Abstract
Prostaglandin E2 (PGE2) has been shown to play important roles in several aspects of tumor development and progression. PGE2 is synthesized from arachidonic acid by cyclooxygenases (COX) and prostaglandin E synthases (PGES) and mediates its biological activity through binding to the four prostanoid receptors EP1 through EP4. In this study, we show for the first time that medulloblastoma (MB), the most common malignant childhood brain tumor, expresses high levels of COX-2, microsomal prostaglandin E synthase-1, and EP1 through EP4 and secretes PGE2. PGE2 and the EP2 receptor agonist butaprost stimulated MB cell proliferation. Treatment of MB cells with COX inhibitors suppressed PGE2 production and induced caspase-dependent apoptosis. Similarly, specific COX-2 silencing by small interfering RNA inhibited MB cell growth. EP1 and EP3 receptor antagonists ONO-8713 and ONO-AE3-240, but not the EP4 antagonists ONO-AE3-208 and AH 23848, inhibited tumor cell proliferation, indicating the significance of EP1 and EP3 but not EP4 for MB growth. Administration of COX inhibitors at clinically achievable nontoxic concentrations significantly inhibited growth of established human MB xenografts. Apoptosis was increased, proliferation was reduced, and angiogenesis was inhibited in MBs treated with COX inhibitors. This study suggests that PGE2 is important for MB growth and that therapies targeting the prostanoid metabolic pathway are potentially beneficial and should be tested in clinical settings for treatment of children with MB.
Keywords: angiogenesis, apoptosis, cyclooxygenase-2, in vivo treatment, medulloblastoma, microsomal prostaglandin E synthase-1, primitive neuroectodermal tumors, proliferation, prostaglandin E2, prostanoid receptors
Medulloblastoma (MB), an embryonic tumor of the cerebellum with high tendency to metastasize, is the most frequent malignant embryonal CMS tumor of childhood.1 Advances in treatment regimens have improved the 5-year survival rates for standard-risk patients to approximately 70%. However, the prognosis for patients with high-risk MB remains poor, and long-term survivors frequently show detrimental physical and neuropsychological sequelae.2,3
Increased levels of prostaglandin E2 (PGE2) have been detected in a variety of malignancies, including brain tumors.4–7 PGE2 is synthesized from arachidonic acid by the sequential action of cyclooxygenases (COX) and prostaglandin E synthases (PGES).6 COX-2, the inducible form of COX, has frequently been detected in both premalignant and malignant tissues.8 COX-2 is functionally coupled to microsomal prostaglandin E synthase-1 (mPGES-1), which converts prostaglandin H2 to PGE2.9 Overexpression of mPGES-1 in cancer tissues has been reported, further supporting an important function of prostanoids in tumorigenesis.10,11 PGE2 exerts its physiological effects by interacting with a subfamily of four distinct G-protein– coupled receptors designated EP1, EP2, EP3, and EP4. PGE2 promotes tumor growth by stimulating EP receptor signaling with subsequent enhancement of cellular proliferation, promotion of angiogenesis, inhibition of apoptosis, stimulation of invasion, and suppression of immune responses.12,13
In this study, we investigated the expression of key enzymes involved in the production of PGE2 as well as its prostanoid EP receptors in MB primary tumors and cell lines. We also studied the effects of PGE2, the EP2 agonist butaprost, and four EP antagonists, ONO-8713, ONO-AE3-240, ONO-AE3-208, and AH 23848, that specifically block the function of EP1, EP3, and EP4, respectively, on MB cell growth. Furthermore, we examined the effect of inhibiting COX-2 using RNA silencing by small interfering RNA or nonsteroidal anti-inflammatory drugs (NSAIDs) on MB cell proliferation, apoptosis, and PGE2 secretion in vitro and in nude mice carrying established MB xenografts.
Materials and Methods
Tumor Material and Patient Characteristics
All tumor tissue samples used in this study were collected at the Department of Oncology and Pathology, Karolinska University Hospital (Stockholm, Sweden), between 1994 and 2005. The diagnoses were confirmed by histological assessment of specimens according to WHO classification criteria.1 Tumor and patient characteristics are summarized in Table 1. Briefly, a total of 40 specimens from 39 patients 0–47 years of age (21 males; 31 of the patients were 0–18 years of age) were investigated. Five of the samples were classified as supratentorial primitive neuroectodermal tumors (sPNETs). Of the 39 patients, 12 had died of their disease (0–31 months from surgery), and 27 had no evidence of disease at the last follow-up (13–30 months from surgery). Eleven patients had metastatic disease, of which six were alive at the last follow-up.
Table 1.
COX-2 protein, mPGES-1, and EP1 through EP4 receptor expression in medulloblastoma (MB) primary tumors
| Sample | Diagnosis | Age (Years) | Gender | Risk Group | Out-come | Survival (Months) | Met | COX-2 | mPGES-1 | EP1 | EP2 | EP3 | EP4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MB | 7 | M | HR | NED | 130+ | Yes | + | + | + | + | + | + |
| 2A | MB | 8 | F | SR | NED | 112+ | No | + | + | + | + | + | + |
| 2B | MB | 13a | F | Relapse | NED | 52+ | No | + | + | + | + | + | + |
| 3 | MB | 8 | F | HR | NED | 112+ | No | + | + | + | + | + | + |
| 4 | sPNET | 8 | F | HR | NED | 110+ | No | + | + | + | + | + | + |
| 5 | MB | 8 | M | SR | NED | 108+ | No | + | + | + | + | + | + |
| 6 | sPNET | 3 | M | HR | DOD | 8 | No | + | + | + | + | + | + |
| 7 | sPNET | 5 | F | SR | NED | 39 | No | + | + | + | + | + | + |
| 8 | MB | 8 | M | SR | DOD | 31 | No | + | + | + | + | + | + |
| 9 | MB | 0 | F | HR | DOD | 0 | No | + | + | + | + | + | + |
| 10 | MB | 2 | M | HR | NED | 101+ | No | + | + | + | + | + | + |
| 11 | MB | 5 | F | HR | NED | 95+ | Yes | + | + | + | + | + | + |
| 12 | MB | 6 | M | HR | DOD | 29 | Yes | + | + | + | + | + | + |
| 13 | MB | 7 | M | SR | NED | 90+ | No | + | + | + | + | + | + |
| 14 | MB | 11 | F | HR | NED | 70+ | Yes | + | + | + | + | + | + |
| 15 | MB | 1 | F | SR | NED | 69+ | No | + | + | + | + | + | + |
| 16 | MB | 4 | M | SR | NED | 65+ | No | + | + | + | + | + | + |
| 17 | MB | 0 | F | HR | DOD | 15 | No | + | + | + | + | + | + |
| 18 | MB | 8 | F | SR | NED | 50+ | No | + | + | + | + | + | + |
| 19 | MB | 1 | F | HR | NED | 39+ | No | + | + | + | + | + | + |
| 20 | MB | 4 | M | SR | NED | 37+ | No | + | + | + | + | + | + |
| 21 | MB | 8 | F | SR | NED | 36+ | No | + | + | + | + | + | + |
| 22 | MB | 7 | M | HR | DOD | 3 | Yes | + | + | + | + | + | + |
| 23 | MB | 11 | M | HR | NED | 22+ | Yes | + | + | + | + | + | + |
| 24 | MB | 3 | F | HR | NED | 19+ | No | + | + | + | + | + | + |
| 25 | MB | 25 | F | SR | NED | 18+ | No | + | + | + | + | + | + |
| 26 | MB | 7 | M | HR | NED | 13+ | Yes | + | + | + | + | + | + |
| 27 | MB | 7 | M | SR | NED | 34+ | No | + | + | + | + | + | + |
| 28 | MB | 6 | M | HR | DOD | 22 | Yes | + | + | + | + | + | + |
| 29 | sPNET | 11 | F | SR | NED | 118+ | No | + | + | + | + | + | + |
| 30 | MB | 23 | M | SR | DOD | 33 | No | + | + | + | + | + | + |
| 31 | MB | 27 | M | SR | NED | 102+ | No | + | + | + | + | + | + |
| 32 | MB | 25 | M | SR | NED | 87+ | No | + | + | + | + | + | + |
| 33 | MB | 16 | M | HR | NED | 84+ | Yes | + | + | + | + | + | + |
| 34 | sPNET | 47 | F | HR | DOD | 68 | Yes | + | + | + | + | + | + |
| 35 | MB | 30 | M | SR | NED | 62+ | No | + | + | + | + | + | + |
| 36 | MB | 19 | F | SR | DOD | 0 | No | + | + | + | + | + | + |
| 37 | MB | 19 | M | HR | DOD | 23 | Yes | + | + | + | + | + | + |
| 38 | MB | 19 | M | SR | NED | 34+ | No | + | + | + | + | + | + |
| 39 | MB | 12 | F | SR | DOD | 6 | No | + | + | + | + | + | + |
Abbreviations: Met, metastatic disease; COX-2, Cyclooxygenase-2; mPGES-1, microsomal prostaglandin E synthase-1; EP, prostanoid receptor; MB, medulloblastoma; M, male; HR, high risk; NED, no evidence of disease; F, female; SR, standard risk; sPNET, supratentorial primitive neuroectodermal tumor; DOD, dead of disease.
Age at surgery for relapse.
Ethical approval was obtained by the Karolinska University Hospital Research Ethics Committee (approval no. 03-708).
Immunohistochemistry
For detection of COX-2 and mPGES-1 in primary tumors, tissue sections were incubated with either a monoclonal mouse anti-COX-2 antibody (Zymed Laboratories Inc., Carlsbad, CA, USA) or a monoclonal mouse anti-mPGES-1 antibody (Cayman Chemicals, Ann Arbor, MI, USA) overnight at 4°C. Prostanoid receptors were detected by incubating tumor sections with rabbit anti-EP1, anti-EP2, anti-EP3, and anti-EP4 (Cayman Chemicals), respectively, overnight at 4°C. The SuperPicture polymer detection kit with appropriate secondary antibodies was used together with a diaminobenzidine (DAB) substrate chromogen system to visualize immunopositivity (Zymed).
Active caspase-3 expression in human MB xenografts isolated from NMRI nu/nu mice was investigated by incubating sections overnight at 4°C with a monoclonal rabbit antibody specifically detecting active caspase-3 (R&D Systems, Abingdon, UK). Proliferation was detected using a specific Ki-67 (SP6) antibody (Neomarkers, Fremont, CA, USA). Immunopositivity was visualized as described above. As a control for nonspecific background staining, corresponding sections were incubated with antimouse immunoglobulin G (IgG) isotype or antirabbit IgG isotype controls (Zymed). Caspase-3 activation in tumor sections was quanitified by counting the number of tumor cells staining positive for the active caspase-3 antibody in six representative regions of the tumor section. Proliferation was assessed by counting the number of Ki-67 positively staining nuclei and total number of cancer cells at ×200 magnification, in six representative regions. For caspase-3 and Ki-67 staining, the results are expressed as the proportion of positively staining cells over the total number of cells.
Biotinylated Bandeiraea simplicifolia-1 (BS-1) lectin (Sigma-Aldrich, Solna, Sweden) was used to visualize endothelial cells. BS-1 was diluted 1:50 and incubated overnight at 4°C. Cells were detected with ABComplex conjugated to horseradish peroxidase (Dako A/S, Glostrup, Denmark). Sections were developed using DAB (SK-4100, Vector Laboratories Inc., Burlingame, CA, USA). Four tumor slides per treatment group and four fields per slide were quantified for microvessel density at ×200 magnification. The results are expressed as an average number of microvessels per field.
Chemicals
Diclofenac (Cayman Chemicals) was dissolved in OptiMEM (Gibco BRL, Sundbyberg, Sweden) to achieve the concentrations desired. Celecoxib (Pfizer, Täby, Sweden); ONO-8713, ONO-AE3-240, and ONO-AE3-208 (a gift from ONO Pharmaceuticals Co., Ltd., Osaka, Japan); AH 23848 and PGE2 (Sigma-Aldrich); and butaprost (Cayman Chemicals) were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and further diluted in OptiMEM or RPMI medium (Gibco BRL) to its final concentration (final DMSO concentration, 0.1%–0.7%).
Cell Lines
Cell lines used were kindly provided by Dr. T. Pietsch (University of Bonn Medical Center, Bonn, Germany), Dr. C. Redfern (Northern Institute for Cancer Research, Newcastle University, Newcastle, UK), and Dr. M. Nister (Karolinska Institutet, Stockholm, Sweden). In total, nine human MB/PNET cell lines were used, although DAOY and D324 MED, originally the same cell line, were obtained from different sources and cultured under slightly different conditions.14 The cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; DAOY and MEB-MED-8A cells), modified essential medium (MEM; D283 MED and D324 MED cells), Richter’s improved MEM with zinc (IMEMZO/DMEM; (D425 MED and D458 MED cells), DMEM/F12 (UW228-3 cells), IMEMZO/N-2 growth factor (D384 MED cells), or RPMI (PFSK-1 cells). Medium was supplemented with 10% (D283 MED, D324 MED, and PFSK-1) or 15% (DAOY, MEB-MED-8A, D425 MED, D458 MED, D384 MED, and UW228-3) heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/ml penicillin G, and 100 μg/ml streptomycin (Life Technologies Inc., Stockholm, Sweden) at 37°C in a humidified 5% CO2 atmosphere. All media were purchased from Gibco BRL.
PGE2 Measurement and COX Activity Assay
The MB cell lines D324 MED and PFSK-1 were seeded in 96-well plates and cultivated in OptiMEM containing 80 μM arachidonic acid (Sigma-Aldrich). Cells were treated with increasing concentrations of either diclofenac (0.78–100 μM) or celecoxib (0.001–30 μM) for 24 h, respectively. Cell supernatants were harvested, and PGE2 levels were measured using a PGE2 ELISA (enzyme-linked immunosorbent assay) kit (Cayman Chemicals) according to the manufacturer’s instructions. For measurements of COX enzymatic activity in MB cells, cell extracts from 1 × 108 D324 MED or PFSK-1 cells were preincubated with 0.001–10 μM celecoxib or 0.001–30 μM diclofenac for 5 min before addition of arachidonic acid. COX enzymatic activity was measured using a COX activity assay (Cayman Chemicals).
Proliferation and Clonogenic Assay
The effects of NSAIDs (diclofenac and celecoxib) and the EP receptor antagonists (ONO-8713, ONO-AE3-240, ONO-AE3-208, and AH 23848) on MB cell growth were determined using a colorimetric 3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay (Sigma-Aldrich) as previously described.15 Eight parallels of each treatment were performed in each experiment. The concentration that inhibited 50% of cell viability (EC50) was calculated.
For assessment of PGE2 and butaprost effects on cell proliferation, 1.0 × 104 cells were seeded in 96-well plates and incubated overnight in RPMI medium containing 10% FBS. Cells were then serum starved for 24 h before treatment with 5 nM to 1 μM PGE2 supplemented with 0.5% bovine serum albumin (Sigma-Aldrich) or 1–10 μM butaprost for 72 h. Proliferation was measured using MTT assay as described above.
To determine colony formation, D283 MED, D324 MED, PFSK-1, DAOY, and UW228-3 cells were seeded in 50 mm2 Cell+ Petri dishes (Sarstedt, Solna, Sweden) at a concentration of 150 cells/dish in triplicate. Cells were allowed to attach to the surface for 5 h before treatment with diclofenac (50 and 100 μM) or celecoxib (10 and 30 μM) for 48 h. After 12 days of incubation in drug-free medium, cell cultures were rinsed with phosphate-buffered saline, fixed in formaldehyde, and stained with Giemsa (Gibco BRL). Colonies (>75 cells) with 50% plate efficiency (PE) were counted manually using a colony counter. For each treatment combination, the surviving fraction was calculated as the ratio of the mean PE of treated cells over the PE of untreated control cells.
Plasmids and Lentivirus Infection
Plasmids containing COX-2 short hairpin RNA (shRNA; pLKO.1 COX-2 shRNA clone 0000045535, Open Biosystems, Huntsville, AL, USA), scrambled shRNA (Addgene pLKO.1, clone 1864, Addgene Inc., Cambridge, MA, USA), or enhanced green fluorescent protein plasmid (peGFP; clone 12257, Addgene Inc.) were cotransfected together with psPAX2 and pDM2.G (Addgene Inc.) into actively growing HEK-293T cells (kindly provided by J. Löfling, Karolinska Institutet) using polyethyleneimine (Polyscience Europe, Eppelheim, Germany); peGFP served as a control for transfection efficiency. MB cells were infected with approximately 1 × 106 lentivirus particles/ml. Western blot analysis on protein extracts and trypan blue dye exclusion to evaluate cell proliferation were carried out 5 days after infection.
Fluorescence-Activated Cell-Sorting Analysis
DNA content was assessed essentially as previously described.16 Briefly, D283 MED, D324 MED, and PFSK-1 cells were treated with the indicated concentrations of diclofenac and celecoxib for 48 h. Cells were harvested, stained with 4′,6-diamidino-2-phenylindole (DAPI), and subjected to cell cycle analysis using single-parameter DNA flow cytometry. The multicycle program for cell cycle analysis (Phoenix Flow Systems, San Diego, CA, USA) was used for histogram analysis.
Western Blotting
Protein was extracted from cells in RIPA buffer (25 mM Tris [pH 7.8], 2 mM EDTA, 20% glycerol, 0.1% Nonidet P-40 [NP-40], 1 mM dithiothreitol, and protease inhibitors [Roche Diagnostic, Mannheim, Germany]). Protein concentration was measured using Bradford reagent (Bio-Rad, Sundbyberg, Sweden). Equal quantities were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nylon membranes (Millipore Inc., Sundbyberg, Sweden), and probed with antibodies against COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); polyclonal anti-mPGES-1 (Cayman Chemicals); EP1 receptor, EP2 receptor, EP3 receptor, and EP4 receptor (Cayman Chemicals); cleaved caspase-9, cleaved caspase-3, poly(ADP-ribose) (PARP), and the BH3 interacting domain death agonist (BID; Cell Signaling Technology, Beverly, MA, USA); and β-actin (Sigma-Aldrich). Antimouse IgG or antirabbit IgG, conjugated with horseradish peroxidase (Pharmacia Biosciences, Uppsala, Sweden), was used as secondary antibody. Pierce Super Signal (Pierce, Rockford, IL, USA) was used for chemiluminescent detection.
Xenografts and In Vivo Administration of NSAIDs
Female NMRI nu/nu mice (Taconic Laboratories, Ejby, Denmark) 4–8 weeks old were maintained at five of each per cage and were given sterile water and food ad libitum. Each NMRI nu/nu mouse was subcutaneously injected with 20 × 106 D283 MED MB cells. Treatment was started on the appearance of palpable tumors reaching the volume of 0.20 ml. The mean tumor volume at the start of the treatment was 0.24 ml. Tumors were measured every day, and tumor volume was calculated as (width)2 × length × 0.44. Tumor volume index was calculated using the measured volume divided by the volume measured at start of treatment. Two independent experiments were carried out. In the first experiment mice were randomly assigned to receive 250 mg/liter diclofenac (n = 8) in drinking water or no treatment (n = 9). In the second experiment, mice were randomized to receive 2 mg celecoxib (n = 7) once daily through a gastric feeding tube or no treatment (n = 10). Tumor weight was recorded at autopsy, after which tumors were fixed in formaldehyde for subsequent immunohistochemical analysis. All animal experiments were approved by the regional ethics committee for animal research (approval N234/05) in accordance with the Animal Protection Law (SFS 1988:534), the Animal Protection Regulation (SFS 1988:539), and the Regulation for the Swedish National Board for Laboratory Animals (SFS 1988:541)
Statistical Analysis
The EC50 values were evaluated from a plot of survival versus the logarithm of the molar drug concentration, using a standard dose–response curve defined by four parameters: the baseline response (Bottom), the maximum response (Top), the slope (Hill slope), and the drug concentration:
| (1) |
where x is the logarithm of the drug concentration. The nonlinear regression analysis was performed by the PCNONLIN program (version 2.0).17 The data were initially fitted to Equation 1 with the Hill slope fixed to a value of −1, but the Hill slope was also fitted along with the other parameters. The choice of the final model was based on the F-ratio test.18
Two independent populations were analyzed for statistically significant differences by the Mann-Whitney U-test; several independent populations were analyzed by the Kruskal-Wallis test (nonparametric analysis of variance) followed by Dunn’s multiple comparison test.
Calculation of median values and their nonparametric approximate 95% confidence intervals (95% CIs) were based on the Wilcoxon sign-rank test as outlined by Tukey.19 The one-sample t-test was used to test whether the mean of a single sample differed significantly from control.
All statistical tests were two-sided.
Results
Expression of COX-2, mPGES-1, and Prostanoid EP Receptors in MB Tissues and Cell Lines
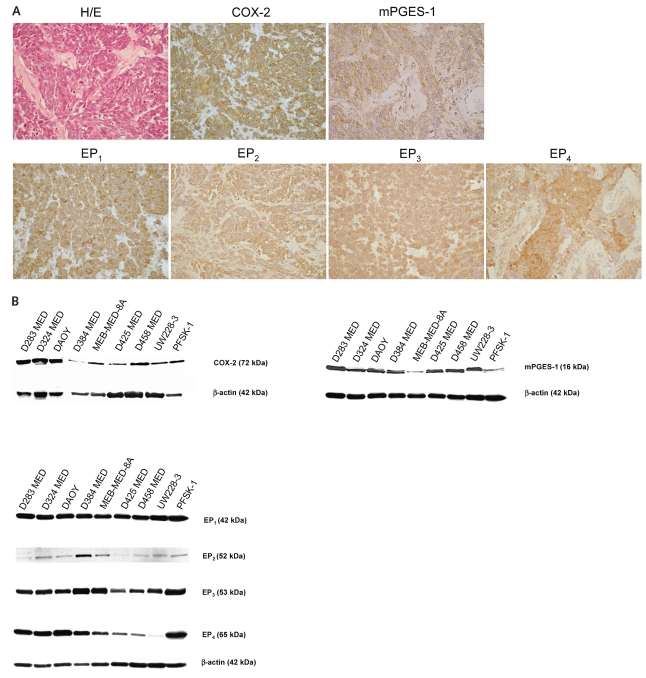
Thirty-nine human MB and sPNET tissue samples derived from different pathological subsets and ages were examined for expression of COX-2 and mPGES-1 by immunohistochemistry (Table 1). All tumors investigated showed specific expression of COX-2 protein in the cytoplasm of the tumor cells. High expression of mPGES-1 was also detected in all clinical MB tumors investigated (Fig. 1A). Neither COX-2 protein nor mPGES-1 was detected in surrounding stromal cerebellar tissue, except for Purkinje cells that also expressed COX-2 and mPGES-1 (not shown; Fig. 1A). Moreover, abundant expression of the four prostanoid EP receptors was detected in all clinical MB tumors analyzed, both in tumor cells and in stromal endothelial cells. Localization of the receptors was shown to be both at outer membranes and within subcellular compartments (Fig. 1A). As a negative control, sections were immunostained with isotype control antibody, which resulted in no staining (data not shown).
Fig. 1.
Cyclooxygenase-2 (COX-2), microsomal prostaglandin E synthase-1 (mPGES-1), and prostanoid receptor (EP1 through EP4) expression in medulloblastoma (MB) primary tumors and cell lines. (A) Immunohistochemistry showing specific expression of COX-2, mPGES-1, and EP1 through EP4 in clinical MB tissue samples. H/E, hematoxylin and eosin staining. Magnification, ×400 magnification. (B) Western blotting detecting COX-2, mPGES-1, and EP receptor proteins in MB cell lines.
Examination of human MB cell lines by Western blotting revealed that all MB cell lines express COX-2, mPGES-1, and EP1 through EP4 receptors (Fig. 1B).
PGE2 Induces Proliferation of MB Cells
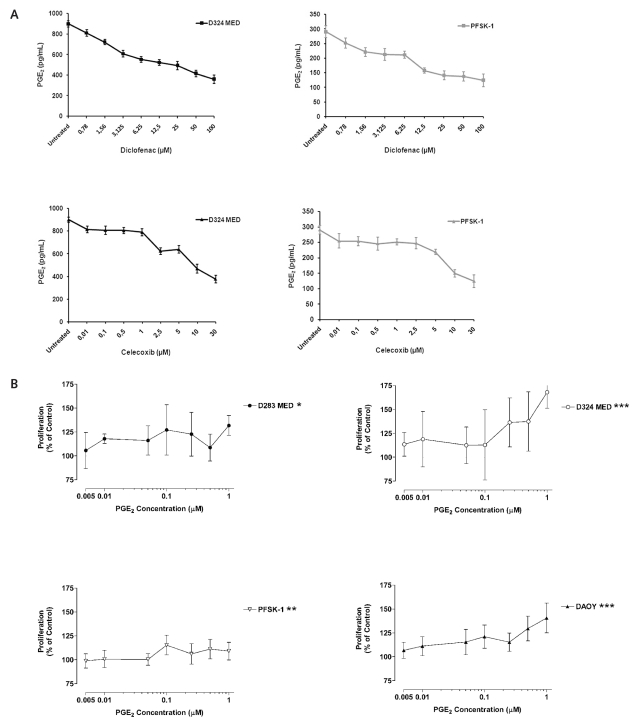
Because most of the MB cell lines investigated expressed high levels of COX-2 as well as mPGES-1, we measured the levels of PGE2 in the supernatant from the MB cell lines D324 MED and PFSK-1. Both cell lines secreted PGE2, levels of which could be significantly reduced in a dose-dependent manner by treatment with the COX inhibitors diclofenac and celecoxib (p < 0.01; Fig. 2A). The concentrations that inhibited 50% of PGE2 secretion (IC50) from D324 MED and PFSK-1 cells were 40 μM diclofenac and 10 μM celecoxib, and 35 μM diclofenac and 12 μM celecoxib, respectively (Fig. 2A). Half-maximal inhibition (IC50) of COX enzymatic activity as measured by the conversion of arachidonic acid to prostaglandin H2 was achieved with 0.1 μM diclofenac or 0.005 μM celecoxib in cell extracts isolated from D324 MED or PFSK-1 cells (data not shown).
Fig. 2.
Prostaglandin E2 (PGE2) induces proliferation of medulloblastoma (MB) cells. (A) PGE2 secretion by D324 MED and PFSK-1 MB cells is inhibited by the nonsteroidal anti-inflammatory drugs (NSAIDs) diclofenac and celecoxib. (B) Effect of PGE2 on MB cell growth: mean ± SD percent proliferation of D283 MED, D324 MED, PFSK-1, and DAOY MB cells incubated with PGE2 compared with untreated cells (100%). *p <0.05, **p <0.01, ***p <0.001.
We also examined the effect of exogenous addition of PGE2 and of the commercially available EP2 receptor agonist butaprost on the proliferation of MB cells. Incubation of MB cell lines with increasing concentration of PGE2 (5 nM to 1 μM) or butaprost (1–10 μM) for 72 h resulted in a significant increase of MB cell proliferation (PGE2: D283 MED, p < 0.05; D324 MED, p < 0.001; PFSK-1, p < 0.01; DAOY, p < 0.001 [Fig. 2B]; butaprost: D283 MED, p < 0.001; D324 MED, p < 0.001; PFSK-1, p < 0.001; DAOY, p < 0.001; data not shown).
Inhibition of COX-2 or EP Receptor Activity Suppresses Both Growth and Clonogenic Capacity of MB Cells
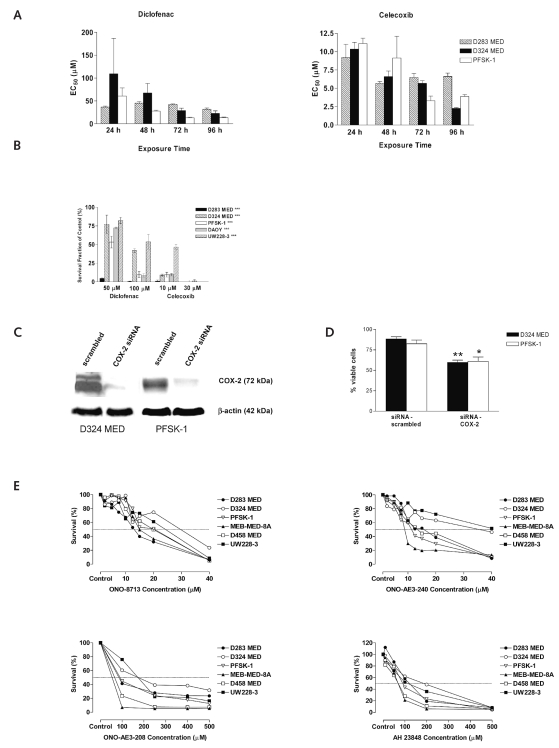
We first investigated the effect of the dual COX-1/COX-2 inhibitor diclofenac and the COX-2–specific inhibitor celecoxib on the growth of nine MB cell lines. The cell lines were treated with increasing concentrations of diclofenac or celecoxib for 48 h, and cell viability was determined by MTT at the end of the treatment period. All MB cell lines investigated demonstrated a concentration- dependent decrease in cell viability after 48 h of incubation (Table 2). The biological EC50 concentrations for the MB cell lines ranged from 27.5 μM to 79.1 μM (median, 47.3 μM) for diclofenac and from 7.8 μM to 13.5 μM (median, 9.3 μM) for celecoxib (Table 2). We also investigated the time-dependent effect of diclofenac and celecoxib on the MB cell lines D283 MED, D324 MED, and PFSK-1 (Fig. 3A). A significant reduction of cell viability was observed in MB cells treated with diclofenac or celecoxib with increasing incubation times (p < 0.001; Fig. 3A). Comparison of the EC50 values for the different MB cell lines incubated for 24–96 h with diclofenac or celecoxib demonstrated that the effect of the dual COX-1/COX-2 inhibitor diclofenac had a more definite time dependency and was more effective upon longer incubation periods compared with celecoxib (Fig. 3A).
Table 2.
Concentrations of diclofenac or celecoxib associated with 50% cell viability inhibition (EC50) in medulloblastoma cell lines
| Diclofenac
|
Celecoxib
|
|||||
|---|---|---|---|---|---|---|
| Cell Line | EC50 (10−6 M) | SD | CV% | EC50 (10−6 M) | SD | CV% |
| D283 MED | 35.7 | 1.7 | 4.7 | 8.8 | 0.7 | 3.3 |
| D324 MED | 65.4 | 2.9 | 4.5 | 13.5 | 3.6 | 10.5 |
| PFSK-1 | 79.1 | 1.8 | 2.3 | 12.2 | 0.5 | 1.5 |
| D425 MED | 59.1 | 10.4 | 17.6 | 8.3 | 1.0 | 4.6 |
| D458 MED | 59.9 | 4.8 | 8.1 | 9.3 | 0.9 | 4.0 |
| DAOY | 59.0 | 16.6 | 28.2 | 12.8 | 0.8 | 2.4 |
| UW228-3 | 27.5 | 0.6 | 2.1 | 8.8 | 0.7 | 3.3 |
| D384 MED | 33.5 | 9.1 | 27.0 | 7.8 | 2.0 | 10.4 |
| MEB-MED-8A | 29.8 | 0.5 | 1.6 | 11.6 | 4.6 | 16.4 |
| Median | 47.35 | 3.85 | 6.4 | 9.3 | 0.95 | 4.3 |
Abbreviation: CV%, coefficient of variation.
Fig. 3.
Inhibition of PGE2 synthesis and its receptor activity suppresses medulloblastoma (MB) growth in vitro. (A) Time-dependent effect of diclofenac and celecoxib treatment on the MB cell lines D283 MED, D324 MED, and PFSK-1: mean ± SD for MB cells treated with nonsteroidal anti-inflammatory drugs (NSAIDs) with increasing incubation times compared with untreated controls. p < 0.001 for all treatments. (B) Effect of NSAIDs on clonogenic survival of MB cells: mean ± SD for MB cells treated with NSAIDs compared with untreated controls. ***p < 0.001. (C) Suppression of cyclooxygenase-2 (COX-2) expression by RNA interference in D324 MED and PFSK-1 cells: Western blotting of cell extracts isolated from D324 MED and PFSK-1 cells infected with lentivirus containing small interfering RNA (siRNA) targeting COX-2 mRNA or with a scrambled siRNA. (D) Suppression of COX-2 expression reduced MB cell proliferation: mean ± SD for MB cells transfected with either COX-2 siRNA or scrambled siRNA. *p <0.05, **p <0.01. (E) Effect of EP receptor antagonists on MB cell proliferation: proliferation assays of MB cells incubated with the EP1 receptor antagonist ONO-8713, the EP3 receptor antagonist ONO-AE3-240, or the EP4 receptor antagonists ONO-AE3-208 and AH 23848.
Clonogenic assay was performed on five of the MB cell lines to further determine the antitumor effects of diclofenac and celecoxib. Treatment showed a significant dose-dependent inhibition of colony formation (p < 0.001; Fig. 3B).
To investigate whether the observed treatment effect of NSAIDs was caused by a direct inhibition of COX-2 or by COX-2–independent mechanisms, we suppressed COX-2 expression in D324 MED and PFSK-1 MB cells using COX-2 small interfering RNA (siRNA). A marked reduction of COX-2 protein, as analyzed by Western blotting, was observed in both MB cell lines infected with lentiviral COX-2 siRNA compared with cells infected with the scrambled siRNA construct (Fig. 3C). Both MB cell lines infected with the COX-2–specific siRNA demonstrated significantly reduced cell proliferation (D324 MED, p < 0.0022; PFSK-1, p < 0.0152; Fig. 3D) after 5 days of incubation, compared with cells infected with the scrambled siRNA construct.
As shown in Fig. 1, A and B, all MB/sPNET primary tumors and cell lines investigated expressed the four PGE2 receptors EP1 through EP4. We therefore examined the effect of EP receptor antagonists on MB cell proliferation using the EP1-specific receptor antagonist ONO-8713, the EP3-specific antagonist ONO-AE3-240, and the EP4-specific antagonists ONO-AE3-208 and AH 23848. The biological EC50 values of the different receptor antagonists ranged from 14 μM to 30 μM for ONO-8713 and from 9 μM to 42 μM for ONO-AE3-240; both EP4 receptor antagonists, ONO-AE3-208 and AH 23848, were less efficient in inhibiting MB proliferation with EC50 concentrations varying from 55 μM to 175 μM for ONO-AE3-208 and from 70 μM to 270 μM for AH 23848 (Fig. 3E).
Inhibition of COX-2 Activity Induces Apoptosis of MB Cells
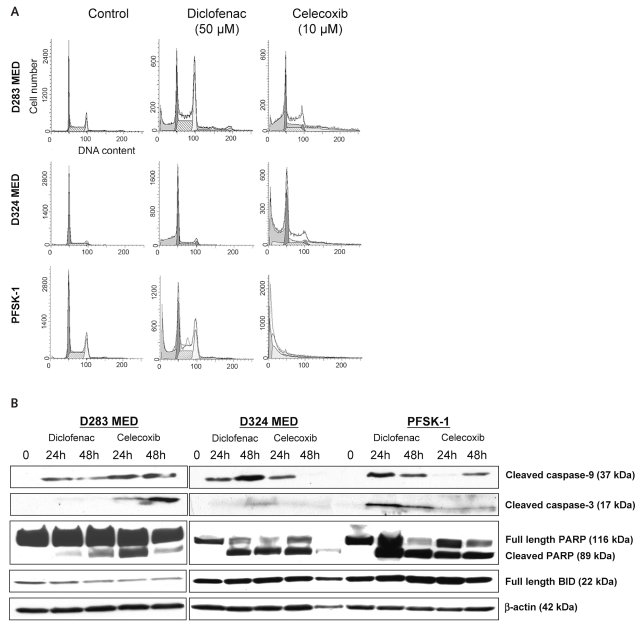
To further study the mechanisms of NSAID-mediated inhibition of MB cell growth, we evaluated the effects of diclofenac and celecoxib on the induction of apoptosis. Cell cycle analyses of D283 MED, D324 MED, and PFSK-1 cells treated with diclofenac or celecoxib for 48 h showed an accumulation of cells in the sub-G1 phase of the cell cycle (Fig. 4A). A pronounced G2 arrest was also observed in MB cells treated with diclofenac (Fig. 4A). Western blot performed on protein extract isolated from MB cells treated with diclofenac or celecoxib for 24 h and 48 h revealed activation of caspase-9 and caspase-3 followed by cleavage of PARP, a downstream substrate of caspase-3 (Fig. 4B). No cleavage of BID (BH3 interacting domain death agonist) was detected in MB cells treated with NSAIDs (Fig. 4B).
Fig. 4.
Effect of diclofenac and celecoxib on apoptosis in medulloblastoma (MB) cells. (A) Cell cycle analysis of MB cells treated with cyclooxygenase (COX) inhibitors. A pronounced accumulation of cells in the sub-G1 phase of the cell cycle was observed for both diclofenac and celecoxib in all three MB cell lines. (B) Analysis of proteins in the apoptotic pathways by Western blotting. In all three MB cell lines, D283 MED, D324 MED, and PFSK-1, activation of caspase-3, caspase-9, and poly(ADP-ribose) polymerase (PARP) after 24 h of drug treatment was observed. No activation of BH3 interacting domain death agonist (BID) was detected. The blot was stripped and probed with β-actin to ensure equal protein loading.
NSAIDs Significantly Inhibit MB Growth In Vivo
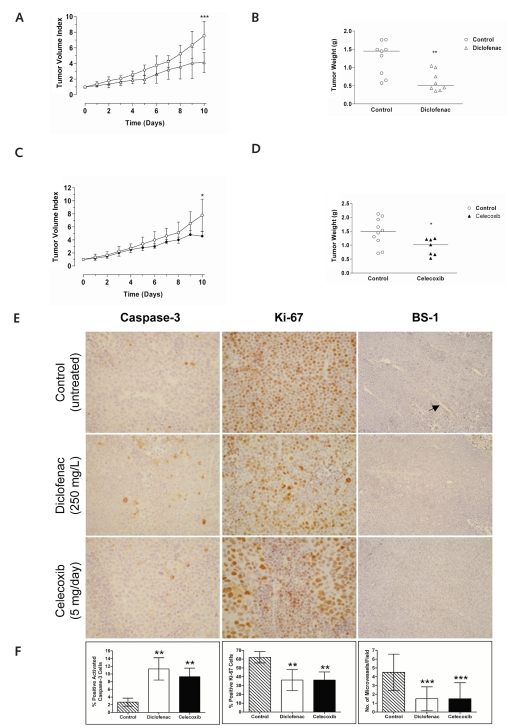
To investigate the therapeutic effects of NSAIDs on MB growth in vivo, we treated NMRI nu/nu mice carrying D283 MED xenografts with either diclofenac or celecoxib. Mice were randomly assigned to receive 250 mg/liter diclofenac in the drinking water (n = 8) or 2 mg celecoxib administered through oral gavage every 24 h for 10 days (n = 7) or to be left untreated (diclofenac control, n = 9; celecoxib control, n = 10). Mean tumor volume at start of treatment was 0.24 ml. Both diclofenac and celecoxib significantly inhibited MB tumor growth after 10 days of treatment compared to untreated controls (diclofenac, p = 0.0006; tumor volume index, 4.4 vs. 7.7; celecoxib, p = 0.0130; tumor volume index, 4.3 vs. 7.4; Fig. 5A,C). The mean tumor weight recorded at autopsy was 0.62 g (95% CI, 0.44–0.88 g) for diclofenac-treated mice, 0.94 g (95% CI, 0.59–1.22 g) for celecoxib-treated mice, 1.30 g (95% CI, 0.95–1.63 g) for untreated diclofenac controls, and 1.44 g (95% CI, 1.09–1.83 g) for untreated celecoxib controls (Fig. 5B,D).
Fig. 5.
Effects of cyclooxygenase (COX) inhibitors on established medulloblastoma (MB) xenografts in vivo. (A) Treatment of nude mice carrying D283 MED xenograft with 250 mg/liter diclofenac significantly inhibited tumor growth (mean ± SD). ***p <0.001. (B) Tumor weight of D283 MED xenografts from mice treated with 250 mg/liter diclofenac and untreated controls. **p < 0.01. (C) Treatment of nude mice carrying D283 MED xenograft with 2 mg celecoxib significantly inhibited tumor growth (mean ± SD). *p < 0.05. (D) Tumor weight of D283 MED xenografts from mice treated with 2 mg celecoxib and untreated controls. *p < 0.05. (E) Immunohistochemical analysis of tumor sections from D283 MED xenografts of nude mice receiving no treatment (control) or treated with diclofenac or celecoxib. Tissue sections from D283 MED xenografts were incubated with a monoclonal antibody detecting activated caspase-3 (left column; ×400 magnification). Proliferation was investigated using a specific antibody detecting the proliferation marker Ki-67 (middle column; ×400 magnification). Endothelial cells were stained using biotinylated Bandeiraea simplicifolia-1 (BS-1) lectin (right column; ×200 magnification). (F) Summary of the findings for caspase-3 activation, proliferative index, and microvessel density in tumors from nude mice treated with diclofenac or celecoxib (mean ± SE): cells staining positive for (left to right) activated caspase-3, Ki-67, and BS-1. Microvessel density was assessed by counting the number of microvessels at ×200 magnification in 16 fields that had the highest vascularization. **p < 0.01, ***p < 0.001.
Immunohistochemistry of tumors from mice treated with either diclofenac or celecoxib showed a significant elevated expression of active caspase-3 (diclofenac, p = 0.0022; celecoxib, p = 0.0022) indicating that these NSAIDs induce apoptosis of MB in vivo (Fig. 5E,F). Since inhibition of angiogenesis can contribute to reduced cell proliferation and increased apoptosis, we also determined the effect of diclofenac and celecoxib on microvessel density. Microvessel density was significantly decreased (33%) in tumors from animals treated with either diclofenac or celecoxib compared with tumors from animals receiving no treatment (diclofenac, p < 0.001; celecoxib, p < 0.001; Fig. 5E,F). Both diclofenac and celecoxib induced a significant reduction (26%) in expression of the nuclear proliferation marker Ki-67 compared with untreated tumors (diclofenac, p = 0.0022; celecoxib, p = 0.0022; Fig. 5E,F).
Discussion
In this study, we examined the role of the PGE2 synthetic pathway in MB and demonstrate that MB primary tumors and cell lines constitutively express COX-2 and mPGES-1 as well as all four prostanoid EP receptors. Moreover, PGE2 stimulates MB cell proliferation, and inhibition of PGE2 production or its receptors has therapeutic efficacy on MB in vitro and in vivo.
MB is considered to originate from primitive pluripotent neuroepithelial stem cells, located in the external germinal layer (EGL) of the cerebellum.20 Nontumorigenic cells located in the EGL do not express COX-2, whereas Purkinje cells, which are unlikely to give rise to MB tumors, have been shown to express COX-2.20–25 Using immunohistochemistry, we demonstrated that MB cells and Purkinje cells express COX-2, whereas in stroma and surrounding nonmalignant brain tissue, COX-2 was not detected (Fig. 1A). Hence, MB cells might acquire COX-2 expression at some stage during the development from pluripotent neuroepithelial progenitor cells. High expression of COX-2 has also been reported in other tumors of CNS origin, including glioma and ependymoma, as well as in meningioma.24,26–28 We also detected constitutive expression of mPGES-1 in both MB primary tumors and cell lines (Fig. 1A,B). Although information on mPGES-1 expression in brain tumors is limited, this finding is consistent with other reports describing increased expression of mPGES-1 in cancers.10,11
Compared to nonmalignant nervous tissue, MB and other tumors of CNS origin contain increased levels of arachidonic acid.4,29 The presence of high levels of COX-2 and mPGES-1 in MB cells may therefore generate a favorable environment for the efficient production of prostanoids.
Measurement of PGE2 using ELISA revealed that MB cells secrete PGE2 that could be reduced by inhibiting COX (Fig. 2A). Furthermore, exogenous addition of PGE2 to serum-starved MB cells resulted in increased proliferation (Fig. 2B). Other reports have shown that patients with malignant brain tumors have significantly higher concentrations of PGE2 in their tumors and plasma compared with patients with benign brain tumors or with nontumoral control patients.6,30 Moreover, the concentration of PGE2 in plasma decreases following neurosurgical resection of both malignant and benign brain tumors.5
Having shown that PGE2 stimulates MB cell growth, we looked for the expression of prostanoid receptors in both clinical MB samples and cell lines and found that all four EP receptors (EP1 through EP4) were abundantly expressed in MB (Fig. 1A,B). Although the precise role of each EP receptor in cancer development has not been fully characterized, the generation of specific EP receptor knockout mice has shown that all four prostanoid receptors may contribute to carcinogenesis, malignant aggressiveness, and tumor progression in a number of cancers.31 Despite structural and sequence similarities among the EP receptors they are coupled to different intracellular signaling pathways, and the receptors exhibit different functions depending on tumor type.12 Of interest, the stimulation of EP receptors by PGE2 has been shown to activate intracellular signaling pathways that are aberrantly regulated in MB. For instance, stimulation of EP2 receptor by PGE2 activates the kinase activity of the phosphatidylinositol-3-kinase (PI3K) oncogene and stimulates the transcription-factor activity of β-catenin, whereas PGE2-mediated activation of EP1 or EP4 induces phosphorylation of the epidermal growth factor receptor resulting in the activation of the Erk signaling path-way.32–34 Hence, activation of EP receptors by PGE2 may contribute to the enhancement of signal transduction by these pathways in MB. In our study, treatment with the EP1 antagonist ONO-8713 or EP3 antagonist ONO-AE3-240 reduced MB cell growth, whereas antagonists targeting the EP4 receptor were less effective in inhibiting MB cell growth. We also found that the EP2 agonist butaprost stimulated MB cell growth. To our knowledge, antagonists targeting the EP2 receptor are not yet commercially available for experimental research. Although more studies need to be performed in order to exactly demonstrate which EP receptors are most important in promoting MB cell proliferation, our data indicate that the prostanoid receptors EP1, EP2, and EP3 are important for PGE2-mediated stimulation of MB cell growth.
Because we showed that MB expressed high levels of both COX-2 and mPGES-1, we decided to investigate the effect of inhibiting PGE2 secretion by targeting COX-2. As shown in Fig. 3A and Table 2, both the dual COX-1/COX-2 NSAID diclofenac and the COX-2–specific NSAID celecoxib, inhibited MB cell growth and clonogenic capacity in all cell lines tested. The biological EC50 values ranged from 27.5 μM to 79.1 μM for diclofenac and from 7.8 μM to 13.5 μM for celecoxib (Table 2) after 48 h of drug incubation, which is comparable to EC50 values found in other cancer cell lines.15,35–38 Although the EC50 doses of diclofenac and celecoxib are higher than the concentrations of NSAIDs required to inhibit the enzymatic activity of COX in MB cells, they correspond to the concentrations required to inhibit 50% of PGE2 secretion from MB cells. These data, together with the demonstration that PGE2 stimulates MB cell proliferation (Fig. 2B), suggest that PGE2 is important for MB growth. The observed growth inhibitory effect of NSAIDs was associated with the induction of caspase- dependent apoptosis (Fig. 4B). To further test the importance of COX-2 expression in MB, we suppressed COX-2 expression by COX-2 siRNA (Fig. 3C). This suppression resulted in inhibition of MB cell proliferation (Fig. 3D). Although a number of other reports have shown that NSAIDs induce cytotoxicity in tumor cells by COX-2 inhibition followed by induction of apoptosis, it cannot be excluded that NSAID targets other than COX-2 are affected in MB cells.39,40 For instance, the COX-2–specific inhibitors, celecoxib and rofecoxib, have been shown to decrease cell survival in colon cancer and prostate cancer cell lines, independent of their COX-2 expression.41,42 In addition to specific COX-2 inhibition, celecoxib has also been shown to direct target protein-dependent kinase-1, the endoplasmic reticulum Ca2+ ATPase complex, cyclin-dependent kinases, and various carbonic anhydrases. Inhibition of these proteins leads to apoptosis or inhibition of cell cycle progression, angiogenesis, and metastasis.40,42–45 The concentration of NSAID required to induce these effects is higher than that achieved in the plasma of patients in clinical studies to obtain anticarcinogenic effect.40
We found that treatment with NSAIDs was associated with the suppression of growth of established human MB xenografts in nude mice (Fig. 5A–D). The doses of NSAID used for the in vivo study were based on our previously obtained results in experimental neuroblastoma, and the dose for celecoxib is significantly lower than the oncological dose of 250 mg/m2 orally twice daily established for children.36,46,47 All mice gained in weight during treatment and showed no signs of toxicity. To better understand the growth inhibitory effects of diclofenac and celecoxib, measurements of apoptosis, microvessel density, and cell proliferation were performed ex vivo (Fig. 5E,F). Immunohistochemistry of tumors from diclofenac- and celecoxib-treated animals demonstrated elevated expression of active caspase-3 compared with untreated tumors, indicating that both NSAIDs induce apoptosis of MB in vivo. We also detected significant reduction in the expression of the cell proliferation marker Ki-67 in tumors from animals treated with NSAIDs. The increased number of caspase-3–positive cells in tumors from animals treated with NSAIDs might be due to a direct effect of COX-2 inhibition. However, we also observed a significant reduction in microvessel density in the treated tumors compared with nontreated tumors (Fig. 5E,F). COX-2 is expressed within neovasculature in tumors, and a number of reports have shown that certain NSAIDs, including celecoxib and diclofenac, suppress angiogenesis.48–51 Thus, both diclofenac and celecoxib may inhibit MB growth by a combination of apoptosis of tumor cells and inhibition of angiogenesis.
The current treatment approach for patients with childhood MB is surgery followed by irradiation and chemotherapy. Inhibition of COX-2 activity has been shown to potentiate the effect of both irradiation and cytotoxic drugs.52–54 Hence, agents that target components in the eicosanoid cascade may prove beneficial, especially as an adjuvant therapy because their advantageous effect may be the synergism with irradiation or conventional cytotoxic treatment or novel agents targeted against other pro-carcinogenic pathways. Particularly the youngest children are at risk for irreversible adverse side effects from irradiation. Since ionizing radiation induces COX-2 expression the concomitant use of agents targeting the production of prostanoids may be beneficial for patients through both improved therapeutic efficacy and the putative limitation of adverse long-term neurological side effects.53
Acknowledgments
This work was supported by grants from the Swedish Children’s Cancer Foundation, Swedish Cancer Society, Märta and Gunnar V Philipson Foundation, Mary Bevé Foundation, and Swedish Research Council.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 3.Rood BR, Macdonald TJ, Packer RJ. Current treatment of medulloblastoma: recent advances and future challenges. Semin Oncol. 2004;31:666–675. doi: 10.1053/j.seminoncol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Kokoglu E, Tuter Y, Yazici Z, et al. Profiles of the fatty acids in the plasma membrane of human brain tumors. Cancer Biochem Biophys. 1998;16:301–312. [PubMed] [Google Scholar]
- 5.Loh JK, Hwang SL, Lieu AS, Huang TY, Howng SL. The alteration of prostaglandin E2 levels in patients with brain tumors before and after tumor removal. J Neurooncol. 2002;57:147–150. doi: 10.1023/a:1015782809966. [DOI] [PubMed] [Google Scholar]
- 6.Nathoo N, Barnett GH, Golubic M. The eicosanoid cascade: possible role in gliomas and meningiomas. J Clin Pathol. 2004;57:6–13. doi: 10.1136/jcp.57.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furstenberger G, Krieg P, Muller-Decker K, Habenicht AJ. What are cyclooxygenases and lipoxygenases doing in the driver’s seat of carcinogenesis? Int J Cancer. 2006;119:2247–2254. doi: 10.1002/ijc.22153. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Naraba H, Tanioka T, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 10.Mehrotra S, Morimiya A, Agarwal B, Konger R, Badve S. Microsomal prostaglandin E2 synthase-1 in breast cancer: a potential target for therapy. J Pathol. 2006;208:356–363. doi: 10.1002/path.1907. [DOI] [PubMed] [Google Scholar]
- 11.van Rees BP, Sivula A, Thoren S, et al. Expression of microsomal prostaglandin E synthase-1 in intestinal type gastric adenocarcinoma and in gastric cancer cell lines. Int J Cancer. 2003;107:551–556. doi: 10.1002/ijc.11422. [DOI] [PubMed] [Google Scholar]
- 12.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Fulton AM, Ma X, Kundu N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer Res. 2006;66:9794–9797. doi: 10.1158/0008-5472.CAN-06-2067. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen PF, Jenkyn DJ, Papadimitriou JM. Establishment of a human medulloblastoma cell line and its heterotransplantation into nude mice. J Neuropathol Exp Neurol. 1985;44:472–485. doi: 10.1097/00005072-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Johnsen JI, Lindskog M, Ponthan F, et al. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210–7215. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- 16.Ponthan F, Johnsen JI, Klevenvall L, Castro J, Kogner P. The synthetic retinoid RO 13-6307 induces neuroblastoma differentiation in vitro and inhibits neuroblastoma tumour growth in vivo. Int J Cancer. 2003;104:418–424. doi: 10.1002/ijc.10954. [DOI] [PubMed] [Google Scholar]
- 17.Statistical Consultants, Inc. PCNONLIN and NONLIN84: software for the statistical analysis of nonlinear models. Am Stat. 1986:40–52. [Google Scholar]
- 18.Boxenbaum HG, Riegelman S, Elashoff RM. Statistical estimations in pharmacokinetics. J Pharmacokinet Pharmacodynam. 1974;2:123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- 19.Daniel WW, editor. Applied Nonparametric Statistics. Boston: Houghton Mifflin; 1978. [Google Scholar]
- 20.Raffel C. Medulloblastoma: molecular genetics and animal models. Neoplasia. 2004;6:310–322. doi: 10.1593/neo.03454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsubokura S, Watanabe Y, Ehara H, et al. Localization of prostaglandin endoperoxide synthase in neurons and glia in monkey brain. Brain Res. 1991;543:15–24. doi: 10.1016/0006-8993(91)91043-z. [DOI] [PubMed] [Google Scholar]
- 23.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 24.Joki T, Heese O, Nikas DC, et al. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. 2000;60:4926–4931. [PubMed] [Google Scholar]
- 25.Pardue S, Rapoport SI, Bosetti F. Co-localization of cytosolic phospholipase A2 and cyclooxygenase-2 in rhesus monkey cerebellum. Brain Res Mol Brain Res. 2003;116:106–114. doi: 10.1016/s0169-328x(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 26.Kim SK, Lim SY, Wang KC, et al. Overexpression of cyclooxygenase-2 in childhood ependymomas: role of COX-2 inhibitor in growth and multi-drug resistance in vitro. Oncol Rep. 2004;12:403–409. [PubMed] [Google Scholar]
- 27.Lin CC, Kenyon L, Hyslop T, et al. Cyclooxygenase-2 (COX-2) expression in human meningioma as a function of tumor grade. Am J Clin Oncol. 2003;26:S98–S102. doi: 10.1097/01.COC.0000074308.97198.D0. [DOI] [PubMed] [Google Scholar]
- 28.Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res. 2001;61:4375–4381. [PubMed] [Google Scholar]
- 29.Reynolds LM, Dalton CF, Reynolds GP. Phospholipid fatty acids and neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci Lett. 2001;309:193–196. doi: 10.1016/s0304-3940(01)02071-7. [DOI] [PubMed] [Google Scholar]
- 30.Kokoglu E, Tuter Y, Sandikci KS, et al. Prostaglandin E2 levels in human brain tumor tissues and arachidonic acid levels in the plasma membrane of human brain tumors. Cancer Lett. 1998;132:17–21. doi: 10.1016/s0304-3835(98)00127-x. [DOI] [PubMed] [Google Scholar]
- 31.Majima M, Amano H, Hayashi I. Prostanoid receptor signaling relevant to tumor growth and angiogenesis. Trends Pharmacol Sci. 2003;24:524–529. doi: 10.1016/j.tips.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol. 2006;207:261–270. doi: 10.1002/jcp.20560. [DOI] [PubMed] [Google Scholar]
- 33.Markowitz SD. Aspirin and colon cancer—targeting prevention? N Engl J Med. 2007;356:2195–2198. doi: 10.1056/NEJMe078044. [DOI] [PubMed] [Google Scholar]
- 34.Pozzi A, Yan X, Macias-Perez I, et al. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J Biol Chem. 2004;279:29797–29804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. Cellular FLICE-inhibitory protein down-regulation contributes to celecoxib-induced apoptosis in human lung cancer cells. Cancer Res. 2006;66:11115–11119. doi: 10.1158/0008-5472.CAN-06-2471. [DOI] [PubMed] [Google Scholar]
- 36.Ponthan F, Wickstrom M, Gleissman H, et al. Celecoxib prevents neuroblastoma tumor development and potentiates the effect of chemotherapeutic drugs in vitro and in vivo. Clin Cancer Res. 2007;13:1036–1044. doi: 10.1158/1078-0432.CCR-06-1908. [DOI] [PubMed] [Google Scholar]
- 37.Shin YK, Park JS, Kim HS, et al. Radiosensitivity enhancement by celecoxib, a cyclooxygenase (COX)-2 selective inhibitor, via COX-2-dependent cell cycle regulation on human cancer cells expressing differential COX-2 levels. Cancer Res. 2005;65:9501–9509. doi: 10.1158/0008-5472.CAN-05-0220. [DOI] [PubMed] [Google Scholar]
- 38.Williams CS, Watson AJ, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–6051. [PubMed] [Google Scholar]
- 39.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 40.Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98:736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- 41.Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 42.Kulp SK, Yang YT, Hung CC, et al. 3-Phosphoinositide-dependent protein kinase-1/Akt signaling represents a major cyclooxygenase-2-independent target for celecoxib in prostate cancer cells. Cancer Res. 2004;64:1444–1451. doi: 10.1158/0008-5472.can-03-2396. [DOI] [PubMed] [Google Scholar]
- 43.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 44.Johnson AJ, Hsu AL, Lin HP, Song X, Chen CS. The cyclo-oxygenase-2 inhibitor celecoxib perturbs intracellular calcium by inhibiting endoplasmic reticulum Ca2+-ATPases: a plausible link with its anti-tumour effect and cardiovascular risks. Biochem J. 2002;366:831–837. doi: 10.1042/BJ20020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JL, Lin KL, Chen JS, et al. Effect of celecoxib on Ca2+ movement and cell proliferation in human osteoblasts. Biochem Pharmacol. 2004;67:1123–1130. doi: 10.1016/j.bcp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Johnsen JI, Lindskog M, Ponthan F, et al. NSAIDs in neuroblastoma therapy. Cancer Lett. 2005;228:195–201. doi: 10.1016/j.canlet.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 47.Stempak D, Gammon J, Klein J, Koren G, Baruchel S. Single-dose and steady-state pharmacokinetics of celecoxib in children. Clin Pharmacol Ther. 2002;72:490–497. doi: 10.1067/mcp.2002.129322. [DOI] [PubMed] [Google Scholar]
- 48.Jones MK, Wang H, Peskar BM, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 49.Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625–1629. [PubMed] [Google Scholar]
- 50.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 51.von Rahden BH, Stein HJ, Puhringer F, et al. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005;65:5038–5044. doi: 10.1158/0008-5472.CAN-04-1107. [DOI] [PubMed] [Google Scholar]
- 52.Cervi D, Klement G, Stempak D, Baruchel S, Koki A, Ben-David Y. Targeting cyclooxygenase-2 reduces overt toxicity toward low-dose vinblastine and extends survival of juvenile mice with Friend disease. Clin Cancer Res. 2005;11:712–719. [PubMed] [Google Scholar]
- 53.Choy H, Milas L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: a rational advance? J Natl Cancer Inst. 2003;95:1440–1452. doi: 10.1093/jnci/djg058. [DOI] [PubMed] [Google Scholar]
- 54.Dandekar DS, Lopez M, Carey RI, Lokeshwar BL. Cyclooxygenase-2 inhibitor celecoxib augments chemotherapeutic drug-induced apoptosis by enhancing activation of caspase-3 and -9 in prostate cancer cells. Int J Cancer. 2005;115:484–492. doi: 10.1002/ijc.20878. [DOI] [PubMed] [Google Scholar]