NA, Copeland NG. Cooperating cancer-gene identification through oncogenic-retrovirus-induced insertional mutagenesis. Blood. 2005;106(7):2498–2505. doi: 10.1182/blood-2004-12-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hur EH, Hur W, Choi JY, et al. Functional identification of the pro-apoptotic effector domain in human Sox4. Biochem Biophys Res Commun. 2004;325(1):59–67. doi: 10.1016/j.bbrc.2004.09.215. [DOI] [PubMed] [Google Scholar]
- 28.Neben K, Korshunov A, Benner A, et al. Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res. 2004;64(9):3103–3111. doi: 10.1158/0008-5472.can-03-3968. [DOI] [PubMed] [Google Scholar]
- 29.Kuhlbrodt K, Herbarth B, Sock E, et al. Cooperative function of POU proteins and SOX proteins in glial cells. J Biol Chem. 1998;273(26):16050–16057. doi: 10.1074/jbc.273.26.16050. [DOI] [PubMed] [Google Scholar]
- 30.Weigle B, Ebner R, Temme A, et al. Highly specific overexpression of the transcription factor SOX11 in human malignant gliomas. Oncol Rep. 2005;13(1):139–144. [PubMed] [Google Scholar]
- 31.Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130(23):5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 32.Foster JW, Dominguez-Steglich MA, Guioli S, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372(6506):525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 33.Kordes U, Hagel C. Expression of SOX9 and SOX10 in central neuroepithelial tumor. J Neurooncol. 2006;80(2):151–155. doi: 10.1007/s11060-006-9180-7. [DOI] [PubMed] [Google Scholar]
- 34.Modena P, Lualdi E, Facchinetti F, et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24(33):5223–5233. doi: 10.1200/JCO.2006.06.3701. [DOI] [PubMed] [Google Scholar]
- 35.Blache P, van de Wetering M, Duluc I, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166(1):37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 37.Schuldiner O, Eden A, Ben-Yosef T, et al. ECA39, a conserved gene regulated by c-Myc in mice, is involved in G1/S cell cycle regulation in yeast. Proc Natl Acad Sci U S A. 1996;93(14):7143–7148. doi: 10.1073/pnas.93.14.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niwa O, Kumazaki T, Tsukiyama T, et al. A cDNA clone overexpressed and amplified in a mouse teratocarcinoma line. Nucleic Acids Res. 1990;18(22):6709. doi: 10.1093/nar/18.22.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eibl RH, Kleihues P, Jat PS, Wiestler OD. A model for primitive neuroectodermal tumors in transgenic neural transplants harboring the SV40 large T antigen. Am J Pathol. 1994;144(3):556–564. [PMC free article] [PubMed] [Google Scholar]
- 40.Weggen S, Preuss U, Pietsch T, et al. Identification of amplified genes from SV40 large T antigen-induced rat PNET cell lines by subtractive cDNA analysis and radiation hybrid mapping. Oncogene. 2001;20(16):2023–2031. doi: 10.1038/sj.onc.1204287. [DOI] [PubMed] [Google Scholar]
- 41.Yoshikawa R, Yanagi H, Shen CS, et al. ECA39 is a novel distant metastasis-related biomarker in colorectal cancer. World J Gastroenterol. 2006;12(36):5884–5889. doi: 10.3748/wjg.v12.i36.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Yosef T, Eden A, Benvenisty N. Characterization of murine BCAT genes: Bcat1, a c-Myc target, and its homolog, Bcat2. Mamm Genome. 1998;9(7):595–597. doi: 10.1007/s003359900825. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Yosef T, Yanuka O, Halle D, Benvenisty N. Involvement of Myc targets in c-myc and N-myc induced human tumors. Oncogene. 1998;17(2):165–171. doi: 10.1038/sj.onc.1201939. [DOI] [PubMed] [Google Scholar]
- 44.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.Hutson SM, Berkich D, Drown P, et al. Role of branched-chain aminotransferase isoenzymes and gabapentin in neurotransmitter metabolism. J Neurochem. 1998;71(2):863–874. doi: 10.1046/j.1471-4159.1998.71020863.x. [DOI] [PubMed] [Google Scholar]
- 46.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3(5):421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 47.Xiao Z, Xue J, Sowin TJ, Zhang H. Differential roles of checkpoint kinase 1, checkpoint kinase 2, and mitogen-activated protein kinase-activated protein kinase 2 in mediating DNA damage-induced cell cycle arrest: implications for cancer therapy. Mol Cancer Ther. 2006;5(8):1935–1943. doi: 10.1158/1535-7163.MCT-06-0077. [DOI] [PubMed] [Google Scholar]
- 48.Zhu P, Martin E, Mengwasser J, et al. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell. 2004;5(5):455–463. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 49.Harms KL, Chen X. Histone deacetylase 2 modulates p53 transcriptional activities through regulation of p53–DNA binding activity. Cancer Res. 2007;67(7):3145–3152. doi: 10.1158/0008-5472.CAN-06-4397. [DOI] [PubMed] [Google Scholar]
- 50.Sano M, Genkai N, Yajima N, et al. Expression level of ECT2 protooncogene correlates with prognosis in glioma patients. Oncol Rep. 2006;16(5):1093–1098. [PubMed] [Google Scholar]
- 51.Salsano E, Croci L, Maderna E, et al. Expression of the neurogenic basic helix-loop-helix transcription factor NEUROG1 identifies a subgroup of medulloblastomas not expressing ATOH1. Neuro-Oncology. 2007;9(3):298–307. doi: 10.1215/15228517-2007-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rostomily RC, Bermingham-McDonogh O, Berger MS, et al. Expression of neurogenic basic helix-loop-helix genes in primitive neuroectodermal tumors. Cancer Res. 1997;57(16):3526–3531. [PubMed] [Google Scholar]