Abstract
Optic pathway/hypothalamic pilocytic astrocytomas in children are usually treated with chemotherapy following a surgical biopsy. In this report, we retrospectively considered the role of surgical intervention. In a series of 25 patients without neurofibromatosis type 1, the median age at initial treatment was 3.1 years (range, 0–15 years). Twenty cases were verified by histology, and five cases were diagnosed by MRI findings. Twenty-three patients received chemotherapy. All patients were alive at median follow-up of 66 months. Aims of surgery at the initiation of treatment were biopsy in 12 cases (1 stereotactic and 11 craniotomies) and debulking in 7 cases. The 11 open biopsies revealed pilocytic astrocytoma; however, noticeable complications occurred in five children after the biopsies. Review of preoperative MRIs showed that all had typical findings indicating pilocytic astrocytoma. The open biopsy offered no noteworthy benefit for the patients despite surgical risk and delay of chemotherapy. The extent of the seven resection surgeries was 70% or less removal, and postoperative adjuvant therapy was needed for six of the seven patients. The remaining six children who did not undergo surgery obtained remission with chemotherapy alone. After relapse in nine patients, 15 bulk-reduction surgeries were performed. Surgical resection was not curative in any patient. In five patients, mostly older children, cystic expansion of tumor was partially resected, resulting in additional remission. In conclusion, considering the risk of open surgery and the effectiveness of chemotherapy, the role of surgical intervention is restricted to bulk-reduction surgery only when it is inevitable, especially at relapse after chemotherapy.
Keywords: biopsy, hypothalamus, optic pathway, pilocytic astrocytoma, surgical removal
Optic pathway/hypothalamic glioma is a rare brain tumor that occurs mostly in young children. Initial manifestations are usually serious visual disturbance, hypothalamic dysfunction including diencephalic syndrome, or both. Although in general this tumor is WHO grade I pilocytic astrocytoma, in some patients, particularly in very young populations, the optic pathway/hypothalamic pilocytic astrocytoma (OPHPA) may show an aggressive clinical course, including dissemination through the cerebrospinal fluid pathway, and these are a variant type known as pilomyxoid astrocytoma.1,2
The literature contains a number of discussions concerning notable progress in the treatment of OPHPA, especially focusing on chemotherapy and radiation therapy.2–15 Neurosurgical management, which seems even now to offer major contributions to control of the tumor, has rarely been given attention.14,16,17
In the young population with neurofibromatosis type 1 (NF-1)–associated OPHPA, decisions to initiate chemotherapy are generally made without biopsy and are guided by ophthalmological and imaging examinations.18,19 In cases of sporadic OPHPA in non-NF-1 patients, a biopsy or a partial resection by craniotomy to confirm histology remains the first mode of treatment.3,14
In addition to cases requiring the histopathological diagnosis, certain cases refractory to chemotherapy, cases with a large mass causing obstructive hydrocephalus, or cases with cystic expansion compressing the optic pathway occasionally require surgical debulking during the long clinical course. This report reconsiders the role of surgical interventions for various stages of OPHPAs, excluding NF-1–associated tumors.
Materials and Methods
We retrospectively assessed a series of 25 consecutive patients who had a clinical diagnosis of a sporadic OPHPA from a review of clinical records since 1992, when high-resolution MRI became routinely available. We excluded NF-1–associated gliomas, single optic nerve gliomas, unilateral hypothalamic gliomas, and quiescent cases found in patients older than 15 years because these tumors have different natural histories and require dissimilar treatment strategies.8,18
Histology (pilocytic astrocytoma, including pilomyxoid type) was verified in 20 of the 25 patients, with 19 patients undergoing surgery at the initiation of therapy and another at relapse. The remaining five patients were diagnosed by pathognomonic radiological appearance and typical clinical manifestations. Fifteen of the 25 patients had newly diagnosed disease; the remaining 10 patients were referred to our hospital after a biopsy or at the time of relapse. The median ages of symptom onset and initial treatment were 1 year (range, 0–14 years) and 3.1 years (range, 0–15 years), respectively.
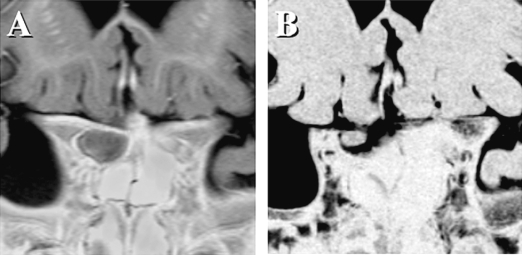
At the time of initial diagnosis of brain tumor, all cases, in retrospect, had a typical appearance suggestive of pilocytic astrocytoma on high-resolution MRI (Fig. 1A,B) and CT, located in the midline involving the optic pathway and hypothalamus. Although the majority of the 25 tumors appeared as apparently well-demarcated masses on MRI, two had a predominantly infiltrative pattern involving the whole optic pathway, including the bilateral optic nerves, chiasm, tracts, geniculate ganglions, internal capsules, and optic radiations. Contrast enhancement on MRI was present for the most part in all but one case; in this latter case, a large tumor (involving the chiasm, right optic tract, and bilateral hypothalamus and occupying the third ventricle) exhibited a scarce enhancement pattern. To resolve obstructive hydrocephalus, the tumor was subtotally resected and found to be pilocytic astrocytoma upon histological examination. The maximum diameters of the 25 tumors, encompassing globular masses but excluding the infiltrating part to the surrounding brain, ranged from 34 to 65 mm. No cases had dissemination at the time of diagnosis.
Fig. 1.
MR images of a 1-year-old patient demonstrate typical appearance of pilocytic astrocytoma. T2-weighted axial image (A) shows a high-signal intensity suprasellar mass that is homogeneously enhanced with gadolinium contrast on T1-weighted image (B). After six cycles of chemotherapy, the tumor almost completely disappeared on usual MR images; however, a coronal image of three-dimensional MR cisternography (C) depicted a tiny residual tumor within the right side of the chiasm.
Initial manifestations and reasons for initiation of therapy were diverse. Fifteen (60%) of the 25 children had their initial symptom before 2 years of age. Twenty children had visual impairment at the time of correct diagnosis. The interval between onset of initial manifestation and diagnosis of brain tumor was longer than 2 years for seven children. Among them, five children who were younger than 3 years at diagnosis showed a long-term history of visual impairment without hypothalamic dysfunction that had not been recognized by their parents. In contrast, 9 of 10 infants presenting recognizable symptoms, including either pendular/fixation nystagmus or diencephalic syndrome such as emaciation, anorexia, and weight loss, had diagnosis without delay. Others were found with headache due to obstructive hydrocephalus, dwarfism, or precocious puberty or had tumors that were found incidentally. An 8-year-old patient whose tumor was found incidentally was initially asymptomatic, but the tumor grew during a 3-year observation period and caused obstructive hydrocephalus and a slight visual field defect. All the patients were therefore symptomatic at the time of initial treatment.
The aims of surgery, at the initiation of treatment, were biopsy (very limited resection) in 12 cases (1 stereotactic surgery and 11 craniotomies) and tumor debulking in 7 cases. If a typical low-grade astrocytoma was encountered during craniotomy and limited resection was then performed, it was evaluated as a “biopsy” case in this report. Partial removal was defined as less than 90% resection. These 19 patients underwent surgery for progressive symptoms or progressive tumor growth. In six patients, the decision to initiate chemotherapy was made without biopsy and was guided by serial MRI examinations. In nine patients with relapse, a total of 15 salvage surgeries were performed. In the present series, 21 (62%) of 34 various craniotomies were performed by the senior author (Y.S.). The surgical procedures applied were pterional frontobasal transsylvian, transcallosal interseptal, transcallosal trans-foramen of Monro, frontal transcortical, and interhemispheric trans-lamina terminalis approaches.
Twenty-three (92%) patients were treated with chemotherapy, and six patients (24%) with a relapsing tumor after chemotherapy received radiation therapy. The remaining two patients underwent surgical resection alone without adjuvant therapy. Generally, three chemotherapeutic regimens were used: cisplatin with vincristine,5 carboplatin with vincristine, and temozolomide.
Neurological and radiological examinations were performed before and after surgery. Assessment of overall response was based on tumor evaluation by MRI and interpreted according to the Response Evaluation Criteria in Solid Tumors (RECIST).20 Complete surgical resection was defined as no visible tumor found on high-resolution postsurgical MRI and was not based on surgical record.
Results
Outcome of Patients
All 25 children tolerated the various therapies well and were alive at median follow-up of 66 months. Among them, only one patient achieved a completely tumor-free status of 9–year duration, after spontaneous complete involution on serial MRI observation. Final outcome of the remaining 24 patients could not be determined due to the short period of observation. At the final observation, Karnofsky performance status was better than or equal to 70% in 20 patients. All children, except one who was completely blind at birth, retained functional vision in at least one eye.
Initial Therapy in Cases with Surgical Biopsy or Resection
At the initiation of treatment, 19 patients underwent surgery, including stereotactic biopsy in 1 case, craniotomy biopsy (limited resection) in 11 cases, and debulking surgery in 7 cases. No endoscopic biopsy was applied in this series. Five children received a ventriculoperitoneal shunt for obstructive hydrocephalus. Because of the location of a tumor at the bottom of the third ventricle, endoscopic third ventriculostomy was not available for any patient.
Eight of 12 biopsies were performed at a previous hospital, with these children then referred to our institution. The 12 biopsies revealed a histological diagnosis of WHO grade I pilocytic astrocytoma, including pilomyxoid type in seven patients. There were no cases of anaplastic tumor. One tumor was initially diagnosed as fibrillary astrocytoma, but pathology review confirmed pilocytic astrocytoma. Because 7 of these 11 children were younger than 4 years, precise assessments of their visual function (including visual field evaluation and minimal change of cognitive function) were not possible. Noticeable postsurgical symptomatic complications were observed in five patients. One patient had deterioration of cognition after consciousness disturbance for 2 weeks. One had worsened bitemporal hemianopsia. One had postsurgical epileptic seizures. One had moderate, but transient, hemiparesis. In two infants who underwent a frontal interhemispheric approach, postsurgical MRI examinations showed medial frontal malacia in the rectal and cingulate gyri on T2-weighted images; one was symptomatic (consciousness disturbance for 2 weeks), and the other was asymptomatic. In another child, a small cerebral infarction was found due to a perforating artery injury originating from the middle cerebral artery, although this child seemed asymptomatic.
For initial treatment, bulk-reduction surgery by craniotomy was performed in seven children with large-volume tumors (Table 1). The senior author (Y.S.) performed two of these craniotomies. Although obstructive hydrocephalus in two children resolved after craniotomy, the extent of resection surgeries appeared to be insufficient, resulting in removal of 70% or less of the tumor volume. Following the surgery, six patients received adjuvant chemotherapy. One child received radiation therapy at 6 years of age, 4 years after initial surgery, due to an aggressive relapse after cycles of chemotherapy with carboplatin and vincristine. As a result, benefits of the first resection surgery were obscure for the seven children; nevertheless, postsurgical complications were considerable, as shown in Table 1.
Table 1.
Results of bulk-reduction surgery at initial treatment
| Patient Age | Route (Approach) | Postsurgical Complications | Brain Tissue Damage | Extent of Surgery (Volume) |
|---|---|---|---|---|
| 2 years | Frontal interhemispheric | Epilepsy | Mesial frontal lobe | <50% |
| 1 year | Anterior transcallosal | Cerebral salt wasting syndrome, slight right hemiparesis, possible cognitive deterioration | Fornix, anterior commissure, right deep frontal, hypothalamus | <50% |
| 3 years | Right frontal transcortical | Hypothalamic dysfunction, body temperature dysregulation, mental deterioration, worsened vision | Right frontal lobe, hypothalamus, visual pathway | ~70% |
| 3 years | Frontal interhemispheric | Panhypopituitarism, epilepsy, right visual loss | Optic nerve, chiasm, hypothalamus, stalk, mesial frontal lobe | <50% |
| 3 years | Anterior transcallosal through foramen of Monro | None | None | <50% |
| 7 months | Frontobasal transsylvian | None (total blindness prior to surgery) | Resection of right optic nerve | <50% |
| 5 months | Frontobasal transsylvian | Right visual loss | Right optic nerve and chiasm | <50% |
Initial Therapy in Cases without Biopsy
Given patient age, initial manifestations, location of tumor, and preoperative radiological appearance, the decision to initiate chemotherapy was made without biopsy and was guided by serial MRI in six patients with typical features of OPHPA at initial diagnosis. All these patients had their disease newly diagnosed at Hokkaido University Hospital. They successfully obtained a durable remission after either six or eight cycles of chemotherapy using cisplatin and vincristine, although no patients achieved a complete response. One patient underwent unilateral optic nerve decompression during first-line chemotherapy. Two patients who had a large mass after completion of initial chemotherapy continually received second-line chemotherapy using either carboplatin/vincristine or temozolomide. Two of the six patients showed relapse: one patient, after a 34-month remission, was treated with temozolomide; the second patient, after a 65-month remission, required subsequent irradiation and then salvage surgery for bulk reduction. At median follow-up of 47 months, all six of these children were alive with stabilized residual mass on MRI.
Fig. 1 shows representative MR images for a 1-year-old patient who presented with diencephalic syndrome, progressive emaciation, and severe visual impairment. This child was given chemotherapy without biopsy. During the period of chemotherapy, the tumor had gradually shrunk toward the optic chiasm and finally disappeared almost completely on usual MR images. However, a coronal image of three-dimensional MR cisternography (three-dimensional constructive interference in steady-state MRI) showed a tiny residual tumor in the right side of the chiasm. The patient’s disease had been stable for 73 months with slightly improved visual acuity. For this child, either biopsy or partial resection would have resulted in additional visual impairment.
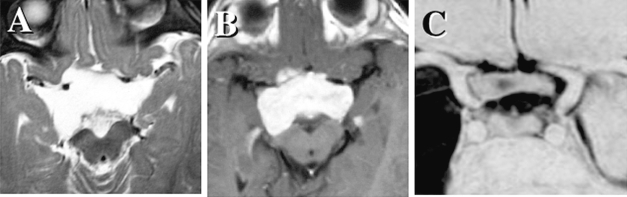
OPHPA occasionally infiltrates the optic nerve in the optic canal and orbit. In our series, only 3 of the 25 cases presented with optic nerve invasion in remarkably expanded optic canal(s). Fig. 2 shows a coronal image of a swollen optic nerve in the right optic canal. This patient had count-finger visual acuity on the right and 2/200 on the left. The right vision occasionally deteriorated to the level of light perception, especially when the patient had a high fever. Because of the deterioration of visual acuity during first-line chemotherapy, the optic canal was unroofed by an emergent craniotomy to decompress the affected nerve without tumor resection. Postoperatively, the patient’s visual acuity was preserved and 3 years later was 4/200 on the right and hand movement to 2/200 on the left. After cycles of chemotherapy, the swollen optic nerve gradually shrank.
Fig. 2.
A coronal image shows a swollen optic nerve in the right optic canal (A; multiple planner reconstruction image). Due to deterioration of visual acuity, the optic canal was unroofed to decompress the affected nerve. After cycles of chemotherapy, the optic nerve gradually shrank (B; three-dimensional MR cisternography).
Salvage Surgery for Relapse
Nine children underwent salvage surgery for relapse: 12 partial resections, 2 gross total removals, and 1 cyst puncture by image-guided stereotactic method. The salvage bulk-reduction surgery was performed to partially remove a relapsing tumor after prolonged chemotherapy and prior to second-line chemotherapy, to reduce a large mass prior to planned radiation therapy to solve obstructive hydrocephalus, or to remove the wall of the expanding cyst(s). Five patients underwent salvage surgery once, and three patients underwent such surgery twice. Another child, who had previously received various chemotherapies and radiation, underwent partial removal twice and then gross total removal twice.
Regarding postsurgical quality of life, complete surgical resection could not be achieved in any patient due to the invasive nature of the tumor into the optic pathway and the bilateral hypothalamus. Median follow-up for the nine patients was 128 months (range, 42–174 months), and all were alive at the final observation.
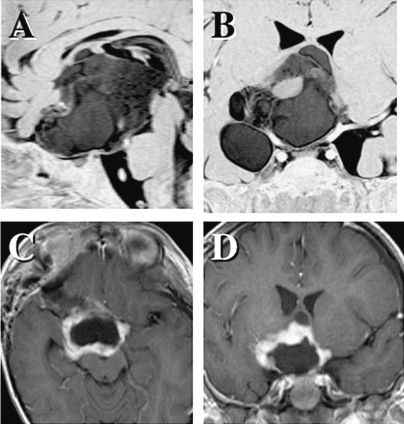
Fig. 3 shows a large pilocytic astrocytoma in a 33-month-old patient who had been given cycles of chemotherapy following initial biopsy when she was 6 months old. Because the second partial removal was insufficient, the patient was referred to our institution. To further reduce the large volume, a radical resection was performed, leaving the tumor margin intact to preserve the residual hypothalamic function and very poor vision of the left eye. Maintenance chemotherapy using temozolomide is ongoing at the time of this writing. Relapsing tumor of a similar size was seen in three children, and all involved the circle of Willis, including its numerous perforators, as well as the optic pathway and the hypothalamus. In these cases, the risk posed by sufficient bulk-reduction surgery that was required prior to either alternative chemotherapy or radiation therapy was extremely high, although we fortunately did not observe any unacceptable postsurgical sequelae.
Fig. 3.
A progressively growing pilocytic astrocytoma in a 2-year-old child who underwent long-term chemotherapy and partial tumor removal twice (A and B). Through the frontobasal transsylvian route, a radical third resection was performed, leaving the tumor margin intact (C and D). Tumors of this size involve the circle of Willis, including its numerous perforators.
Spontaneous involution after partial resection was observed in three patients. In the case shown in Fig. 4, the mass filling in the third ventricle was selectively removed, leaving a part of the tumor infiltrating the chiasm and the hypothalamus, because the patient still retained good vision and pituitary function without diabetes insipidus. Although the selective excision was technically hard, intraoperative observation revealed that the proliferating part of a relapsing tumor was very soft and easily resectable, while the tumor infiltrating the brain tissue was relatively firm, probably due to a mixed gliofibrous component.
Fig. 4.
A relapsing tumor (A) in a 7-year-old boy, who had received chemotherapy for 7 years following a partial resection. MR image shows a homogeneous tumor, but the tumor infiltrating the chiasm and the hypothalamus was relatively firm, and only the soft mass filling in the third ventricle was selectively resected (B). Five years later, when the patient was 13 years old, the residual tumor had spontaneously shrunk, and visual function was preserved (C and D).
For one child who had received chemotherapy, radiation therapy, and partial resections with transection of the left optic nerve over a period of 8 years, a radical total resection of a relapsing tiny tumor adherent on the chiasm was attempted. The result of surgery was evaluated as a gross total removal on MRI. Three years later, the tumor eventually recurred on the left optic tract and was radically resected again. This patient had been receiving maintenance chemotherapy using temozolomide, and he was tumor free on MRI for an additional 2 years, maintaining half nasal-side vision of the right eye. Whether this final surgery could lead to a disease cure is not clear because of the short observation period.
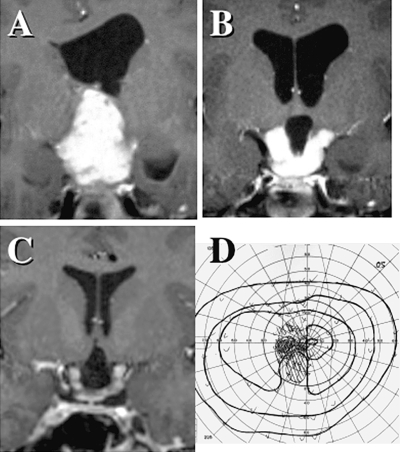
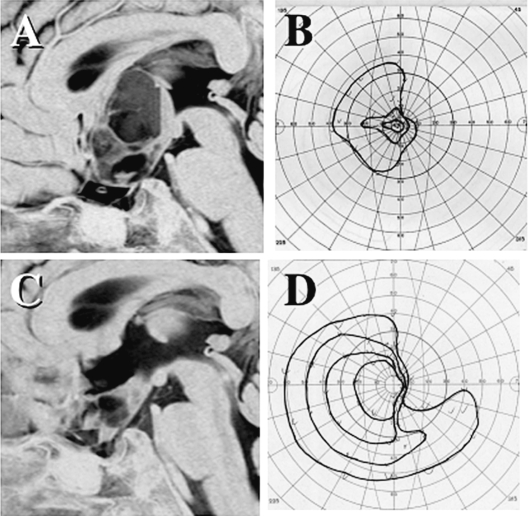
Multi- or single-cystic expansion of the tumor was observed in five older patients after a long disease course, with enlargement of tumor cyst(s) occurring at 4, 8, 10, 15, and 18 years of age. Four of the five patients underwent partial resection of the cyst wall by craniotomy, and the fifth patient underwent an image-guided stereotactic puncture of a subcutaneous reservoir. Only one experienced a further relapse of multiple cysts and was treated by craniotomy again. These surgical treatments resulted in further remission in all five patients, and three resulted in improvement of deteriorating vision. Among them, a spontaneous complete involution of the residual enhancing mass was observed in the child who received stereotactic puncture of the cyst. Pathological examinations showed predominantly degenerative changes of pilocytic astrocytoma, in which the index for Ki-67 staining was less than 1%. Fig. 5 shows a multicystic expansion of a pilocytic astrocytoma. The volume of tumor parenchyma enhanced with gadolinium contrast had been stable. The visual function of this 10-year-old patient was rapidly deteriorating. Partial removal of the cyst wall by craniotomy resulted in collapse of most of the cysts and improvement in the patient’s vision. A selective resection of cystic walls preserving the remaining optic pathway always requires precise presurgical MRI investigation with three-dimensional MR cisternography and careful surgical planning.
Fig. 5.
A multicystic expansion of a pilocytic astrocytoma after 9-year remission following initial chemotherapy and radiation therapy (A). The visual function was deteriorated (B). A partial removal of the cyst wall by a craniotomy achieved collapse of most of the cysts (C) and improvement of vision (D).
Discussion
All 25 children tolerated the various therapies well, and all were alive at the time of final observation. Among them, only one patient definitely achieved a tumor-free status. The long-term final outcome of these patients will be clearer 10 or more years after this report. Therefore, we here focus on the efficacy of the surgical interventions.
Biopsy at the Initiation of Therapy
In 1995, Sutton et al.14 reported the long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. In their series, all patients who had globular suprasellar masses without involvement of optic nerves or optic radiations underwent surgical exploration. The goal of this surgery was primarily to establish a histological diagnosis, and if a typical low-grade astrocytoma was encountered, a biopsy and limited resection were performed. Large masses obstructing the foramen of Monro were debulked to relieve ventricular obstruction, but generally no attempt at gross total excision was made. This concept may have been widely accepted as a standard surgical strategy for OPHPA at initial treatment.3,21
Currently, OPHPA in children with usual clinical and radiographic appearance using modern MRI technique can be more correctly diagnosed without surgical biopsy. The majority of such patients respond to platinum-based chemotherapy.3–7,9–11 Serious visual dysfunction can be improved by chemotherapy, and a chemotherapy-first strategy can preserve the intellectual outcome of patients who thereby avoid the need for radiotherapy.22,23 In our series, 11 biopsies (limited resections) by craniotomy in fact caused remarkable complications related to surgical procedure. We speculate that these were attributable to incorrect preoperative diagnosis or insufficient experience of the surgeon. For example, two young children had a preoperative diagnosis of craniopharyngioma; however, pilocytic astrocytoma was suggested by frozen section during the craniotomy. In these children, a bifrontal interhemispheric approach by a large craniotomy was used; nevertheless, the surgery produced unnecessary complications. In contrast to chemotherapy, the result of surgical intervention depends on the surgeon’s skill and experience. The benefit of surgical biopsy at initiation of therapy may therefore be ambiguous considering surgical morbidities, cost, and delay of chemotherapy. Silva et al.11 treated 14 young children, including four patients after endoscopic biopsy and five patients without biopsy, and recommended chemotherapy as a primary treatment for optic pathway/hypothalamic gliomas.
Gliomas in similar locations, such as NF-1–associated gliomas, single optic nerve gliomas, hypothalamic hamartomas, and unilateral hypothalamic gliomas, require a treatment strategy distinct from that for OPHPA. Excluding these instances by MRI examination, the vast majority of gliomas involving the optic pathway and bilateral hypothalamus in children are pilocytic astrocytomas.8,14,24 Other tumors in the same region, however, may mimic OPHPA and would necessitate different therapeutic approaches. In addition to routine imaging studies and endocrinological assessments, examinations of tumor markers in the serum and cerebrospinal fluid and high-resolution MR images, such as MR cisternography or multiple planner reconstruction image, should suitably assist the diagnosis, to rule out craniopharyngioma, various germ cell tumors, Langer-hans cell histiocytosis, hypothalamic hamartoma, diffuse astrocytoma, or ganglioglioma. In some cases, surgical biopsy is indispensable to confirm histological diagnosis. In some instances, image-guided stereotactic biopsy or endoscopic biopsy may be feasible and valuable. These procedures carry some risk, and difficulty may be experienced in small children without dilatation of lateral ventricles.
Pilomyxoid astrocytoma, an infantile variant with known aggressive potential, may be more susceptible to chemotherapy and exhibit more typical features on MRI compared with classical pilocytic astrocytomas found in older children,2,4,6–10,15,21,24 given that children younger than 1 year have a higher risk for tumor progression than do older children.6,8,15,17 The surgical risk of craniotomy for very small children is clearly high. Furthermore, a combination of surgical operation and histological examination may delay initiation of chemotherapy. Moreover, surgical removal might introduce tumor cell seeds into the cerebrospinal fluid pathway. There may be no place for surgical exploration in the treatment of young children, except for endoscopic biopsy.
Partial Resection at the Initiation of Therapy
In addition to the use of biopsy for diagnosis, some investigators have advocated resection for large tumors, and certain children can obtain long-term amelioration by initial surgical resection alone.16,17 In 2002, Steinbok et al.25 reported surgical results for 18 chiasmatic-hypothalamic astrocytomas; eight patients had subtotal resections, six had partial resections, three had limited resections, and one had no surgery. Fewer complications were associated with the limited resections, especially with respect to hypothalamic dysfunction. There was no correlation between the extent of resection and the time to tumor progression. The chiasmatic-hypothalamic tumors caused more morbidity than did the chiasmatic tumors, and radical resections did not prolong time to progression compared with more limited resections. The authors concluded that if surgery is performed, it may be appropriate to do a surgical procedure that strives only to provide a tissue diagnosis and to decompress the optic apparatus and/or ventricular system.
We agree with the conclusion of Steinbok et al.25 Selective decompression of the optic apparatus, however, appeared to be difficult. Precise estimation of visual dysfunction as a consequence of partial resection is difficult to nearly impossible, especially in young children. Surgical morbidity in the previous reports might have been underestimated with respect to visual function in children. In addition, as demonstrated in Fig. 1, if the tumor is located mainly in the chiasm, a partial excision of the tumor will injure the visual pathway.
Concerning the complications and invasiveness of such debulking surgery, decompression of the ventricular system by a partial resection can be replaced with a ventriculoperitoneal shunt, with or without endoscopic septotomy. Only when chemotherapy fails to induce or maintain remission should salvage debulking surgery be planned.
Salvage Resection Surgery
Chemotherapy is increasingly being used and is occasionally curative, and it provides a stabilizing role in most cases.3,6,8,9,11 Following initial induction chemotherapy, even if it was effective and given for a long period, many children with OPHPA experience tumor relapse. The second-line treatment may be alternative chemotherapy, and radiation therapy might then be considered at further progression.6 Because the long-term effectiveness of conventional fractionated radiation therapy, including recent stereotactic techniques, is superior to that of chemotherapy,3,26–28 radiation therapy is available for older children with a localized relapse. However, some OPHPAs are “surgically amenable” in a subset of patients with recurrent disease.14,17
It is known that optic gliomas in non-NF-1 patients often regress spontaneously.13,17,29 If most pilocytic astrocytomas have a limited time span for growing and lower age is a worse prognostic factor,8,21,30 a partial resection to ameliorate symptoms or to reduce mass effect of a growing tumor may be worthwhile. In our series of patients, spontaneous involution after partial resection or cyst puncture of a relapsing tumor was observed in four patients. This result may warrant a salvage maximal (partial) excision for relapsing tumor with parenchymal growth, especially in older children.
Radical Surgery and Complication
Radical surgery for patients with OPHPA carries the risk of damage to the hypothalamus, visual apparatus, and vascular structures.17 In addition, no complete surgical resection could be achieved when the functional outcome was seriously considered.2,14,24 Neurosurgeons, however, seldom have the opportunity to operate on OPHPA, which is an uncommon childhood brain tumor. In addition, the surgical strategy and technique are extremely complex. In our series of patients, the complication rates from biopsy and bulk-reduction surgery at the initiation of therapy seemed to be unexpectedly high compared with literature reports.2,3,11,14,17,21 Definitive permanent deficits occurred in two children after 11 open biopsies and in four children after 7 bulk-reduction surgeries. Few of these complications, however, resulted from the surgeries performed by the senior author, suggesting an effect of surgeon experience.
It is of note that results of such surgical excisions may vary and that the results greatly depend on the experience of the neurosurgeon. Although OPHPA should be operated on by an experienced surgeon, results will vary with the specific conditions in each case. Radical surgical resection of OPHPA will therefore generally be offered only when the tumor has progressed despite feasible chemotherapy or, in certain cases, radiation therapy.
Partial Removal for Cystic Expansion
Cystic tumor expansion without parenchymal growth occurred mostly in older children. The formation of cysts may be a consequence of tumor degeneration and may occur prior to spontaneous involution, as observed in cases of vestibular schwannoma. The growing cyst (containing proteinaceous fluid) appeared to be refractory to both chemotherapy and radiation therapy. An intended nonaggressive partial resection of cyst wall(s) or stereotactic puncture can ameliorate progressive visual disturbance and produce a certain term of remission.
Optic Nerve Decompression
OPHPA occasionally involves the optic nerves in the optic canal. The swollen optic nerve expands the bony optic canal, especially in very young children (younger than ~4 years of age) and may result in entrapment neuropathy of the optic nerve itself. To our knowledge, no report has described the efficacy of optic nerve decompression for such cases. Although we had a solitary case of successful decompression in the present series, this surgical procedure may be applied to rescue very poor vision caused by optic nerve invasion by tumor cells.
In conclusion, to treat OPHPA in young children, surgical biopsy appears to be dispensable for clinically or radiologically typical cases. A curative resection is rarely achieved when the functional outcome of patients is seriously respected. The role of surgical intervention may be restricted to bulk-reduction surgery only when it is inevitable. However, during the long clinical course of OPHPA in children, especially at relapse, both chemotherapy and radiation therapy have to be selected considering a variety of available surgical treatments with neurosurgeons who have profound experience with this unique tumor.
Acknowledgment
This study was supported partly by Clinical Cancer Research and Health and Labor Sciences research grants (H17-ganrinsyou-ippan-005) from the Ministry of Health, Labour and Welfare.
References
- 1.Chikai K, Ohnishi A, Kato T, et al. Clinico-pathological features of pilomyxoid astrocytoma of the optic pathway. Acta Neuropathol (Berl) 2004;108:109–114. doi: 10.1007/s00401-004-0858-7. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez C, Figarella-Branger D, Girard N, et al. Pilocytic astrocytomas in children: prognostic factors—a retrospective study of 80 cases. Neurosurgery. 2003;53:544–555. doi: 10.1227/01.neu.0000079330.01541.6e. [DOI] [PubMed] [Google Scholar]
- 3.Fouladi M, Wallace D, Langston JW, et al. Survival and functional outcome of children with hypothalamic/chiasmatic tumors. Cancer. 2003;97:1084–1092. doi: 10.1002/cncr.11119. [DOI] [PubMed] [Google Scholar]
- 4.Gururangan S, Cavazos CM, Ashley D, et al. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;13:2951–2958. doi: 10.1200/JCO.2002.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Kato T, Sawamura Y, Tada M, Ikeda J, Ishii N, Abe H. Cisplatin/vincristine chemotherapy for hypothalamic/visual pathway astrocytomas in young children. J Neurooncol. 1998;37:263–270. doi: 10.1023/a:1005866021835. [DOI] [PubMed] [Google Scholar]
- 6.Laithier V, Grill J, Le Deley MC, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy—results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 7.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 8.Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42:1807–1816. doi: 10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 10.Packer RJ, Sutton LN, Bilaniuk LT, et al. Treatment of chiasmatic/hypothalamic gliomas of childhood with chemotherapy: an update. Ann Neurol. 1988;23:79–85. doi: 10.1002/ana.410230113. [DOI] [PubMed] [Google Scholar]
- 11.Silva MM, Goldman S, Keating G, Marymont MA, Kalapurakal J, Tomita T. Optic pathway hypothalamic gliomas in children under three years of age: the role of chemotherapy. Pediatr Neurosurg. 2000;33:151–158. doi: 10.1159/000028996. [DOI] [PubMed] [Google Scholar]
- 12.Strong JA, Hatten HP, Jr, Brown MT, et al. Pilocytic astrocytoma: correlation between the initial imaging features and clinical aggressiveness. AJR Am J Roentgenol. 1993;161:369–372. doi: 10.2214/ajr.161.2.8333380. [DOI] [PubMed] [Google Scholar]
- 13.Suarez JC, Viano JC, Zunino S. Management of child optic pathway gliomas: new therapeutical option. Childs Nerv Syst. 2006;22:679–684. doi: 10.1007/s00381-005-0021-3. [DOI] [PubMed] [Google Scholar]
- 14.Sutton LN, Molloy PT, Sernyak H, et al. Long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. J Neurosurg. 1995;83:583–589. doi: 10.3171/jns.1995.83.4.0583. [DOI] [PubMed] [Google Scholar]
- 15.Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999;58:1061–1068. doi: 10.1097/00005072-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman HJ, Humphreys RP, Drake JM, et al. Optic pathway/hypothalamic gliomas: a dilemma in management. Pediatr Neurosurg. 1993;19:186–195. doi: 10.1159/000120729. [DOI] [PubMed] [Google Scholar]
- 17.Wisoff JH, Abbott R, Epstein F. Surgical management of exophytic chiasmatic-hypothalamic tumors of childhood. J Neurosurg. 1990;73:661–667. doi: 10.3171/jns.1990.73.5.0661. [DOI] [PubMed] [Google Scholar]
- 18.Grill J, Laithier V, Rodriguez D, Raquin MA, Pierre-Kahn A, Kalifa C. When do children with optic pathway tumours need treatment? An oncological perspective in 106 patients treated in a single centre. Eur J Pediatr. 2000;159:692–696. doi: 10.1007/s004310000531. [DOI] [PubMed] [Google Scholar]
- 19.Leonard JR, Perry A, Rubin JB, King AA, Chicoine MR, Gutmann DH. The role of surgical biopsy in the diagnosis of glioma in individuals with neurofibromatosis-1. Neurology. 2006;67:1509–1512. doi: 10.1212/01.wnl.0000240076.31298.47. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Chan MY, Foong AP, Heisey DM, Harkness W, Hayward R, Michalski A. Potential prognostic factors of relapse-free survival in childhood optic pathway glioma: a multivariate analysis. Pediatr Neurosurg. 1998;29:23–28. doi: 10.1159/000028680. [DOI] [PubMed] [Google Scholar]
- 22.Lacaze E, Kieffer V, Streri A, et al. Neuropsychological outcome in children with optic pathway tumours when first-line treatment is chemotherapy. Br J Cancer. 2003;89:2038–2044. doi: 10.1038/sj.bjc.6601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell AE, Elder JE, Mackey DA, Waters KD, Ashley DM. Visual improvement despite radiologically stable disease after treatment with carboplatin in children with progressive low-grade optic/thalamic gliomas. J Pediatr Hematol Oncol. 2001;23:572–577. doi: 10.1097/00043426-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Komotar RJ, Burger PC, Carson BS, et al. Pilocytic and pilomyxoid hypothalamic/chiasmatic astrocytomas. Neurosurgery. 2004;54:72–79. doi: 10.1227/01.neu.0000097266.89676.25. [DOI] [PubMed] [Google Scholar]
- 25.Steinbok P, Hentschel S, Almqvist P, Cochrane DD, Poskitt K. Management of optic chiasmatic/hypothalamic astrocytomas in children. Can J Neurol Sci. 2002;29:132–138. [PubMed] [Google Scholar]
- 26.Erkal HS, Serin M, Cakmak A. Management of optic pathway and chiasmatic-hypothalamic gliomas in children with radiation therapy. Radiother Oncol. 1997;45:11–15. doi: 10.1016/s0167-8140(97)00102-3. [DOI] [PubMed] [Google Scholar]
- 27.Gnekow AK, Kortmann RD, Pietsch T, Emser A. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy—report from the multicenter treatment study for children and adolescents with a low grade glioma—HIT-LGG 1996—of the Society of Pediatric Oncology and Hematology (GPOH) Klin Padiatr. 2004;216:331–342. doi: 10.1055/s-2004-832355. [DOI] [PubMed] [Google Scholar]
- 28.Marcus KJ, Goumnerova L, Billett AL, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61:374–379. doi: 10.1016/j.ijrobp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Parsa CF, Hoyt CS, Lesser RL, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001;119:516–529. doi: 10.1001/archopht.119.4.516. [DOI] [PubMed] [Google Scholar]
- 30.Palma L, Celli P, Mariottini A. Long-term follow-up of childhood cerebellar astrocytomas after incomplete resection with particular reference to arrested growth or spontaneous tumour regression. Acta Neurochir (Wien) 2004;146:581–588. doi: 10.1007/s00701-004-0257-9. [DOI] [PubMed] [Google Scholar]